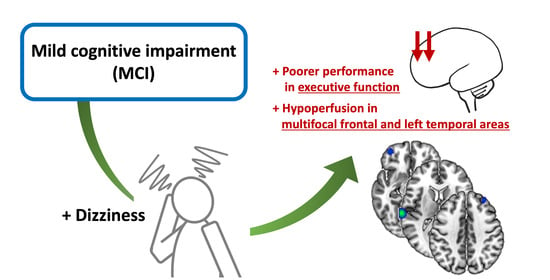
Altered Regional Cerebral Blood Perfusion in Mild Cognitive Impairment Patients with Dizziness
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessment
2.3. SPECT Acquisition and Analysis
2.4. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics
3.2. SPECT Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Neuhauser, H.K. Chapter 5—The epidemiology of dizziness and vertigo. In Handbook of Clinical Neurology; Furman, J.M., Lempert, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 137, pp. 67–82. [Google Scholar]
- Goldberg, J.M.; Wilson, V.J.; Angelaki, D.E.; Cullen, K.E.; Fukushima, K.; Buttner-Ennever, J. The Vestibular System: A Sixth Sense; Chapter 2; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Smith, P.F. The vestibular system and cognition. Curr. Opin. Neurol. 2017, 30, 84–89. [Google Scholar] [CrossRef]
- Bigelow, R.T.; Semenov, Y.R.; du Lac, S.; Hoffman, H.J.; Agrawal, Y. Vestibular vertigo and comorbid cognitive and psychiatric impairment: The 2008 National Health Interview Survey. J. Neurol. Neurosurg. Psychiatry 2016, 87, 367–372. [Google Scholar] [CrossRef]
- Bigelow, R.T.; Agrawal, Y. Vestibular involvement in cognition: Visuospatial ability, attention, executive function, and memory. J. Vestib. Res. 2015, 25, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Breinbauer, H.A.; Contreras, M.D.; Lira, J.P.; Guevara, C.; Castillo, L.; Ruëdlinger, K.; Muñoz, D.; Delano, P.H. Spatial Navigation Is Distinctively Impaired in Persistent Postural Perceptual Dizziness. Front. Neurol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Deroualle, D.; Borel, L.; Tanguy, B.; Bernard-Demanze, L.; Devèze, A.; Montava, M.; Lavieille, J.-P.; Lopez, C. Unilateral vestibular deafferentation impairs embodied spatial cognition. J. Neurol. 2019, 266, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Dobbels, B.; Peetermans, O.; Boon, B.; Mertens, G.; Van de Heyning, P.; Van Rompaey, V. Impact of bilateral Vestibulopathy on spatial and nonspatial cognition: A systematic review. Ear Hear. 2019, 40, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Popp, P.; Wulff, M.; Finke, K.; Rühl, M.; Brandt, T.; Dieterich, M. Cognitive deficits in patients with a chronic vestibular failure. J. Neurol. 2017, 264, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Wei, E.X.; Oh, E.S.; Harun, A.; Ehrenburg, M.; Agrawal, Y. Vestibular loss predicts poorer spatial cognition in patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 61, 995–1003. [Google Scholar] [CrossRef]
- Tangen, G.G.; Engedal, K.; Bergland, A.; Moger, T.A.; Mengshoel, A.M. Relationships between balance and cognition in patients with subjective cognitive impairment, mild cognitive impairment, and Alzheimer disease. Phys. Ther. 2014, 94, 1123–1134. [Google Scholar] [CrossRef]
- Sugaya, N.; Arai, M.; Goto, F. Changes in cognitive function in patients with intractable dizziness following vestibular rehabilitation. Sci. Rep. 2018, 8, 9984. [Google Scholar] [CrossRef]
- Brandt, T.; Schautzer, F.; Hamilton, D.A.; Brüning, R.; Markowitsch, H.J.; Kalla, R.; Darlington, C.; Smith, P.; Strupp, M. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain 2005, 128, 2732–2741. [Google Scholar] [CrossRef] [PubMed]
- Indovina, I.; Riccelli, R.; Chiarella, G.; Petrolo, C.; Augimeri, A.; Giofre, L.; Lacquaniti, F.; Staab, J.P.; Passamonti, L. Role of the insula and vestibular system in patients with chronic subjective dizziness: An fMRI study using sound-evoked vestibular stimulation. Front. Behav. Neurosci. 2015, 9, 334. [Google Scholar] [CrossRef] [PubMed]
- Kremmyda, O.; Hüfner, K.; Flanagin, V.L.; Hamilton, D.A.; Linn, J.; Strupp, M.; Jahn, K.; Brandt, T. Beyond dizziness: Virtual navigation, spatial anxiety and hippocampal volume in bilateral vestibulopathy. Front. Hum. Neurosci. 2016, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Wurthmann, S.; Naegel, S.; Schulte Steinberg, B.; Theysohn, N.; Diener, H.C.; Kleinschnitz, C.; Obermann, M.; Holle, D. Cerebral gray matter changes in persistent postural perceptual dizziness. J. Psychosom. Res. 2017, 103, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-O.; Lee, E.-S.; Kim, J.-S.; Lee, Y.-B.; Jeong, Y.; Choi, B.S.; Kim, J.-H.; Staab, J.P. Altered brain function in persistent postural perceptual dizziness: A study on resting state functional connectivity. Hum. Brain Mapp. 2018, 39, 3340–3353. [Google Scholar] [CrossRef]
- Na, S.; Im, J.J.; Jeong, H.; Lee, E.-S.; Lee, T.-K.; Chung, Y.-A.; Song, I.-U. Cerebral perfusion abnormalities in patients with persistent postural-perceptual dizziness (PPPD): A SPECT study. J. Neural Transm. 2019, 126, 123–129. [Google Scholar] [CrossRef]
- Nigro, S.; Indovina, I.; Riccelli, R.; Chiarella, G.; Petrolo, C.; Lacquaniti, F.; Staab, J.P.; Passamonti, L. Reduced cortical folding in multi-modal vestibular regions in persistent postural perceptual dizziness. Brain Imaging Behav. 2019, 13, 798–809. [Google Scholar] [CrossRef]
- Warwick, J.M. Imaging of brain function using SPECT. Metab. Brain Dis. 2004, 19, 113–123. [Google Scholar] [CrossRef]
- Camargo, E.E. Brain SPECT in neurology and psychiatry. J. Nucl. Med. 2001, 42, 611–623. [Google Scholar]
- Alegret, M.; Vinyes-Junque, G.; Boada, M.; Martinez-Lage, P.; Cuberas, G.; Espinosa, A.; Roca, I.; Hernandez, I.; Valero, S.; Rosende-Roca, M.; et al. Brain perfusion correlates of visuoperceptual deficits in mild cognitive impairment and mild Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 21, 557–567. [Google Scholar] [CrossRef]
- Huang, C.; Eidelberg, D.; Habeck, C.; Moeller, J.; Svensson, L.; Tarabula, T.; Julin, P. Imaging markers of mild cognitive impairment: Multivariate analysis of CBF SPECT. Neurobiol. Aging 2007, 28, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Hufner, K.; Stephan, T.; Hamilton, D.; Kalla, R.; Glasauer, S.; Strupp, M.; Brandt, T. Gray-matter atrophy after chronic complete unilateral vestibular deafferentation. Ann. New York Acad. Sci. 2009, 1164, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Ventre-Dominey, J. Vestibular function in the temporal and parietal cortex: Distinct velocity and inertial processing pathways. Front. Integr. Neurosci. 2014, 8, 53. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Na, D.L.; Hahn, S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J. Korean Neurol. Assoc. 1997, 15, 300. [Google Scholar]
- Yesavage, J.A.; Sheikh, J.I. Geriatric depression scale (GDS) recent evidence and development of a shorter version. Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Kang, Y.; Jahng, S.; Na, D. Seoul Neuropsychological Screening Battery; (SNSB-II): Professional Manual; Human Brain Research and Consulting: Incheon, Korea, 2012. [Google Scholar]
- Jacobson, G.P.; Newman, C.W. The development of the dizziness handicap inventory. Arch. Otolaryngol. Head Neck Surg. 1990, 116, 424–427. [Google Scholar] [CrossRef]
- Risey, J.; Briner, W. Dyscalculia in patients with vertigo. J. Vestib. Res. 1990, 1, 31–37. [Google Scholar]
- Harun, A.; Oh, E.S.; Bigelow, R.T.; Studenski, S.; Agrawal, Y. Vestibular impairment in dementia. Otol. Neurotol. 2016, 37, 1137. [Google Scholar] [CrossRef]
- Nakamagoe, K.; Fujimiya, S.; Koganezawa, T.; Kadono, K.; Shimizu, K.; Fujizuka, N.; Takiguchi, S.; Ueno, T.; Monzen, T.; Tamaoka, A. Vestibular function impairment in Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 47, 185–196. [Google Scholar] [CrossRef]
- Melrose, R.J.; Campa, O.M.; Harwood, D.G.; Osato, S.; Mandelkern, M.A.; Sultzer, D.L. The neural correlates of naming and fluency deficits in Alzheimer’s disease: An FDG-PET study. Int. J. Geriatr. Psychiatry 2009, 24, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Brandt, T. Vestibular cortex: Its locations, functions, and disorders. In Vertigo; Springer: Berlin/Heidelberg, Germany, 2003; pp. 219–231. [Google Scholar]
- Friberg, L.; Olsen, T.S.; Roland, P.E.; Paulson, O.B.; Lassen, N.A. Focal increase of blood flow in the cerebral cortex of man during vestibular stimulation. Brain 1985, 108 Pt 3, 609–623. [Google Scholar] [CrossRef]
- Bottini, G.; Sterzi, R.; Paulesu, E.; Vallar, G.; Cappa, S.F.; Erminio, F.; Passingham, R.E.; Frith, C.D.; Frackowiak, R.S. Identification of the central vestibular projections in man: A positron emission tomography activation study. Exp. Brain Res. 1994, 99, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Falconer, C.J.; Mast, F.W. Balancing the mind: Vestibular induced facilitation of egocentric mental transformations. Exp. Psychol. 2012, 59, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Hitier, M.; Besnard, S.; Smith, P.F. Vestibular pathways involved in cognition. Front. Integr. Neurosci. 2014, 8, 59. [Google Scholar] [CrossRef]
- Leff, A.P.; Schofield, T.M.; Crinion, J.T.; Seghier, M.L.; Grogan, A.; Green, D.W.; Price, C.J. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: Evidence from 210 patients with stroke. Brain 2009, 132, 3401–3410. [Google Scholar] [CrossRef]
- Rajah, M.N.; D’Esposito, M. Region-specific changes in prefrontal function with age: A review of PET and fMRI studies on working and episodic memory. Brain 2005, 128, 1964–1983. [Google Scholar] [CrossRef]
- Rajah, M.N.; Languay, R.; Grady, C.L. Age-related changes in right middle frontal gyrus volume correlate with altered episodic retrieval activity. J. Neurosci. 2011, 31, 17941–17954. [Google Scholar] [CrossRef]
- Elliott, R.; Dolan, R.J.; Frith, C.D. Dissociable functions in the medial and lateral orbitofrontal cortex: Evidence from human neuroimaging studies. Cereb. Cortex 2000, 10, 308–317. [Google Scholar] [CrossRef]
- Bisdorff, A.; Von Brevern, M.; Lempert, T.; Newman-Toker, D.E. Classification of vestibular symptoms: Towards an international classification of vestibular disorders. J. Vestib. Res. 2009, 19, 1–13. [Google Scholar] [CrossRef]
- Zu Eulenburg, P.; Caspers, S.; Roski, C.; Eickhoff, S.B. Meta-analytical definition and functional connectivity of the human vestibular cortex. Neuroimage 2012, 60, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Chang, R. Anatomy of the vestibular system: A review. NeuroRehabilitation 2013, 32, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Mirza, S.S.; Ikram, M.A.; Bos, D.; Mihaescu, R.; Hofman, A.; Tiemeier, H. Mild cognitive impairment and risk of depression and anxiety: A population-based study. Alzheimer’s Dement. 2017, 13, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Lahmann, C.; Henningsen, P.; Brandt, T.; Strupp, M.; Jahn, K.; Dieterich, M.; Eckhardt-Henn, A.; Feuerecker, R.; Dinkel, A.; Schmid, G. Psychiatric comorbidity and psychosocial impairment among patients with vertigo and dizziness. J. Neurol. Neurosurg. Psychiatry 2015, 86, 302–308. [Google Scholar] [CrossRef]

| Inclusion Criteria |
|---|
|
| Exclusion Criteria |
|
| Characteristics | Dizziness Group (n = 18) | Non-Dizziness Group (n = 32) | p |
|---|---|---|---|
| Age (years) | 78.3 ± 4.8 | 74.1 ± 10.3 | 0.11 |
| Sex (male:female) | 5:13 | 7:25 | 0.64 |
| Education (years) | 5.8 ± 5.0 | 6.7 ± 4.4 | 0.53 |
| MMSE | 23.3 ± 4.0 | 23.5 ± 3.9 | 0.89 |
| GDS-SF | 8.2 ± 4.2 | 6.2 ± 4.0 | 0.10 |
| NPI | 4.4 ± 7.5 | 1.4 ± 3.1 | 0.18 |
| Hypertension | 14 (77.8%) | 32 (56.3%) | 0.24 |
| Diabetes mellitus | 8 (44.5%) | 9 (28.1%) | 0.13 |
| Dyslipidemia | 8 (44.5%) | 12 (37.5%) | 0.63 |
| Sedative, hypnotic, or anxiolytic drugs | 4 (22.2%) | 5 (15.6%) | 0.56 |
| Observed hearing loss | 1 (5.6%) | 3 (9.4%) | 0.63 |
| Migraine | 1 (5.6%) | 1 (3.1%) | 0.67 |
| DHI | 16.3 ± 14.6 | ||
| Precipitating events of dizziness (number, %) | |||
| Vestibular neuritis | 1 (5.6%) | ||
| Benign paroxysmal positional vertigo | 1 (5.6%) | ||
| Orthostatic intolerance | 2 (11.1%) | ||
| Persistent postural-perceptual dizziness | 5 (27.8%) | ||
| Medications | 1 (5.6%) |
| Characteristics | Dizziness Group (n = 18) | Non-Dizziness Group (n = 32) | p |
|---|---|---|---|
| Digit Span Forward | 3.0 ± 1.4 | 3.3 ± 1.0 | 0.91 |
| K-BNT | 33.4 ± 11.2 | 33.3 ± 1.7 | 0.98 |
| RCFT-copy | 24.3 ± 9.1 | 27.3 ± 8.7 | 0.17 |
| SVLT-delayed recall | 1.9 ± 1.7 | 2.2 ± 2.4 | 0.97 |
| RCFT-delayed recall | 5.4 ± 6.5 | 6.0 ± 6.3 | 0.48 |
| COWAT-animal | 10.9 ± 3.3 | 11.8 ± 4.3 | 0.42 |
| COWAT-phonemic | 9.7 ± 9.3 | 14.8 ± 9.1 | 0.04 * |
| Stroop test | 51.2 ± 28.4 | 58.8 ± 30.2 | 0.40 |
| Region | t | p | Coordinates * (x, y, z) | Cluster Size (Voxels) |
|---|---|---|---|---|
| Dizziness group < Non-dizziness group | ||||
| L superior temporal gyrus | 4.70 | <0.001 | −68, −22, 16 | 659 |
| L lateral orbital gyrus | 3.29 | 0.001 | −40, 52, −16 | 130 |
| R middle frontal gyrus | 3.16 | 0.001 | 34, 46, 40 | 114 |
| Dizziness group > Non-dizziness group | ||||
| None | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, S.; Im, J.J.; Jeong, H.; Lee, E.-S.; Lee, T.-K.; Chung, Y.-A.; Song, I.-U. Altered Regional Cerebral Blood Perfusion in Mild Cognitive Impairment Patients with Dizziness. Diagnostics 2020, 10, 777. https://doi.org/10.3390/diagnostics10100777
Na S, Im JJ, Jeong H, Lee E-S, Lee T-K, Chung Y-A, Song I-U. Altered Regional Cerebral Blood Perfusion in Mild Cognitive Impairment Patients with Dizziness. Diagnostics. 2020; 10(10):777. https://doi.org/10.3390/diagnostics10100777
Chicago/Turabian StyleNa, Seunghee, Jooyeon Jamie Im, Hyeonseok Jeong, Eek-Sung Lee, Tae-Kyeong Lee, Yong-An Chung, and In-Uk Song. 2020. "Altered Regional Cerebral Blood Perfusion in Mild Cognitive Impairment Patients with Dizziness" Diagnostics 10, no. 10: 777. https://doi.org/10.3390/diagnostics10100777
APA StyleNa, S., Im, J. J., Jeong, H., Lee, E.-S., Lee, T.-K., Chung, Y.-A., & Song, I.-U. (2020). Altered Regional Cerebral Blood Perfusion in Mild Cognitive Impairment Patients with Dizziness. Diagnostics, 10(10), 777. https://doi.org/10.3390/diagnostics10100777