High Frequency of PIK3CA Mutations in Low-Grade Serous Ovarian Carcinomas of Japanese Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tumor Aamples
2.2. Microdissection and DNA Extraction
2.3. Direct Sequence Analysis
2.4. Immunostaining of p53 and ARID1A
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Kurman, R.J.; Shih, I.M. The dualistic model of ovarian carcinogenesis: Revisited, revised, and expanded. Am. J. Pathol. 2016, 186, 733–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.K.; Tsang, Y.T.; Deavers, M.T.; Mok, S.C.; Zu, Z.; Sun, C.; Malpica, A.; Wolf, J.K.; Lu, K.H.; Gershenson, D.M. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am. J. Pathol. 2010, 177, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Wang, T.L.; Kurman, R.J.; Nakayama, K.; Velculescu, V.E.; Vogelstein, B.; Kinzler, K.W.; Papadopoulos, N.; Shih, I.M. Low-grade serous carcinomas of the ovary contain very few point mutations. J. Pathol. 2012, 226, 413–420. [Google Scholar] [CrossRef]
- Singer, G.; Kurman, R.J.; Chang, H.W.; Cho, S.K.; Shih, I.M. Diverse tumorigenic pathways in ovarian serous carcinoma. Am. J. Pathol. 2002, 160, 1223–1228. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Nakayama, K.; Ishibashi, T.; Ishikawa, N.; Ishikawa, M.; Katagiri, H.; Minamoto, T.; Sato, E.; Sanuki, K.; Yamashita, H.; et al. KRAS/BRAF analysis in ovarian low-grade serous carcinoma having synchronous all pathological precursor regions. Int. J. Mol. Sci. 2016, 17, 625. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, K.; Nakayama, N.; Kurman, R.J.; Cope, L.; Pohl, G.; Samuels, Y.; Velculescu, V.E.; Wang, T.L.; Shih, I.M. Sequence mutations and amplification of PIK3CA and AKT2 genes in purified ovarian serous neoplasms. Cancer Biol. Ther. 2006, 5, 779–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, S.A.; Beare, D.; Boutselakis, H.; Bamford, S.; Bindal, N.; Tate, J.; Cole, C.G.; Ward, S.; Dawson, E.; Ponting, L.; et al. COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids Res. 2017, 45, D777–D783. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, A.; Nakayama, K.; Rahman, M.T.; Rahman, M.; Katagiri, H.; Nakayama, N.; Ishikawa, M.; Ishibashi, T.; Iida, K.; Kobayashi, H.; et al. Loss of ARID1A expression is related to shorter progression-free survival and chemoresistance in ovarian clear cell carcinoma. Mod. Pathol. 2012, 25, 282–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, K.; Takebayashi, Y.; Nakayama, S.; Hata, K.; Fujiwaki, R.; Fukumoto, M.; Miyazaki, K. Prognostic value of overexpression of p53 in human ovarian carcinoma patients receiving cisplatin. Cancer Lett. 2003, 192, 227–235. [Google Scholar] [CrossRef]
- Xu, Y.; Bi, R.; Xiao, Y.; Tu, X.; Li, M.; Li, A.; Shan, L.; Zhou, S.; Yang, W. Low frequency of BRAF and KRAS mutations in Chinese patients with low-grade serous carcinoma of the ovary. Diagn. Pathol. 2017, 12, 87. [Google Scholar] [CrossRef] [Green Version]
- Van Nieuwenhuysen, E.; Busschaert, P.; Laenen, A.; Moerman, P.; Han, S.N.; Neven, P.; Lambrechts, D.; Vergote, I. Loss of 1p36.33 frequent in low-grade serous ovarian cancer. Neoplasia 2019, 21, 582–590. [Google Scholar] [CrossRef]
- Hunter, S.M.; Anglesio, M.S.; Ryland, G.L.; Sharma, R.; Chiew, Y.E.; Rowley, S.M.; Doyle, M.A.; Li, J.; Gilks, C.B.; Moss, P.; et al. Molecular profiling of low grade serous ovarian tumours identifies novel candidate driver genes. Oncotarget 2015, 6, 37663–37677. [Google Scholar] [CrossRef] [Green Version]
- Kurman, R.J.; Shih, I.M. Pathogenesis of ovarian cancer: Lessons from morphology and molecular biology and their clinical implications. Int. J. Gynecol. Pathol. 2008, 27, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Kohno, T. Implementation of “clinical sequencing” in cancer genome medicine in Japan. Cancer Sci. 2018, 109, 507–512. [Google Scholar] [CrossRef] [Green Version]
- Ardighieri, L.; Zeppernick, F.; Hannibal, C.G.; Vang, R.; Cope, L.; Junge, J.; Kjaer, S.K.; Kurman, R.J.; Shih, I.M. Mutational analysis of BRAF and KRAS in ovarian serous borderline (atypical proliferative) tumours and associated peritoneal implants. J. Pathol. 2014, 232, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Tsang, Y.T.; Deavers, M.T.; Sun, C.C.; Kwan, S.Y.; Kuo, E.; Malpica, A.; Mok, S.C.; Gershenson, D.M.; Wong, K.K. KRAS (but not BRAF) mutations in ovarian serous borderline tumour are associated with recurrent low-grade serous carcinoma. J. Pathol. 2013, 231, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Emmanuel, C.; Chiew, Y.E.; George, J.; Etemadmoghadam, D.; Anglesio, M.S.; Sharma, R.; Russell, P.; Kennedy, C.; Fereday, S.; Hung, J.; et al. Australian Ovarian Cancer Study (AOCS). Genomic classification of serous ovarian cancer with adjacent borderline differentiates RAS pathway and TP53-mutant tumors and identifies NRAS as an oncogenic driver. Clin. Cancer Res. 2014, 20, 6618–6630. [Google Scholar] [CrossRef] [Green Version]
- Gershenson, D.M.; Sun, C.C.; Wong, K.K. Impact of mutational status on survival in low-grade serous carcinoma of the ovary or peritoneum. Br. J. Cancer 2015, 113, 1254–1258. [Google Scholar] [CrossRef] [Green Version]
- Farley, J.; Brady, W.E.; Vathipadiekal, V.; Lankes, H.A.; Coleman, R.; Morgan, M.A.; Mannel, R.; Yamada, S.D.; Mutch, D.; Rodgers, W.H.; et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: An open-label, single-arm, phase 2 study. Lancet Oncol. 2013, 14, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Mendivil, A.A.; Tung, P.K.; Bohart, R.; Bechtol, K.; Goldstein, B.H. Dramatic clinical response following dabrafenib and trametinib therapy in a heavily pretreated low grade serous ovarian carcinoma patient with a BRAF V600E mutation. Gynecol. Oncol. Rep. 2018, 26, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Champer, M.; Miller, D.; Kuo, D.Y. Response to trametinib in recurrent low-grade serous ovarian cancer with NRAS mutation: A case report. Gynecol. Oncol. Rep. 2019, 28, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Freixinos, V.; Lheureux, S.; Mandilaras, V.; Clarke, B.; Dhani, N.C.; Mackay, H.; Butler, M.O.; Wang, L.; Siu, L.L.; Kamel-Reid, S.; et al. Impact of somatic molecular profiling on clinical trial outcomes in rare epithelial gynecologic cancer patients. Gynecol. Oncol. 2019, 153, 304–311. [Google Scholar] [CrossRef] [PubMed]
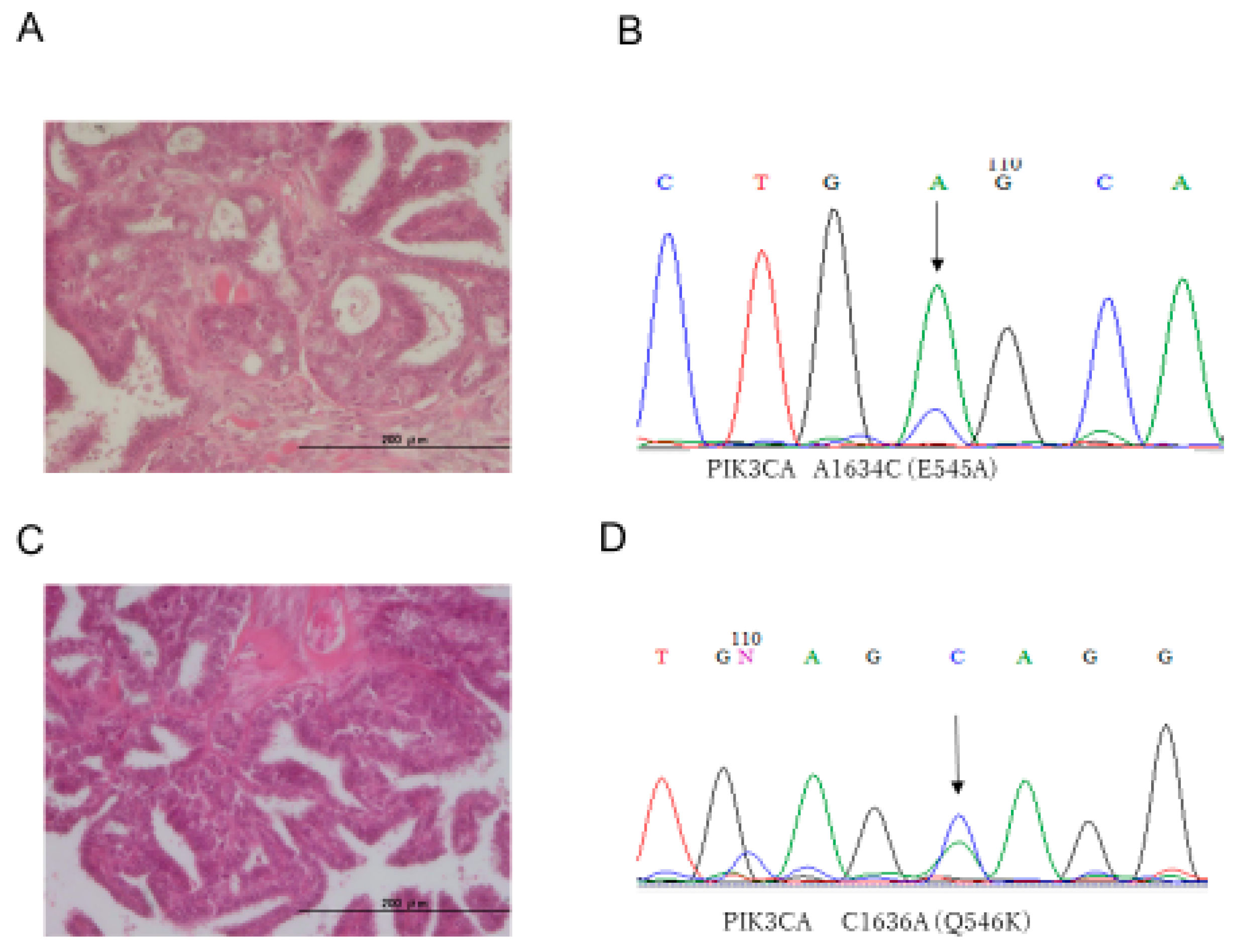

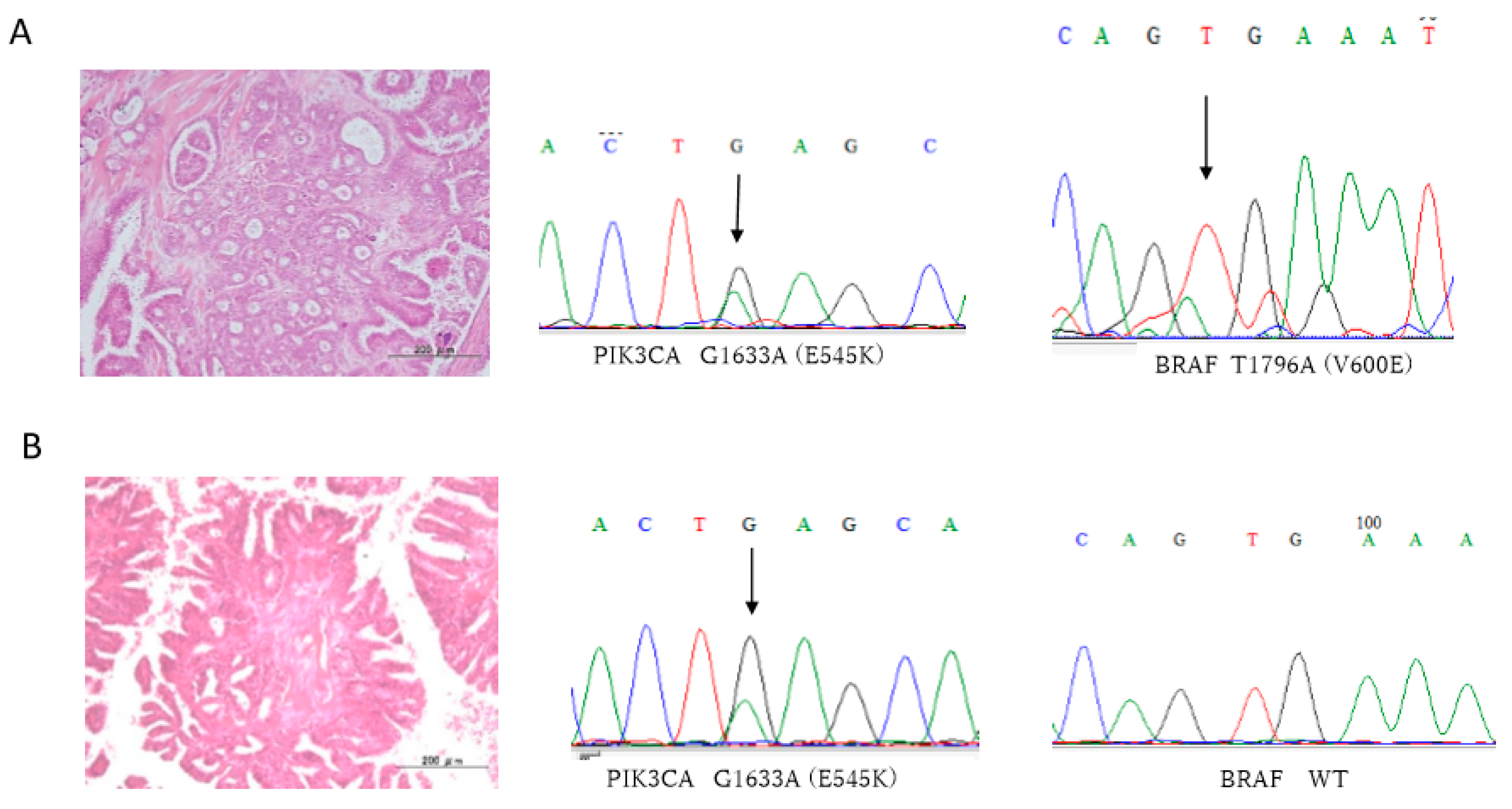

| No. | Age | FIGO Stage | KRAS | BRAF | PI3KCA E9 | PI3KCA E20 | ERBB2 | P53 | ARID1A |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | II c | WT | WT | WT | WT | A2384G (Q795R) | Normal | Normal |
| 2 | 61 | IV b | WT | WT | G1633A (E545K) | WT | WT | Normal | Normal |
| 3 | 83 | I a | WT | WT | A1634C (E545A) | WT | WT | Normal | Normal |
| 4 | 61 | I c | WT | WT | WT | WT | A2384G (Q795R) | Normal | Normal |
| 5 | 37 | III c | WT | WT | G1633C (E545Q) | WT | WT | Normal | Normal |
| 6 | 27 | I c | WT | T1796A (V600E) | A1634C (E545A) | WT | A2384G (Q795R) | Normal | Normal |
| 7 | 61 | III c | WT | WT | WT | WT | WT | Normal | Normal |
| 8 | 48 | I c | WT | WT | WT | WT | WT | Normal | Normal |
| 9 | 26 | III c | WT | WT | G1633A (E545K) | WT | WT | Normal | Normal |
| 10 | 40 | I c | WT | T1796A (V600E) | A1634C (E545A) | WT | WT | Normal | Normal |
| No. | Age | FIGO Stage | KRAS | BRAF | PI3KCA E9 | PI3KCA E20 | ERBB2 | P53 | ARID1A |
|---|---|---|---|---|---|---|---|---|---|
| 11 | 32 | I a | WT | WT | WT | WT | WT | Normal | Normal |
| 12 | 39 | I c | WT | WT | C1636A (Q546K) | WT | WT | Normal | Normal |
| 13 | 44 | III c | WT | WT | WT | WT | WT | Normal | Normal |
| 14 | 45 | I c | WT | WT | C1636A (Q546K) | WT | WT | Normal | Normal |
| 15 | 45 | III c | WT | WT | G1633C (E545Q) | WT | WT | Normal | Normal |
| 16 | 38 | I a | WT | WT | A1634C (E545A) | WT | WT | Positive | Normal |
| 17 | 25 | I a | WT | WT | WT | WT | WT | Normal | Normal |
| 18 | 48 | I a | WT | WT | C1636A (Q546K) | WT | WT | Normal | Normal |
| 19 | 69 | I a | WT | WT | C1636A (Q546K) | WT | WT | Normal | Normal |
| 20 | 57 | I a | WT | WT | C1636A (Q546K) | WT | WT | Normal | Normal |
| 21 | 66 | I c | WT | WT | WT | WT | WT | Normal | Normal |
| No. | Age | KRAS | BRAF | PI3KCA E9 | PI3KCA E20 | ERBB2 | P53 | ARID1A |
|---|---|---|---|---|---|---|---|---|
| 22 | 25 | WT | WT | WT | WT | WT | Normal | Normal |
| 23 | 73 | WT | WT | WT | WT | WT | Normal | Normal |
| 24 | 81 | WT | WT | WT | WT | WT | Normal | Normal |
| 25 | 47 | WT | WT | WT | WT | WT | Normal | Normal |
| 26 | 52 | WT | WT | WT | WT | A2384G (Q795R) | Normal | Normal |
| 27 | 71 | WT | WT | WT | WT | WT | Normal | Normal |
| 28 | 72 | WT | WT | WT | WT | WT | Normal | Normal |
| 29 | 75 | WT | WT | A1634C (E545A) | WT | WT | Normal | Normal |
| 30 | 55 | WT | WT | WT | WT | A2384G (Q795R) | Normal | Normal |
| 31 | 63 | WT | WT | WT | WT | WT | Normal | Normal |
| 32 | 63 | WT | WT | WT | WT | WT | Normal | Normal |
| 33 | 26 | WT | WT | WT | WT | WT | Normal | Normal |
| No. | Age | Stage | LGSC/SBT | KRAS | BRAF | PI3KCA E9 | PI3KCA E20 | ERBB2 | P53 | ARID1A |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | III c | LGSC SBT | WT WT | WT WT | WT WT | WT WT | A2384G (Q795R) A2384G (Q795R) | Normal Normal | Normal Normal |
| 6 | 27 | I c | LGSC SBT | WT WT | T1796A (V600E) WT | A1634G (E545G) WT | WT WT | A2384G (Q795R) WT | Normal Normal | Normal Normal |
| 8 | 48 | I c | LGSC SBT | WT WT | WT WT | WT WT | WT WT | WT WT | Normal Normal | Normal Normal |
| 10 | 40 | I c | LGSC SBT | WT WT | T1796A (V600E) WT | A1634C (E545A) A1634C (E545A) | WT WT | WT WT | Normal Normal | Normal Normal |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishibashi, T.; Nakayama, K.; Razia, S.; Ishikawa, M.; Nakamura, K.; Yamashita, H.; Dey, P.; Iida, K.; Kurioka, H.; Nakayama, S.; et al. High Frequency of PIK3CA Mutations in Low-Grade Serous Ovarian Carcinomas of Japanese Patients. Diagnostics 2020, 10, 13. https://doi.org/10.3390/diagnostics10010013
Ishibashi T, Nakayama K, Razia S, Ishikawa M, Nakamura K, Yamashita H, Dey P, Iida K, Kurioka H, Nakayama S, et al. High Frequency of PIK3CA Mutations in Low-Grade Serous Ovarian Carcinomas of Japanese Patients. Diagnostics. 2020; 10(1):13. https://doi.org/10.3390/diagnostics10010013
Chicago/Turabian StyleIshibashi, Tomoka, Kentaro Nakayama, Sultana Razia, Masako Ishikawa, Kohei Nakamura, Hitomi Yamashita, Puja Dey, Koji Iida, Hiroko Kurioka, Satoru Nakayama, and et al. 2020. "High Frequency of PIK3CA Mutations in Low-Grade Serous Ovarian Carcinomas of Japanese Patients" Diagnostics 10, no. 1: 13. https://doi.org/10.3390/diagnostics10010013
APA StyleIshibashi, T., Nakayama, K., Razia, S., Ishikawa, M., Nakamura, K., Yamashita, H., Dey, P., Iida, K., Kurioka, H., Nakayama, S., Otsuki, Y., Ishikawa, N., & Kyo, S. (2020). High Frequency of PIK3CA Mutations in Low-Grade Serous Ovarian Carcinomas of Japanese Patients. Diagnostics, 10(1), 13. https://doi.org/10.3390/diagnostics10010013






