Lyme Neuroborreliosis in a Patient with Breast Cancer: MRI and PET/CT Findings
Abstract
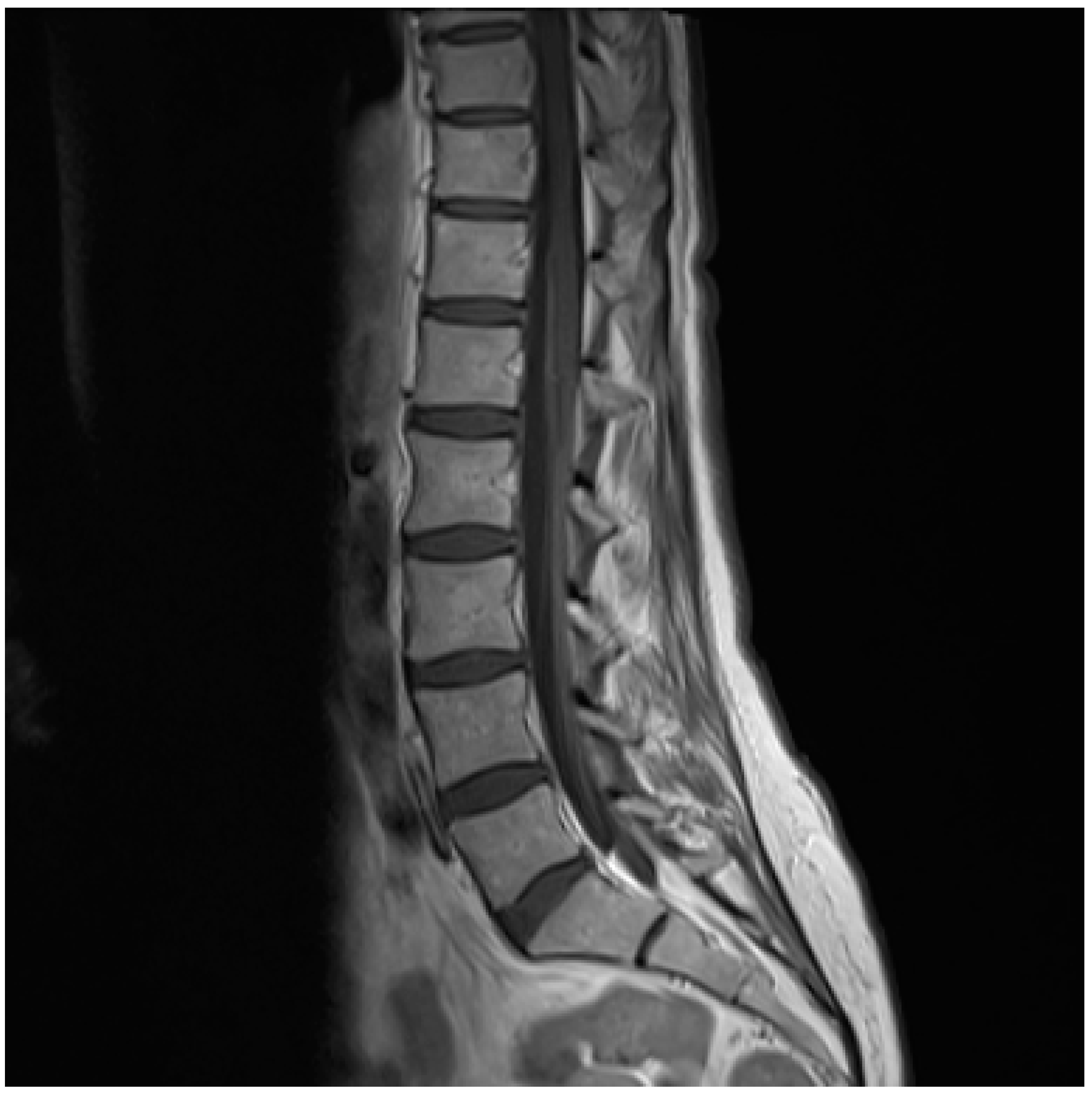
:

Consent for Publication
Conflicts of Interest
References
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Stanek, G.; Strle, F. Lyme borreliosis-from tick bite to diagnosis and treatment. FEMS Microbiol. Rev. 2018, 42, 233–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dessau, R.B.; Espenhain, L.; Molbak, K.; Krause, T.G.; Voldstedlund, M. Improving national surveillance of Lyme neuroborreliosis in Denmark through electronic reporting of specific antibody index testing from 2010 to 2012. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2015, 20. [Google Scholar] [CrossRef] [PubMed]
- Dahl, V.; Wisell, K.T.; Giske, C.G.; Tegnell, A.; Wallensten, A. Lyme neuroborreliosis epidemiology in Sweden 2010 to 2014: Clinical microbiology laboratories are a better data source than the hospital discharge diagnosis register. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2019, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindland, E.S.; Solheim, A.M.; Andreassen, S.; Quist-Paulsen, E.; Eikeland, R.; Ljostad, U.; Mygland, A.; Elsais, A.; Nygaard, G.O.; Lorentzen, A.R.; et al. Imaging in Lyme neuroborreliosis. Insights Imaging 2018, 9, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.E.; Rothberg, M.; Ferencz, G.; Wujack, D. Lyme disease of the CNS: MR imaging findings in 14 cases. Ajnr. Am. J. Neuroradiol. 1990, 11, 479–481. [Google Scholar] [PubMed]
- Oksi, J.; Kalimo, H.; Marttila, R.J.; Marjamaki, M.; Sonninen, P.; Nikoskelainen, J.; Viljanen, M.K. Inflammatory brain changes in Lyme borreliosis. A report on three patients and review of literature. Brain 1996, 119 Pt 6, 2143–2154. [Google Scholar] [CrossRef]
- Agosta, F.; Rocca, M.A.; Benedetti, B.; Capra, R.; Cordioli, C.; Filippi, M. MR imaging assessment of brain and cervical cord damage in patients with neuroborreliosis. Ajnr. Am. J. Neuroradiol. 2006, 27, 892–894. [Google Scholar] [PubMed]
- Hattingen, E.; Weidauer, S.; Kieslich, M.; Boda, V.; Zanella, F.E. MR imaging in neuroborreliosis of the cervical spinal cord. Eur. Radiol. 2004, 14, 2072–2075. [Google Scholar] [CrossRef] [PubMed]
- Niksefat, M.; Albashiti, B.; Burke, D.; Moshayedi, P.; Patira, R.; Knepper, L. Extensive meningeal enhancement in acute central nervous system Lyme: Case series and review of literature. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas 2019, 64, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Glaudemans, A.W.; de Vries, E.F.; Galli, F.; Dierckx, R.A.; Slart, R.H.; Signore, A. The use of (18)F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin. Dev. Immunol. 2013, 2013, 623036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newberg, A.; Hassan, A.; Alavi, A. Cerebral metabolic changes associated with Lyme disease. Nuclear Med. Commun. 2002, 23, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Blanc, F.; Philippi, N.; Cretin, B.; Kleitz, C.; Berly, L.; Jung, B.; Kremer, S.; Namer, I.J.; Sellal, F.; Jaulhac, B.; et al. Lyme neuroborreliosis and dementia. J. Alzheimer’s Dis. 2014, 41, 1087–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plotkin, M.; Hautzel, H.; Krause, B.J.; Mohr, S.; Langen, K.J.; Muller, H.W. Fluorine-18-labeled fluorodeoxyglucose-positron emission tomography studies of acute brainstem Lyme neuroborreliosis [corrected] Case report. J. Neurosurg. 2005, 102, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Kalina, P.; Decker, A.; Kornel, E.; Halperin, J.J. Lyme disease of the brainstem. Neuroradiology 2005, 47, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Eiffert, H.; Karsten, A.; Schlott, T.; Ohlenbusch, A.; Laskawi, R.; Hoppert, M.; Christen, H.J. Acute peripheral facial palsy in Lyme disease—A distal neuritis at the infection site. Neuropediatrics 2004, 35, 267–273. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ørbæk, M.; Klausen, C.; Lebech, A.-M.; Mens, H. Lyme Neuroborreliosis in a Patient with Breast Cancer: MRI and PET/CT Findings. Diagnostics 2020, 10, 36. https://doi.org/10.3390/diagnostics10010036
Ørbæk M, Klausen C, Lebech A-M, Mens H. Lyme Neuroborreliosis in a Patient with Breast Cancer: MRI and PET/CT Findings. Diagnostics. 2020; 10(1):36. https://doi.org/10.3390/diagnostics10010036
Chicago/Turabian StyleØrbæk, Mathilde, Camilla Klausen, Anne-Mette Lebech, and Helene Mens. 2020. "Lyme Neuroborreliosis in a Patient with Breast Cancer: MRI and PET/CT Findings" Diagnostics 10, no. 1: 36. https://doi.org/10.3390/diagnostics10010036
APA StyleØrbæk, M., Klausen, C., Lebech, A.-M., & Mens, H. (2020). Lyme Neuroborreliosis in a Patient with Breast Cancer: MRI and PET/CT Findings. Diagnostics, 10(1), 36. https://doi.org/10.3390/diagnostics10010036




