Papillary Thyroid Cancer in a Struma Ovarii in a 17-Year-Old Nulliparous Patient: A Case Report
Abstract
:1. Introduction
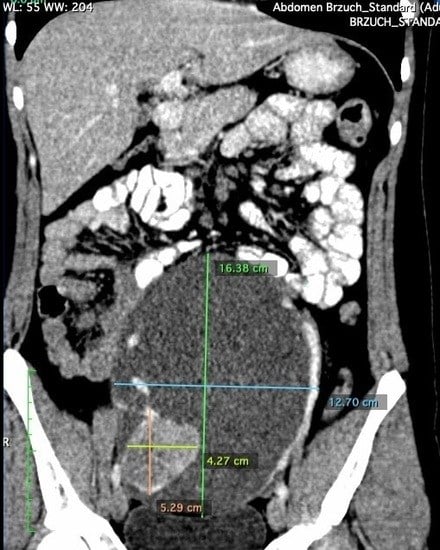
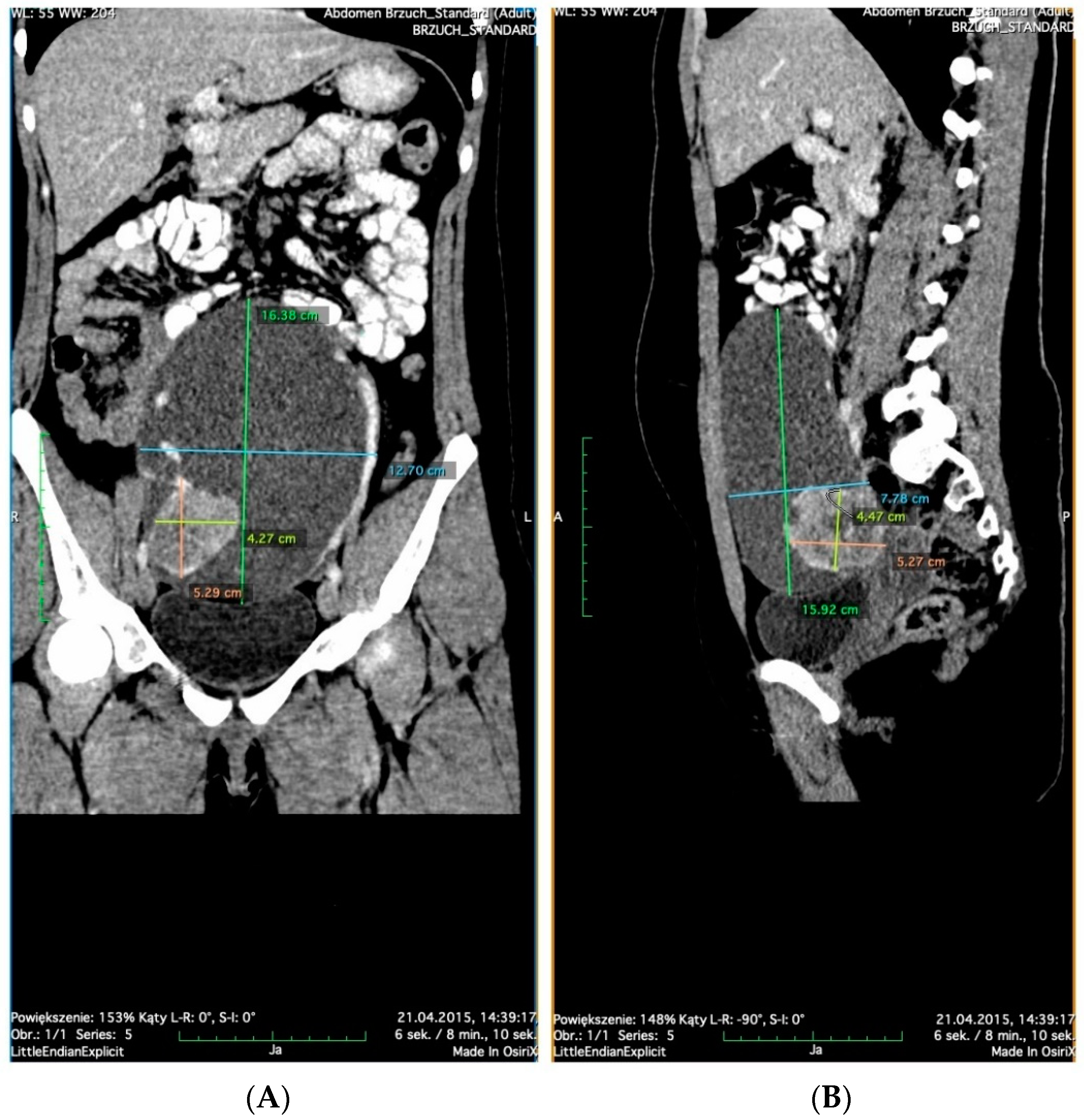
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kabukcuoglu, F.; Baksu, A.; Yilmaz, B.; Aktumen, A.; Evren, I. Malignant Struma Ovarii. Pathol. Oncol. Res. 2002, 8, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Yassa, L.; Sadow, P.; Marqusee, E. Malignant Struma Ovarii. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.C.; Chang, K.H.; Lyu, M.O.; Chang, S.J.; Ryu, H.S.; Kim, H.S. Clinical Characteristics of Struma Ovarii. J. Gynecol. Oncol. 2008, 19, 135–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondi-Pafiti, A.; Mavrigiannaki, P.; Grigoriadis, C.; Kontogianni-Katsarou, K.; Mellou, A.; Kleanthis, C.K.; Liapis, A. Monodermal Teratomas (Struma Ovarii). Clinicopathological Characteristics of 11 Cases and Literature Review. Eur. J. Gynaecol. Oncol. 2011, 32, 657–659. [Google Scholar] [PubMed]
- Llueca, A.; Maazouzi, Y.; Herraiz, J.L.; Medina, M.C.; Piquer, D.; Segarra, B.; del Moral, R.; Serra, A.; Bassols, G. Treatment and Follow-up in an Asymptomatic Malignant Struma Ovarii: A Case Report. Int. J. Surg. Case Rep. 2017, 40, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, B.; Grischke, E.M.; Staebler, A.; Hirides, P.; Rothmund, R. Laparoscopic Excision of Malignant Struma Ovarii and 1 Year Follow-up without Further Treatment. Fertil. Steril. 2011, 95, 2124.e9–2124.e12. [Google Scholar] [CrossRef]
- Goffredo, P.; Sawka, A.M.; Pura, J.; Adam, M.A.; Roman, S.A.; Sosa, J.A. Malignant Struma Ovarii: A Population-Level Analysis of a Large Series of 68 Patients. Thyroid 2015, 25, 211–215. [Google Scholar] [CrossRef]
- McGill, J.F.; Sturgeon, C.; Angelos, P. Metastatic Struma Ovarii Treated with Total Thyroidectomy and Radioiodine Ablation. Endocr. Pract. 2009, 15, 167–173. [Google Scholar] [CrossRef]
- DeSimone, C.P.; Lele, S.M.; Modesitt, S.C. Malignant Struma Ovarii: A Case Report and Analysis of Cases Reported in the Literature with Focus on Survival and I131 Therapy. Gynecol. Oncol. 2003, 89, 543–548. [Google Scholar] [CrossRef]
- Al Hassan, M.S.; Saafan, T.; el Ansari, W.; al Ansari, A.A.; Zirie, M.A.; Farghaly, H.; Abdelaal, A. The Largest Reported Papillary Thyroid Carcinoma Arising in Struma Ovarii and Metastasis to Opposite Ovary: Case Report and Review of Literature. Thyroid Res. 2018, 11, 10. [Google Scholar] [CrossRef]
- Zhang, X.; Axiotis, C. Thyroid-Type Carcinoma of Struma Ovarii. Arch. Pathol. Lab. Med. 2010, 134, 786–791. [Google Scholar]
- Hatami, M.; Breining, D.; Owers, R.L.; del Priore, G.; Goldberg, G.L. Malignant Struma Ovarii—A Case Report and Review of the Literature. Gynecol. Obstet. Investig. 2008, 65, 104–107. [Google Scholar] [CrossRef]
- Brusca, N.; del Duca, S.C.; Salvatori, R.; D’Agostini, A.; Cannas, P.; Santaguida, M.G.; Virili, C.; Bianchi, L.; Gargano, L.; Centanni, M. A Case Report of Thyroid Carcinoma Confined to Ovary and Concurrently Occult in the Thyroid: Is Conservative Treatment Always Advised? Int. J. Endocrinol. Metab. 2015, 13, e18220. [Google Scholar] [CrossRef] [Green Version]
- Makani, S.; Kim, W.; Gaba, A.R. Struma Ovarii with a Focus of Papillary Thyroid Cancer: A Case Report and Review of the Literature. Gynecol. Oncol. 2004, 94, 835–839. [Google Scholar] [CrossRef]
- Marti, J.L.; Clark, V.E.; Harper, H.; Chhieng, D.C.; Sosa, J.A.; Roman, S.A. Optimal Surgical Management of Well-Differentiated Thyroid Cancer Arising in Struma Ovarii: A Series of 4 Patients and a Review of 53 Reported Cases. Thyroid 2012, 22, 400–406. [Google Scholar] [CrossRef]
- Selvaggi, F.; Risio, D.; Waku, M.; Simo, D.; Angelucci, D.; D’Aulerio, A.; Cotellese, R.; Innocenti, P. Struma Ovarii with Follicular Thyroid-Type Carcinoma and Neuroendocrine Component: Case Report. World J. Surg. Oncol. 2012, 10, 93. [Google Scholar] [CrossRef] [Green Version]
- Leong, A.; Roche, P.J.; Paliouras, M.; Rochon, L.; Trifiro, M.; Tamilia, M. Coexistence of Malignant Struma Ovarii and Cervical Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2013, 98, 4599–4605. [Google Scholar] [CrossRef]
- Gonzalez Aguilera, B.; Vazquez, R.G.; Herguido, N.G.; Gallego, F.S.; Gonzalez, E.N. The Lack of Consensus in Management of Malignant Struma Ovarii. Gynecol. Endocrinol. 2015, 31, 258–259. [Google Scholar] [CrossRef]
- Cibas, E.S.; Ali, S.Z. NCI Thyroid FNA State of the Science Conference. The Bethesda System for Reporting Thyroid Cytopathology. Am. J. Clin. Pathol. 2009, 132, 658–665. [Google Scholar] [CrossRef] [Green Version]
- Momesso, D.P.; Tuttle, R.M. Update on Differentiated Thyroid Cancer Staging. Endocrinol. Metab. Clin. N. Am. 2014, 43, 401–421. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Alamdari, M.I.; Habibzadeh, A.; Pakrouy, H.; Chaichi, P.; Sheidaei, S. An Unusual Presentation of a Papillary Thyroid Carcinoma in the Struma Ovarii in a 10 Year-Old Girl: A Case Report. Int. J. Surg. Case Rep. 2018, 51, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Szczepanek-Parulska, E.; Pioch, A.; Cyranska-Chyrek, E.; Wolinski, K.; Jarmolowska-Jurczyszyn, D.; Janicka-Jedynska, M.; Majewski, P.; Zabel, M.; Ruchala, M. The Role of Immunohistochemical Examination in Diagnosis of Papillary Thyroid Cancer in Struma Ovarii. Folia Histochem. Cytobiol. 2019, 57, 35–42. [Google Scholar] [CrossRef]
- O’Neill, J.P.; Burns, P.; Kinsella, J. Papillary Type Thyroid Carcinoma in an Ovarian Struma. Ir. J. Med. Sci. 2012, 181, 115–117. [Google Scholar] [CrossRef]
- Ning, Y.; Kong, F.; Cragun, J.M.; Zheng, W. Struma Ovarii Simulating Ovarian Sertoli Cell Tumor: A Case Report with Literature Review. Int. J. Clin. Exp. Pathol. 2013, 6, 516–520. [Google Scholar]
- Doganay, M.; Gungor, T.; Cavkaytar, S.; Sirvan, L.; Mollamahmutoglu, L. Malignant Struma Ovarii with a Focus of Papillary Thyroid Cancer: A Case Report. Arch. Gynecol. Obstet. 2008, 277, 371–373. [Google Scholar] [CrossRef]
- Pardo-Mindan, F.J.; Vazquez, J.J. Malignant Struma Ovarii. Light and Electron Microscopic Study. Cancer 1983, 51, 337–343. [Google Scholar] [CrossRef]
- Roth, L.M.; Talerman, A. The Enigma of Struma Ovarii. Pathology 2007, 39, 139–146. [Google Scholar] [CrossRef]
- Trovisco, V.; de Castro, I.V.; Soares, P.; Maximo, V.; Silva, P.; Magalhaes, J.; Abrosimov, A.; Guiu, X.M.; Sobrinho-Simoes, M. Braf Mutations Are Associated with Some Histological Types of Papillary Thyroid Carcinoma. J. Pathol. 2004, 202, 247–251. [Google Scholar] [CrossRef]
- Schmidt, J.; Derr, V.; Heinrich, M.C.; Crum, C.P.; Fletcher, J.A.; Corless, C.L.; Nose, V. Braf in Papillary Thyroid Carcinoma of Ovary (Struma Ovarii). Am. J. Surg. Pathol. 2007, 31, 1337–1343. [Google Scholar] [CrossRef]
- Flavin, R.; Smyth, P.; Crotty, P.; Finn, S.; Cahill, S.; Denning, K.; Jinghuan, L.; O’Regan, E.; O’Leary, J.; Sheils, O. Braf T1799a Mutation Occurring in a Case of Malignant Struma Ovarii. Int. J. Surg. Pathol. 2007, 15, 116–120. [Google Scholar] [CrossRef]
- Wolff, E.F.; Hughes, M.; Merino, M.J.; Reynolds, J.C.; Davis, J.L.; Cochran, C.S.; Celi, F.S. Expression of Benign and Malignant Thyroid Tissue in Ovarian Teratomas and the Importance of Multimodal Management as Illustrated by a Braf-Positive Follicular Variant of Papillary Thyroid Cancer. Thyroid 2010, 20, 981–987. [Google Scholar] [CrossRef] [Green Version]
- Celestino, R.; Magalhaes, J.; Castro, P.; Triller, M.; Vinagre, J.; Soares, P.; Sobrinho-Simoes, M. A Follicular Variant of Papillary Thyroid Carcinoma in Struma Ovarii. Case Report with Unique Molecular Alterations. Histopathology 2009, 55, 482–487. [Google Scholar] [CrossRef]
- Gomes-Lima, C.J.; Nikiforov, Y.E.; Lee, W.; Burman, K.D. Synchronous Independent Papillary Thyroid Carcinomas in Struma Ovarii and the Thyroid Gland with Different Ras Mutations. J. Endocr. Soc. 2018, 2, 944–948. [Google Scholar] [CrossRef]
- Coyne, C.; Nikiforov, Y.E. Ras Mutation-Positive Follicular Variant of Papillary Thyroid Carcinoma Arising in a Struma Ovarii. Endocr. Pathol. 2010, 21, 144–147. [Google Scholar] [CrossRef]
- Boutross-Tadross, O.; Saleh, R.; Asa, S.L. Follicular Variant Papillary Thyroid Carcinoma Arising in Struma Ovarii. Endocr. Pathol. 2007, 18, 182–186. [Google Scholar] [CrossRef]
- Janszen, E.W.; van Doorn, H.C.; Ewing, P.C.; de Krijger, R.R.; de Wilt, J.H.; Kam, B.L.; de Herder, W.W. Malignant Struma Ovarii: Good Response after Thyroidectomy and I Ablation Therapy. Clin. Med. Oncol. 2008, 2, 147–152. [Google Scholar] [CrossRef]
- Tzelepis, E.G.; Barengolts, E.; Garzon, S.; Shulan, J.; Eisenberg, Y. Unusual Case of Malignant Struma Ovarii and Cervical Thyroid Cancer Preceded by Ovarian Teratoma: Case Report and Review of the Literature. Case Rep. Endocrinol. 2019, 2019, 7964126. [Google Scholar] [CrossRef] [Green Version]
- Boyd, J.C.; Williams, B.A.; Rigby, M.H.; Kieser, K.; Offman, S.; Shirsat, H.; Trites, J.R.B.; Taylor, S.M.; Hart, R.D. Malignant Struma Ovarii in a 30-Year Old Nulliparous Patient. Thyroid Res. 2017, 10, 3. [Google Scholar] [CrossRef]
- Jean, S.; Tanyi, J.L.; Montone, K.; McGrath, C.; Lage-Alvarez, M.M.; Chu, C.S. Papillary Thyroid Cancer Arising in Struma Ovarii. J. Obstet. Gynaecol. 2012, 32, 222–226. [Google Scholar] [CrossRef]
- Pacini, F.; Schlumberger, M.; Harmer, C.; Berg, G.G.; Cohen, O.; Duntas, L.; Jamar, F.; Jarzab, B.; Limbert, E.; Lind, P.; et al. Post-Surgical Use of Radioiodine (131i) in Patients with Papillary and Follicular Thyroid Cancer and the Issue of Remnant Ablation: A Consensus Report. Eur. J. Endocrinol. 2005, 153, 651–659. [Google Scholar] [CrossRef] [Green Version]
- Mango, L.; Filesi, M.; Ronga, G. Radiotherapy with Iodine-131 in Recurrent Malignant Struma Ovarii. Eur. J. Nucl. Med. 1997, 24, 233. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonet, A.; Ślusarczyk, R.; Gąsior-Perczak, D.; Kowalik, A.; Kopczyński, J.; Kowalska, A. Papillary Thyroid Cancer in a Struma Ovarii in a 17-Year-Old Nulliparous Patient: A Case Report. Diagnostics 2020, 10, 45. https://doi.org/10.3390/diagnostics10010045
Gonet A, Ślusarczyk R, Gąsior-Perczak D, Kowalik A, Kopczyński J, Kowalska A. Papillary Thyroid Cancer in a Struma Ovarii in a 17-Year-Old Nulliparous Patient: A Case Report. Diagnostics. 2020; 10(1):45. https://doi.org/10.3390/diagnostics10010045
Chicago/Turabian StyleGonet, Agnieszka, Rafał Ślusarczyk, Danuta Gąsior-Perczak, Artur Kowalik, Janusz Kopczyński, and Aldona Kowalska. 2020. "Papillary Thyroid Cancer in a Struma Ovarii in a 17-Year-Old Nulliparous Patient: A Case Report" Diagnostics 10, no. 1: 45. https://doi.org/10.3390/diagnostics10010045
APA StyleGonet, A., Ślusarczyk, R., Gąsior-Perczak, D., Kowalik, A., Kopczyński, J., & Kowalska, A. (2020). Papillary Thyroid Cancer in a Struma Ovarii in a 17-Year-Old Nulliparous Patient: A Case Report. Diagnostics, 10(1), 45. https://doi.org/10.3390/diagnostics10010045