Diagnostic Accuracy of Biomarkers for Early-Onset Neonatal Bacterial Infections: Evaluation of Serum Procalcitonin Reference Curves
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design and Subjects
2.2. Suspected or Confirmed Diagnosis of an Early-Onset Bacterial Infection
2.3. Definitions of Culture-Proven Sepsis, Clinical Sepsis, and Systemic Inflammatory Response Syndrome
2.4. Methods of Measuring Each Biomarker and Culture
2.5. Study Methods
2.5.1. Clinical Characteristics of Each Group and the Causative Organisms of Confirmed Infections
2.5.2. Validation of Serum PCT Reference Curves
2.5.3. Most Useful Biomarker for Early-Onset Neonatal Bacterial Infection
2.5.4. Statistical Analysis
3. Results
3.1. Patient Characteristics and Pathogens Detected in the Confirmed Infection Group
3.1.1. Preterm Infants
3.1.2. Term Infants
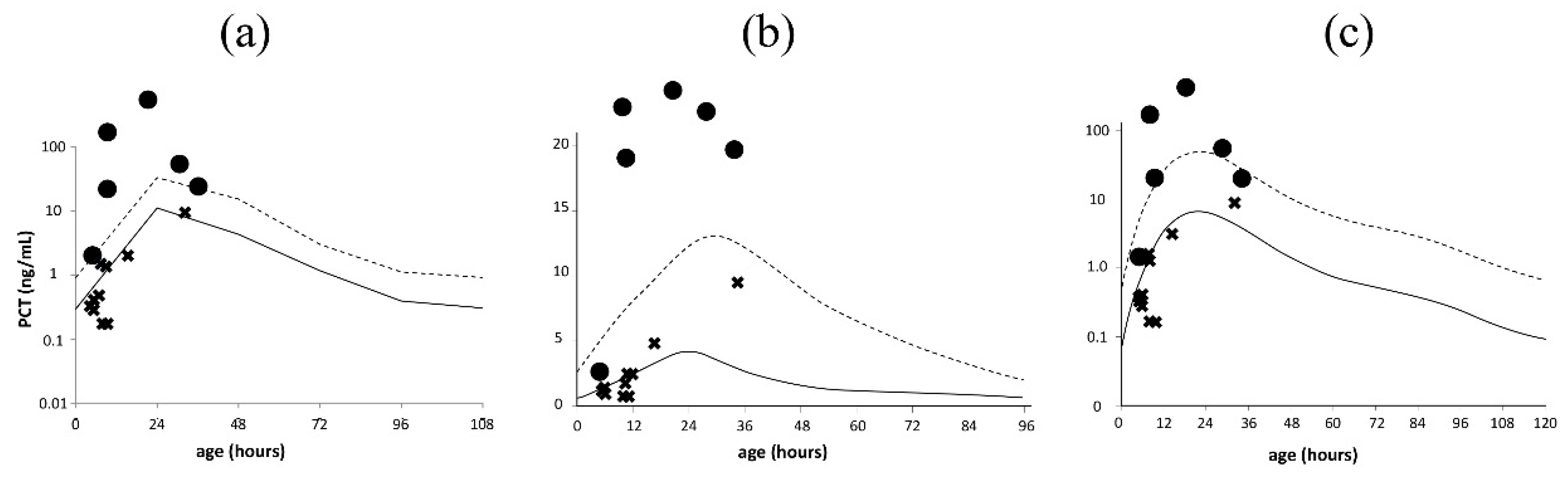
3.2. Validation of Serum PCT Reference Curves
3.3. Useful Biomarkers for Detection of Early-Onset Neonatal Bacterial Infections
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| WBC | White blood cell |
| CRP | C-reactive protein |
| IgM | Immunoglobulin M |
| PCT | Procalcitonin |
| GBS | Group B Streptococcus |
| PROM | Premature rupture of membranes |
| SIRS | Systemic inflammatory response syndrome |
References
- Chiesa, C.; Pacifico, L.; Osborn, J.F.; Bonci, E.; Hofer, N.; Resch, B. Early-onset Neonatal Sepsis: Still room for improvement in procalcitonin diagnostic accuracy studies. Medicine 2015, 94, e1230. [Google Scholar] [CrossRef] [PubMed]
- Schrag, S.J.; Farley, M.M.; Petit, S.; Reingold, A.; Weston, E.J.; Pondo, T.; Jain, J.H.; Lynfield, R. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics 2016, 138, e20162013. [Google Scholar] [CrossRef]
- Morioka, I.; Morikawa, S.; Miwa, A.; Minami, H.; Yoshii, K.; Kugo, M.; Kitsunezuka, Y.; Enomoto, M.; Jikimoto, T.; Nakamura, M.; et al. Culture-proven neonatal sepsis in Japanese neonatal care units in 2006–2008. Neonatology 2012, 102, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect. Dis. 2016, 4, 249. [Google Scholar] [CrossRef] [PubMed]
- Vasilcan, G.; Avasiloaiei, A.L.; Moscalu, M.; Dimitriu, A.G.; Stamatin, M. Procalcitonine–early marker of neonatal infection. Rev. Med. Chir. Soc. Med. Nat. Iasi 2012, 115, 1243–1250. [Google Scholar]
- Weitkamp, J.-H.; Aschner, J.L. Diagnostic Use of C-Reactive protein (CRP) in assessment of Neonatal Sepsis. NeoReviews 2005, 6, e508–e515. [Google Scholar] [CrossRef]
- Krishna, B.V.; Nadgir, S.D.; Tallur, S.S. Immunoglobulin-M estimation and C-reactive protein detection in neonatal septicemia. Indian J. Pathol. Microbiol. 2000, 43, 35–40. [Google Scholar] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Woll, C.; Neuman, M.I.; Aronson, P.L. Management of the febrile young infant. Pediatr. Emerg. Care 2017, 33, 748–753. [Google Scholar] [CrossRef]
- Rodwell, R.L.; Leslie, A.L.; Tudehope, D.I. Early diagnosis of neonatal sepsis using a hematologic scoring system. J. Pediatr. 1988, 112, 761–767. [Google Scholar] [CrossRef]
- Khassawneh, M.; Hayajneh, W.A.; Kofahi, H.; Khader, Y.; Amarin, Z.; Daoud, A. Diagnostic markers for neonatal sepsis: Comparing C-reactive protein, Interleukin-6 and immunoglobulin M. Scand. J. Immunol. 2007, 65, 171–175. [Google Scholar] [CrossRef]
- Pierce, R.; Bigham, M.T.; Giuliano, J.S., Jr. Use of procalcitonin for the prediction and treatment of acute bacterial infection in children. Curr. Opin. Pediatr. 2014, 26, 292–298. [Google Scholar] [CrossRef]
- So-Ngern, A.; Leelasupasri, S.; Chulavatnatol, S.; Pummangura, C.; Bunupuradah, P.; Montakantikul, P. Prognostic value of serum procalcitonin level for the diagnosis of bacterial infections in critically-ill patients. Infect. Chemother. 2019, 51, 263–273. [Google Scholar] [CrossRef]
- Park, I.H.; Lee, S.H.; Yu, S.T.; Oh, Y.K. Serum procalcitonin as a diagnostic marker of neonatal sepsis. Korean J. Pediatr. 2014, 57, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Assumma, M.; Signore, F.; Pacifico, L.; Rossi, N.; Osborn, J.F.; Chiesa, C. Serum procalcitonin concentrations in term delivering mothers and their healthy offspring: A longitudinal study. Clin. Chem. 2000, 46, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, M.; Karastaneva, A.; Besheva, M.; Deneva, T.; Davcheva, D. Dynamics in levels of procalcitonin in healthy term newborns during the first 24 h of life. Akush Ginekol (Sofiia) 2013, 52, 23–28. [Google Scholar]
- Fukuzumi, N.; Osawa, K.; Sato, I.; Iwatani, S.; Ishino, R.; Hayashi, N.; Iijima, K.; Saegusa, J.; Morioka, I. Age-specific percentile-based reference curve of serum procalcitonin concentrations in Japanese preterm infants. Sci. Rep. 2016, 6, 23871. [Google Scholar] [CrossRef]
- Turner, D.; Hammerman, C.; Rudensky, B.; Schlesinger, Y.; Goia, C.; Schimmel, M.S. Procalcitonin in preterm infants during the first few days of life: Introducing an age related nomogram. Arch. Dis. Child. Fetal Neonatal Ed. 2006, 91, F283–F286. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, C.; Natale, F.; Pascone, R.; Osborn, J.F.; Pacifico, L.; Bonci, E.; De Curtis, M. C reactive protein and procalcitonin: Reference intervals for preterm and term newborns during the early neonatal period. Clin. Chim. Acta 2011, 412, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, C.; Panero, A.; Rossi, N.; Stegagno, M.; De Giusti, M.; Osborn, J.F.; Pacifico, L. Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. Clin. Infect. Dis. 1998, 26, 664–672. [Google Scholar] [CrossRef]
- Ochi, F.; Higaki, T.; Ohta, M.; Yamauchi, T.; Tezuka, M.; Chisaka, T.; Moritani, T.; Tauchi, H.; Ishii, E. Procalcitonin as a marker of respiratory disorder in neonates. Pediatr. Int. 2014, 57, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Stocker, M.; Hop, W.C.J.; Van Rossum, A.M.C. Neonatal Procalcitonin Intervention Study (NeoPInS): Effect of procalcitonin-guided decision making on duration of antibiotic therapy in suspected neonatal early-onset sepsis: A multi-centre randomized superiority and non-inferiority intervention study. BMC Pediatr. 2010, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Oeser, C.; Lutsar, I.; Metsvaht, T.; Turner, M.A.; Heath, P.T.; Sharland, M. Clinical trials in neonatal sepsis. J. Antimicrob. Chemother. 2013, 68, 2733–2745. [Google Scholar] [CrossRef] [PubMed]
- Krueger, M.; Nauck, M.; Sang, S.; Hentschel, R.; Wieland, H.; Berner, R. Cord blood levels of interleukin-6 and interleukin-8 for the immediate diagnosis of early-onset infection in premature infants. Biol. Neonate 2001, 80, 118–123. [Google Scholar] [CrossRef]
- Goldstein, B.; Giroir, B.; Randolph, A.; International consensus conference on pediatric sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care. Med. 2005, 6, 2–8. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the youden index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef]
- Müller, W.; Resch, B.; Hofer, N. Definitions of SIRS and sepsis in correlation with early and late onset neonatal sepsis. J. Pediatr. Intensiv. Care 2015, 1, 17–23. [Google Scholar] [CrossRef]
- Hofer, N.; Zacharias, E.; Müller, W.; Resch, B. Performance of the definitions of the systemic inflammatory response syndrome and sepsis in neonates. J. Périnat. Med. 2012, 40, 587–590. [Google Scholar] [CrossRef]
- Yan, S.T.; Sun, L.C.; Jia, H.B.; Gao, W.; Yang, J.P.; Zhang, G.Q. Procalcitonin levels in bloodstream infections caused by different sources and species of bacteria. Am. J. Emerg. Med. 2017, 35, 579–583. [Google Scholar] [CrossRef]
- Kawamura, M.; Nishida, H. The usefulness of serial C-reactive protein measurement in managing neonatal infection. Acta Paediatr. 1995, 84, 10–13. [Google Scholar] [CrossRef]
- Doellner, H.; Arntzen, K.; Haereid, P.E.; Aag, S.; Austgulen, R. Interleukin-6 concentrations in neonates evaluated for sepsis. J. Pediatr. 1998, 132, 295–299. [Google Scholar] [CrossRef]
- Hornik, C.P.; Benjamin, D.K.; Becker, K.C.; Benjamin, D.K., Jr.; Li, J.; Clark, R.H.; Cohen-Wolkowiez, M.; Smith, P.B. Use of the complete blood cell count in early-onset neonatal sepsis. Pediatr. Infect. Dis. J. 2012, 31, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Hofer, N.; Müller, W.; Resch, B. Non-infectious conditions and gestational age influence C-reactive protein values in newborns during the first 3 days of life. Clin. Chem. Lab. Med. 2011, 49, 297–302. [Google Scholar] [CrossRef] [PubMed]

| Confirmed Infection n = 6 | Non-Infection n = 10 | p-Value | |
|---|---|---|---|
| A. Maternal characteristics | |||
| Gestational diabetes ** | 0 (0) | 0 (0) | - |
| Hypertensive disorders of pregnancy ** | 0 (0) | 1 (10) | 1.00 |
| Delivery mode ** | 0.52 | ||
| Vaginal delivery | 2 (33) | 1 (10) | |
| Cesarean section | 4 (67) | 9 (90) | |
| GBS colonization ** | 3 (50) | 2 (20) | 0.30 |
| PROM ** | 4 (67) | 3 (30) | 0.30 |
| Intrapartum antimicrobial treatment ** | 5 (83) | 9 (90) | 1.00 |
| B. Neonatal characteristics | |||
| Birth weight, g * | 1697 (538–2226) | 1551 (549–2422) | 0.96 |
| Gestational age, weeks * | 31.5 (23.9–35.6) | 32.0 (24.0–36.7) | 0.83 |
| Male sex ** | 5 (83) | 5 (50) | 0.31 |
| Apgar score | |||
| at 1 min * | 4.0 (1–8) | 6.5 (1–9) | 0.47 |
| at 5 min * | 6.5 (4–9) | 7.5 (1–9) | 0.34 |
| Umbilical cord blood pH * | 7.235 (7.109–7.360) | 7.304 (6.888–7.427) | 0.30 |
| Severe neonatal asphyxia ** | 3 (50) | 3 (30) | 0.61 |
| Respiratory disorder ** | 6 (100) | 9 (90) | 1.00 |
| Intracranial hemorrhage ** | 2 (33) | 0 (0) | 0.13 |
| Patent ductus arteriosus that required indomethacin or surgical treatment ** | 2 (33) | 5 (50) | 0.63 |
| SIRS ** | 6 (100) | 0 (0) | <0.001 |
| Antimicrobial treatment ** | 6 (100) | 9 (90) | 1.00 |
| ABPC and AMK | 3 (50) | 8 (80) | |
| ABPC and CTX | 2 (33) | 0 (0) | |
| ABPC and CMZ | 0 (0) | 1 (10) | |
| ABPC | 1 (17) | 0 (0) | |
| Use of ventilator ** | 6 (100) | 6 (60) | 0.23 |
| Use of catecholamine ** | 6 (100) | 6 (60) | 0.23 |
| Use of PMX-DHP ** | 2 (33) | 0 (0) | 0.13 |
| Death ** | 1 (17) | 0 (0) | 0.38 |
| Age at measurement of biomarkers, days * | 0.5 (0–2) | 0 (0–2) | 0.13 |
| PCT, ng/mL * | 43.7 (1.45–200) | 0.6 (0.25–8.25) | 0.004 |
| CRP, mg/dL * | 1.42 (0.1–5.82) | 0.1 (0.1–5.67) | 0.01 |
| WBC, /µL * | 6350 (2800–9900) | 9900 (7600–18,000) | 0.01 |
| IgM, mg/dL * | 7.5 (2–14) | 8.5 (4–25) | 0.51 |
| No. | Pathogens Detected | Samples | Clinical Diagnosis |
|---|---|---|---|
| 1 | Streptococcus agalactiae | Nasopharyngeal swab, stool, and gastric aspirate | Clinical sepsis |
| 2 | Escherichia coli | Gastric aspirate | Clinical sepsis |
| 3 | Bacillus cereus | Blood | Culture-proven sepsis |
| 4 | Enterococcus faecalis | Nasopharyngeal swab, stool, and gastric aspirate | Clinical sepsis |
| 5 | Enterococcus faecalis | Blood, nasopharyngeal swab, and gastric aspirate | Culture-proven sepsis |
| 6 | Enterococcus faecalis, Enterobacter aerogenes, Klebsiella pneumoniae | Urine and gastric aspirate | Clinical sepsis |
| Confirmed Infection n = 6 | Non-Infection n = 17 | p-Value | |
|---|---|---|---|
| A. Maternal characteristics | |||
| Gestational diabetes ** | 0 (0) | 0 (0) | - |
| Hypertensive disorders of pregnancy ** | 0 (0) | 0 (0) | - |
| Delivery mode ** | 0.61 | ||
| Vaginal delivery | 5 (83) | 13 (76) | |
| Cesarean section | 1 (17) | 4 (24) | |
| GBS colonization ** | 2 (33) | 2 (12) | 0.27 |
| PROM ** | 3 (50) | 7 (41) | 1.00 |
| Intrapartum antimicrobial treatment ** | 3 (50) | 8 (47) | 1.00 |
| B. Neonatal characteristics | |||
| Birth weight, g * | 2959 (2485–3572) | 3165 (2424–3732) | 0.38 |
| Gestational age, weeks * | 39.5 (37.1–41.3) | 40.0 (37.7–40.9) | 0.79 |
| Male sex ** | 4 (67) | 8 (47) | 0.41 |
| Apgar score | |||
| at 1 min * | 9.0 (7–9) | 8.0 (1–9) | 0.27 |
| at 5 min * | 9.0 (8–10) | 9.0 (5–10) | 0.82 |
| Umbilical cord blood pH * | 7.340 (7.254–7.400) | 7.306 (7.100–7.390) | 0.31 |
| Severe neonatal asphyxia ** | 0 (0) | 2 (12) | 1.00 |
| Respiratory disorder ** | 3 (50) | 6 (35) | 0.64 |
| Intracranial hemorrhage ** | 0 (0) | 3 (18) | 0.54 |
| SIRS ** | 1 (17) | 0 (0) | 0.26 |
| Antimicrobial treatment ** | 6 (100) | 17 (100) | - |
| ABPC and AMK | 5 (83) | 17 (100) | |
| ABPC and CTX | 1 (17) | 0 (0) | |
| Use of ventilator ** | 1 (17) | 0 (0) | 0.26 |
| Use of catecholamine ** | 1 (17) | 1 (6) | 0.46 |
| Use of PMX-DHP ** | 1 (17) | 0 (0) | 0.26 |
| Death ** | 0 (0) | 0 (0) | - |
| Age at measurement of biomarkers, days * | 0.5 (0–1) | 1.0 (0–3) | 0.76 |
| PCT, ng/mL * | 40.4 (1.26–94.1) | 6.37 (0.79–65.6) | 0.25 |
| CRP, mg/dL * | 5.15 (0.65–10.31) | 4.52 (1.02–14.62) | 0.70 |
| WBC, /µL * | 20,650 (1900–36,300) | 18,100 (11,300–42,500) | 1.00 |
| IgM, mg/dL * | 7.5 (5–11) | 13 (6–34) | 0.05 |
| No. | Detected Pathogens | Samples | Clinical Diagnosis |
|---|---|---|---|
| 1 | Streptococcus agalactiae | Stool | Clinical sepsis |
| 2 | Streptococcus agalactiae, Escherichia coli | Nasopharyngeal swab, stool, and gastric aspirate | Clinical sepsis |
| 3 | Streptococcus agalactiae, Escherichia coli | Nasopharyngeal swab, stool, and gastric aspirate | Clinical sepsis |
| 4 | Streptococcus agalactiae, Escherichia coli | Urine | Clinical sepsis |
| 5 | Escherichia coli | Nasopharyngeal swab, stool, and gastric aspirate | Clinical sepsis |
| 6 | Streptococcus pyogenes | Blood and nasopharyngeal swab | Culture-proven sepsis |
| Reference Curve | Sensitivity | Specificity | Accuracy | Youden Index |
|---|---|---|---|---|
| A. Preterm infants | ||||
| (a) | 1.000 | 1.000 | 1.000 | 1.000 |
| (b) | 0.833 | 1.000 | 1.000 | 0.833 |
| (c) | 0.667 | 1.000 | 1.000 | 0.667 |
| B. Term infants | ||||
| (d) | 0.500 | 0.824 | 0.739 | 0.324 |
| (e) | 0.500 | 0.667 | 0.619 | 0.167 |
| (f) | 0.500 | 0.588 | 0.565 | 0.088 |
| Reference Curve | Sensitivity | Specificity | Accuracy | Youden Index |
|---|---|---|---|---|
| A. Preterm infants | ||||
| PCT | 1.000 | 1.000 | 1.000 | 1.000 |
| CRP | 0.667 | 0.800 | 0.750 | 0.467 |
| WBC | 0.167 | 1.000 | 0.688 | 0.167 |
| IgM | 0.000 | 0.900 | 0.563 | −0.100 |
| PCT and/or CRP | 1.000 | 1.000 | 1.000 | 1.000 |
| PCT and/or WBC | 1.000 | 1.000 | 1.000 | 1.000 |
| PCT and/or IgM | 1.000 | 1.000 | 1.000 | 1.000 |
| CRP and/or WBC | 0.667 | 1.000 | 0.875 | 0.667 |
| CRP and/or IgM | 0.667 | 1.000 | 0.875 | 0.667 |
| WBC and/or IgM | 0.167 | 1.000 | 0.688 | 0.167 |
| PCT, CRP, and/or WBC | 1.000 | 1.000 | 1.000 | 1.000 |
| PCT, CRP, and/or IgM | 1.000 | 1.000 | 1.000 | 1.000 |
| PCT, WBC, and/or IgM | 1.000 | 1.000 | 1.000 | 1.000 |
| CRP, WBC, and/or IgM | 0.667 | 1.000 | 0.875 | 0.667 |
| PCT, CRP, WBC, and/or IgM | 1.000 | 1.000 | 1.000 | 1.000 |
| B. Term infants | ||||
| PCT | 0.500 | 0.824 | 0.739 | 0.324 |
| CRP | 0.833 | 0.000 | 0.217 | −0.167 |
| WBC | 0.500 | 0.824 | 0.739 | 0.324 |
| IgM | 0.000 | 0.765 | 0.565 | −0.235 |
| PCT and/or CRP | 1.000 | 0.824 | 0.870 | 0.824 |
| PCT and/or WBC | 0.667 | 1.000 | 0.913 | 0.667 |
| PCT and/or IgM | 0.500 | 1.000 | 0.870 | 0.500 |
| CRP and/or WBC | 1.000 | 0.824 | 0.870 | 0.824 |
| CRP and/or IgM | 0.833 | 0.765 | 0.783 | 0.598 |
| WBC and/or IgM | 0.500 | 0.882 | 0.783 | 0.382 |
| PCT, CRP, and/or WBC | 1.000 | 1.000 | 1.000 | 1.000 |
| PCT, CRP, and/or IgM | 1.000 | 1.000 | 1.000 | 1.000 |
| PCT, WBC, and/or IgM | 0.667 | 1.000 | 0.913 | 0.667 |
| CRP, WBC, and/or IgM | 1.000 | 0.882 | 0.913 | 0.882 |
| PCT, CRP, WBC, and/or IgM | 1.000 | 1.000 | 1.000 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Go, H.; Nagano, N.; Katayama, D.; Akimoto, T.; Imaizumi, T.; Aoki, R.; Hijikata, M.; Seimiya, A.; Kato, R.; Okahashi, A.; et al. Diagnostic Accuracy of Biomarkers for Early-Onset Neonatal Bacterial Infections: Evaluation of Serum Procalcitonin Reference Curves. Diagnostics 2020, 10, 839. https://doi.org/10.3390/diagnostics10100839
Go H, Nagano N, Katayama D, Akimoto T, Imaizumi T, Aoki R, Hijikata M, Seimiya A, Kato R, Okahashi A, et al. Diagnostic Accuracy of Biomarkers for Early-Onset Neonatal Bacterial Infections: Evaluation of Serum Procalcitonin Reference Curves. Diagnostics. 2020; 10(10):839. https://doi.org/10.3390/diagnostics10100839
Chicago/Turabian StyleGo, Hidetoshi, Nobuhiko Nagano, Daichi Katayama, Takuya Akimoto, Takayuki Imaizumi, Ryoji Aoki, Midori Hijikata, Ayako Seimiya, Ryota Kato, Aya Okahashi, and et al. 2020. "Diagnostic Accuracy of Biomarkers for Early-Onset Neonatal Bacterial Infections: Evaluation of Serum Procalcitonin Reference Curves" Diagnostics 10, no. 10: 839. https://doi.org/10.3390/diagnostics10100839
APA StyleGo, H., Nagano, N., Katayama, D., Akimoto, T., Imaizumi, T., Aoki, R., Hijikata, M., Seimiya, A., Kato, R., Okahashi, A., & Morioka, I. (2020). Diagnostic Accuracy of Biomarkers for Early-Onset Neonatal Bacterial Infections: Evaluation of Serum Procalcitonin Reference Curves. Diagnostics, 10(10), 839. https://doi.org/10.3390/diagnostics10100839





