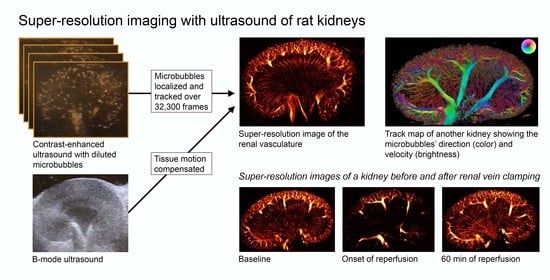
Super-Resolution Imaging with Ultrasound for Visualization of the Renal Microvasculature in Rats Before and After Renal Ischemia: A Pilot Study
Abstract
1. Introduction
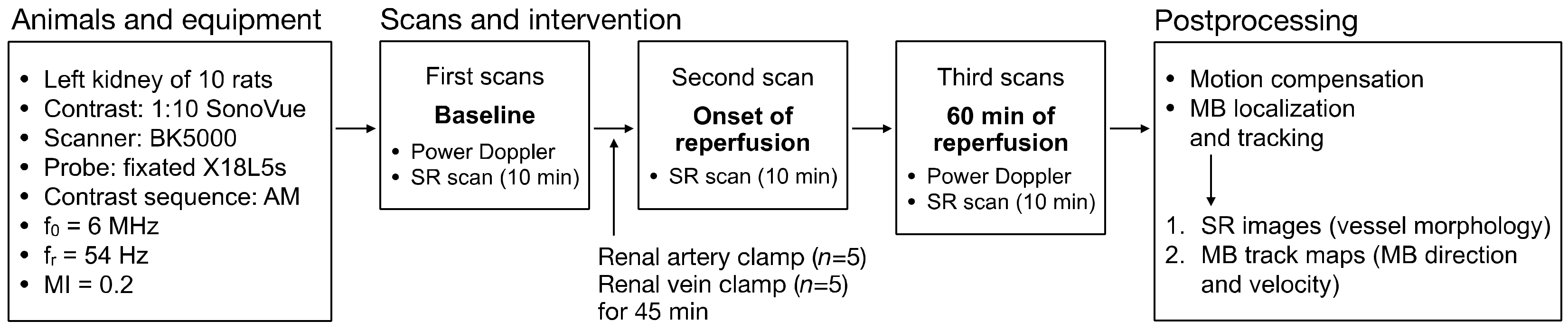
2. Materials and Methods
2.1. Animal Ethics and Preparation
2.2. Ultrasound Scanning, Ischemia-Reperfusion Procedure, and Histopathology
2.3. SR Imaging Technique and Data Processing
2.4. Evaluation of SR Images and Blood Pressure Data
2.5. Ex Vivo MRI of the Microvasculature
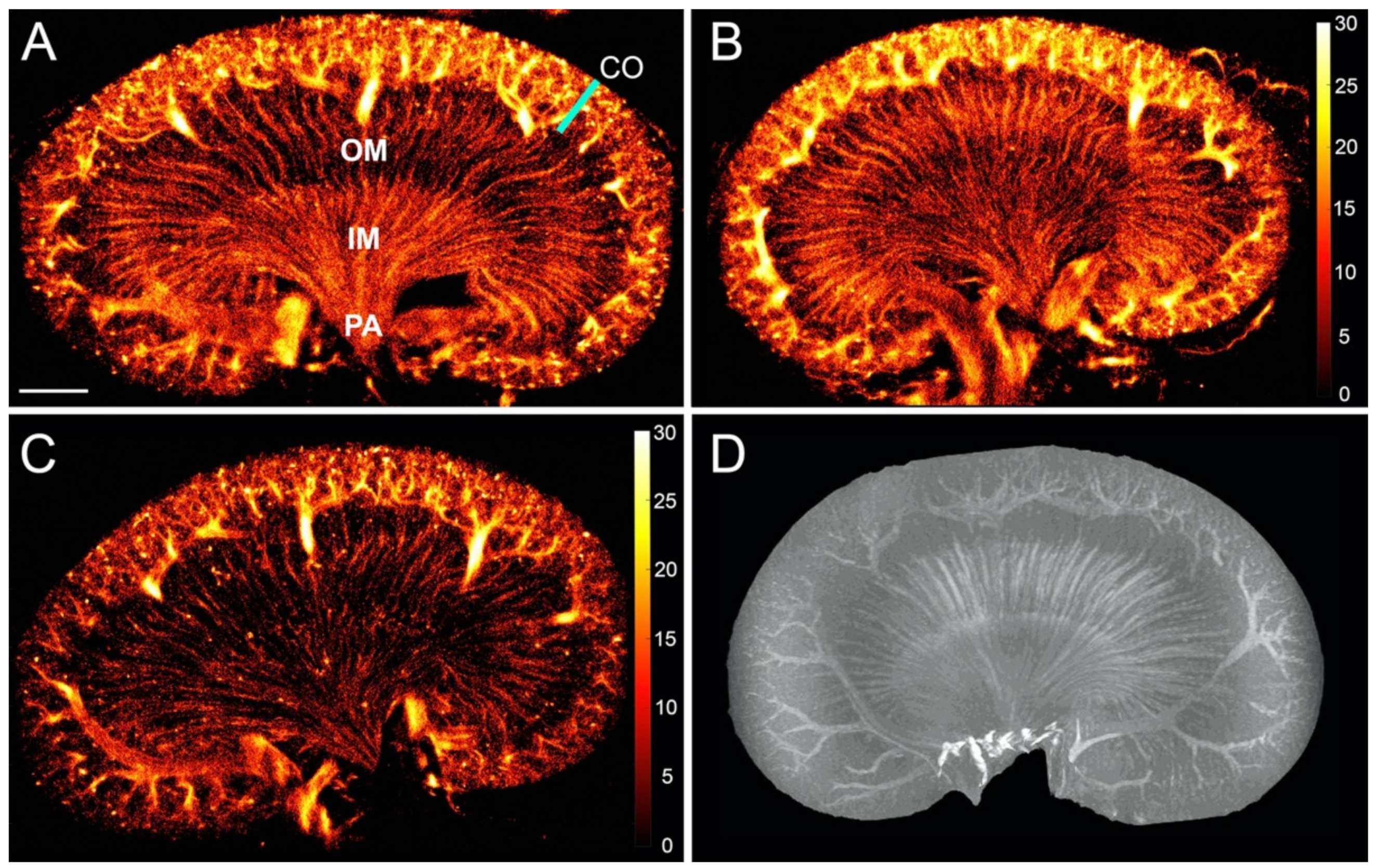
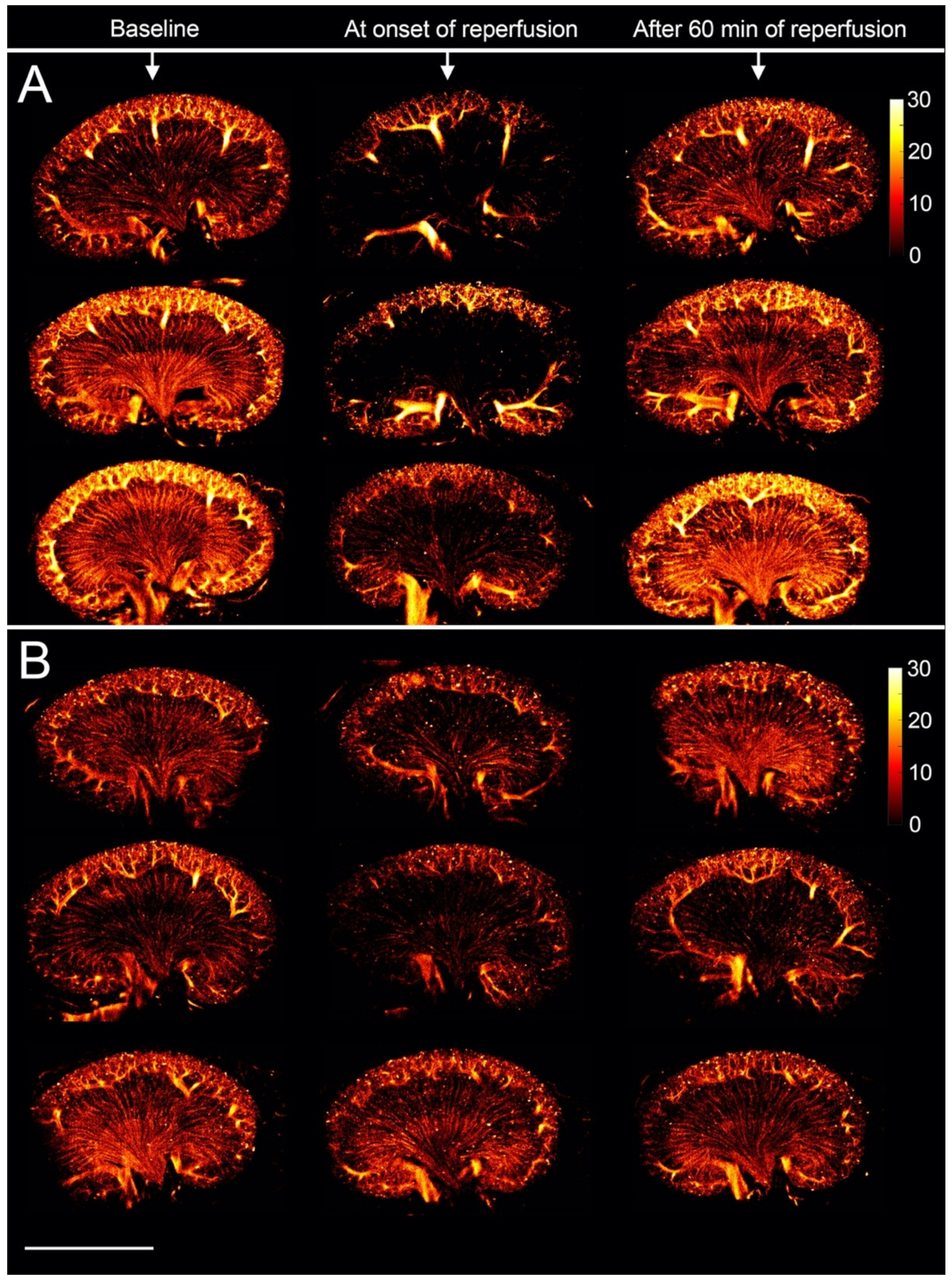
3. Results
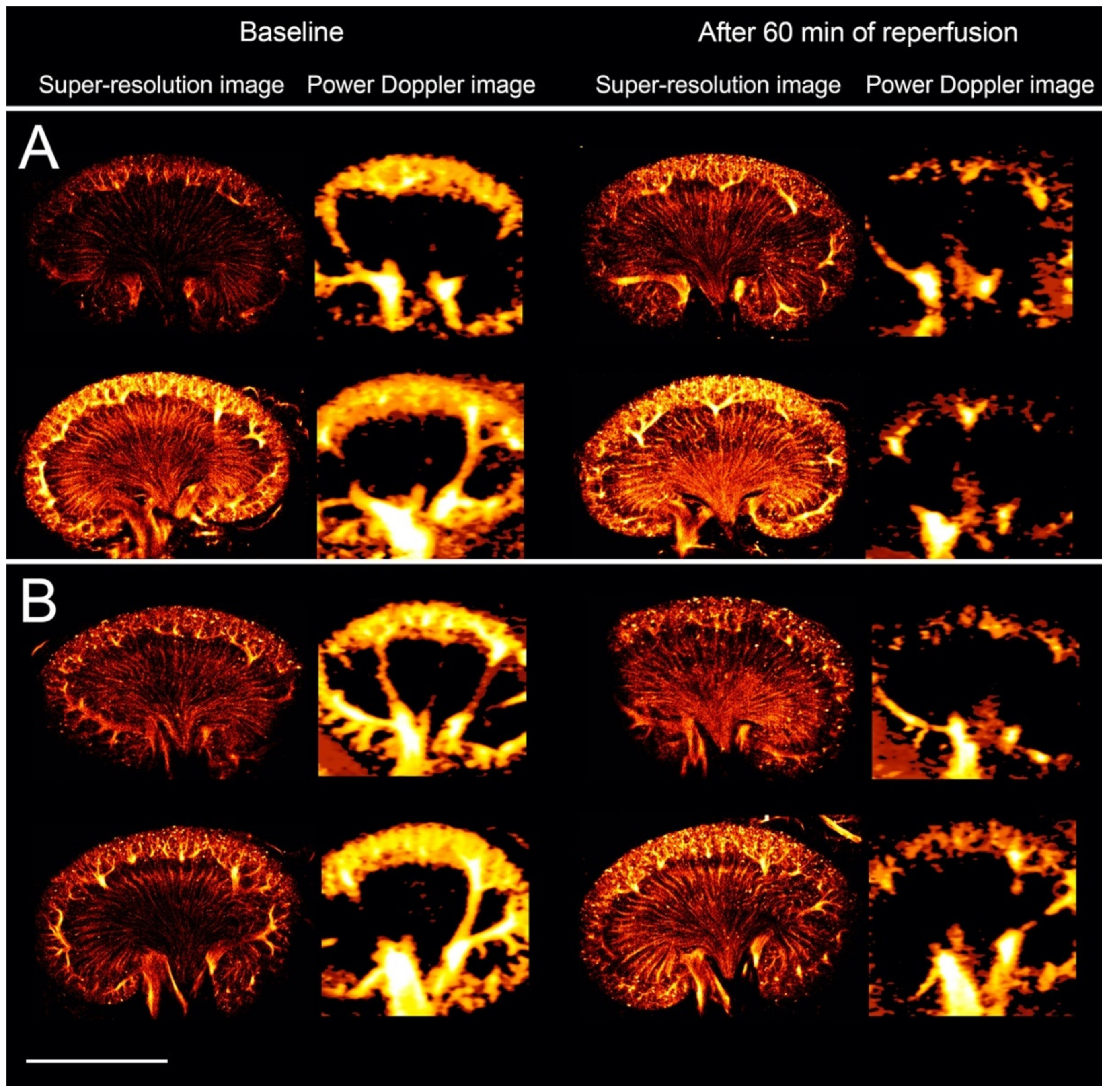
3.1. The Healthy Renal Microvasculature
3.2. The Renal Microvasculature after Ischemia with Reperfusion
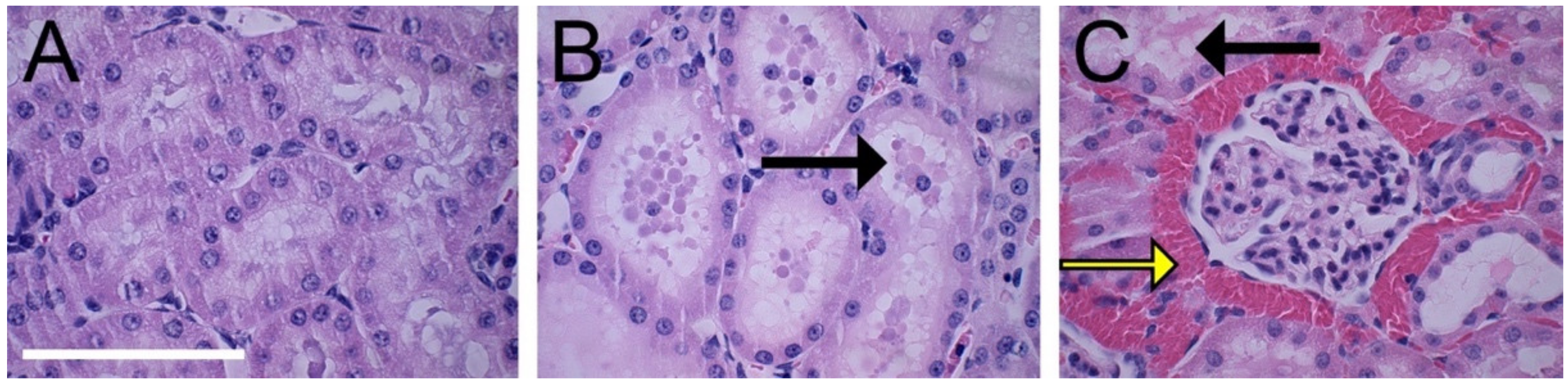
3.3. Histopathological Evaluation
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maric-Bilkan, C.; Flynn, E.R.; Chade, A.R. Microvascular disease precedes the decline in renal function in the streptozotocin-induced diabetic rat. Am. J. Physiol. Physiol. 2012, 302, F308–F315. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Donohoe, D.; Roethe, K.; Osborn, J.L. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am. J. Physiol. Ren. Physiol. 2001, 281, F887–F899. [Google Scholar] [CrossRef]
- Ehling, J.; Bábícková, J.; Gremse, F.; Klinkhammer, B.M.; Baetke, S.; Knuechel, R.; Kiessling, F.; Floege, J.; Lammers, T.; Boor, P. Quantitative Micro-Computed Tomography Imaging of Vascular Dysfunction in Progressive Kidney Diseases. J. Am. Soc. Nephrol. 2016, 27, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.A.; Duncan, M.S.; Damrauer, S.M.; Wells, Q.S.; Barnett, J.V.; Wasserman, D.H.; Bedimo, R.J.; Butt, A.A.; Marconi, V.C.; Sico, J.J.; et al. Microvascular Disease, Peripheral Artery Disease, and Amputation. Circulation 2019, 140, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Ehling, J.; Theek, B.; Gremse, F.; Baetke, S.; Möckel, D.; Maynard, J.; Ricketts, S.A.; Grüll, H.; Neeman, M.; Knuechel, R.; et al. Micro-CT imaging of tumor angiogenesis: Quantitative measures describing micromorphology and vascularization. Am. J. Pathol. 2014, 184, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Demidov, V.; Maeda, A.; Sugita, M.; Madge, V.; Sadanand, S.; Flueraru, C.; Vitkin, I.A. Preclinical longitudinal imaging of tumor microvascular radiobiological response with functional optical coherence tomography. Sci. Rep. 2018, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Jonasson, H.; Bergstrand, S.; Fredriksson, I.; Larsson, M.; Östgren, C.J.; Strömberg, T. Normative data and the influence of age and sex on microcirculatory function in a middle-aged cohort: Results from the SCAPIS study. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H908–H915. [Google Scholar] [CrossRef]
- Errico, C.; Pierre, J.; Pezet, S.; Desailly, Y.; Lenkei, Z.; Couture, O.; Tanter, M. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature 2015, 527, 499–502. [Google Scholar] [CrossRef]
- Christensen-Jeffries, K.; Browning, R.J.; Tang, M.X.; Dunsby, C.; Eckersley, R.J. In Vivo Acoustic Super-Resolution and Super-Resolved Velocity Mapping Using Microbubbles. IEEE Trans. Med. Imaging 2015, 34, 433–440. [Google Scholar] [CrossRef]
- Foiret, J.; Zhang, H.; Ilovitsh, T.; Mahakian, L.; Tam, S.; Ferrara, K.W. Ultrasound localization microscopy to image and assess microvasculature in a rat kidney. Sci. Rep. 2017, 7, 13662. [Google Scholar] [CrossRef]
- Yu, J.; Lavery, L.; Kim, K. Super-resolution ultrasound imaging method for microvasculature in vivo with a high temporal accuracy. Sci. Rep. 2018, 8, 13918. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Rowland, E.M.; Harput, S.; Riemer, K.; Leow, C.H.; Clark, B.; Cox, K.; Lim, A.; Christensen-Jeffries, K.; Zhang, G.; et al. 3D Super-Resolution US Imaging of Rabbit Lymph Node Vasculature in Vivo by Using Microbubbles. Radiology 2019, 291, 642–650. [Google Scholar] [CrossRef]
- Kierski, T.M.; Espindola, D.; Newsome, I.G.; Cherin, E.; Yin, J.; Foster, F.S.; Demore, C.E.M.; Pinton, G.F.; Dayton, P.A. Superharmonic Ultrasound for Motion-Independent Localization Microscopy: Applications to Microvascular Imaging from Low to High Flow Rates. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Opacic, T.; Dencks, S.; Theek, B.; Piepenbrock, M.; Ackermann, D.; Rix, A.; Lammers, T.; Stickeler, E.; Delorme, S.; Schmitz, G.; et al. Motion model ultrasound localization microscopy for preclinical and clinical multiparametric tumor characterization. Nat. Commun. 2018, 9, 1527. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Shelton, S.E.; Espíndola, D.; Rojas, J.D.; Pinton, G.; Dayton, P.A. 3-D Ultrasound Localization Microscopy for Identifying Microvascular Morphology Features of Tumor Angiogenesis at a Resolution Beyond the Diffraction Limit of Conventional Ultrasound. Theranostics 2017, 7, 196–204. [Google Scholar] [CrossRef]
- Lowerison, M.R.; Huang, C.; Lucien, F.; Chen, S.; Song, P. Ultrasound localization microscopy of renal tumor xenografts in chicken embryo is correlated to hypoxia. Sci. Rep. 2020, 10, 2478. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, J.; Rush, B.M.; Stocker, S.D.; Tan, R.J.; Kim, K. Ultrasound super-resolution imaging provides a noninvasive assessment of renal microvasculature changes during mouse acute kidney injury. Kidney Int. 2020, 98, 355–365. [Google Scholar] [CrossRef]
- Moffat, D.B.; Fourman, J. A vascular pattern of the rat kidney. 1963. J. Am. Soc. Nephrol. 2001, 12, 624–632. [Google Scholar]
- Cao, W.; Cui, S.; Yang, L.; Wu, C.; Liu, J.; Yang, F.; Liu, Y.; Bin, J.; Hou, F.F. Contrast-Enhanced Ultrasound for Assessing Renal Perfusion Impairment and Predicting Acute Kidney Injury to Chronic Kidney Disease Progression. Antioxid. Redox Signal. 2017, 27, 1397–1411. [Google Scholar] [CrossRef]
- Fischer, K.; Meral, F.C.; Zhang, Y.; Vangel, M.G.; Jolesz, F.A.; Ichimura, T.; Bonventre, J.V. High-resolution renal perfusion mapping using contrast-enhanced ultrasonography in ischemia-reperfusion injury monitors changes in renal microperfusion. Kidney Int. 2016, 89, 1388–1398. [Google Scholar] [CrossRef]
- Mahoney, M.; Sorace, A.; Warram, J.; Samuel, S.; Hoyt, K. Volumetric contrast-enhanced ultrasound imaging of renal perfusion. J. Ultrasound Med. 2014, 33, 1427–1437. [Google Scholar] [CrossRef]
- Yamamoto, T.; Tada, T.; Brodsky, S.V.; Tanaka, H.; Noiri, E.; Kajiya, F.; Goligorsky, M.S. Intravital videomicroscopy of peritubular capillaries in renal ischemia. Am. J. Physiol. Physiol. 2002, 282, F1150–F1155. [Google Scholar] [CrossRef] [PubMed]
- Hingot, V.; Errico, C.; Heiles, B.; Rahal, L.; Tanter, M.; Couture, O. Microvascular flow dictates the compromise between spatial resolution and acquisition time in Ultrasound Localization Microscopy. Sci. Rep. 2019, 9, 2456. [Google Scholar] [CrossRef] [PubMed]
- Lohmaier, S.; Ghanem, A.; Veltmann, C.; Sommer, T.; Bruce, M.; Tiemann, K. In vitro and in vivo studies on continuous echo-contrast application strategies using SonoVue in a newly developed rotating pump setup. Ultrasound Med. Biol. 2004, 30, 1145–1151. [Google Scholar] [CrossRef]
- Owji, S.M.; Nikeghbal, E.; Moosavi, S.M. Comparison of ischaemia-reperfusion-induced acute kidney injury by clamping renal arteries, veins or pedicles in anaesthetized rats. Exp. Physiol. 2018, 103, 1390–1402. [Google Scholar] [CrossRef]
- Park, Y.; Hirose, R.; Dang, K.; Xu, F.; Behrends, M.; Tan, V.; Roberts, J.P.; Niemann, C.U. Increased severity of renal ischemia-reperfusion injury with venous clamping compared to arterial clamping in a rat model. Surgery 2008, 143, 243–251. [Google Scholar] [CrossRef]
- Jensen, J.A.; Andersen, S.B.; Hoyos, C.A.V.; Hansen, K.L.; Sørensen, C.M.; Nielsen, M.B. Tissue Motion Estimation and Correction in Super Resolution Imaging. In Proceedings of the 2019 IEEE International Ultrasonics Symposium (IUS), Glasgow, UK, 6–9 October 2019; pp. 1–4. [Google Scholar]
- Nitta, N.; Takakusagi, Y.; Kokuryo, D.; Shibata, S.; Tomita, A.; Higashi, T.; Aoki, I.; Harada, M. Intratumoral evaluation of 3D microvasculature and nanoparticle distribution using a gadolinium-dendron modified nano-liposomal contrast agent with magnetic resonance micro-imaging. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Yoldas, A.; Dayan, M.O. Morphological Characteristics of Renal Artery and Kidney in Rats. Sci. World J. 2014, 2014, 468982. [Google Scholar] [CrossRef]
- Wagner, R.; Van Loo, D.; Hossler, F.; Czymmek, K.; Pauwels, E.; Van Hoorebeke, L. High-Resolution Imaging of Kidney Vascular Corrosion Casts with Nano-CT. Microsc. Microanal. 2011, 17, 215–219. [Google Scholar] [CrossRef]
- Yamamoto, K.; Wilson, D.R.; Baumal, R. Blood supply and drainage of the outer medulla of the rat kidney: Scanning electron microscopy of microvascular casts. Anat. Rec. 1984, 210, 273–277. [Google Scholar] [CrossRef]
- Holliger, C.; Lemley, K.V.; Schmitt, S.L.; Thomas, F.C.; Robertson, C.R.; Jamison, R.L. Direct Determination of Vasa Recta Blood Flow in the Rat Renal Papilla. Circ. Res. 1983, 53, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Morel, D.R.; Schwieger, I.; Hohn, L.; Terrettaz, J.; Llull, J.B.; Cornioley, Y.A.; Schneider, M. Human Pharmacokinetics and Safety Evaluation of SonoVueTM, a New Contrast Agent for Ultrasound Imaging. Investig. Radiol. 2000, 35, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Broillet, A.; Tardy, I.; Pochon, S.; Bussat, P.; Bettinger, T.; Helbert, A.; Costa, M.; Tranquart, F. Use of Intravital Microscopy to Study the Microvascular Behavior of Microbubble-Based Ultrasound Contrast Agents. Microcirculation 2012, 19, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, Q.; Zhang, H.; Qiu, L.; Qian, L.; Lee, F.-F.; Liu, Z.; Luo, J. Assessment of Diabetic Kidney Disease Using Ultrasound Localization Microscopy: An in Vivo Feasibility Study in Rats. In Proceedings of the 2018 IEEE International Ultrasonics Symposium (IUS), Kobe, Japan, 22–25 October 2018; pp. 1–4. [Google Scholar]
- Vetterlein, F.; Pethö, A.; Schmidt, G. Distribution of capillary blood flow in rat kidney during postischemic renal failure. Am. J. Physiol. 1986, 251, H510–H519. [Google Scholar] [CrossRef]
- Conesa, E.L.; Valero, F.; Nadal, J.C.; Fenoy, F.J.; López, B.; Arregui, B.; Salom, M.G. N-acetyl-l-cysteine improves renal medullary hypoperfusion in acute renal failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R730–R737. [Google Scholar] [CrossRef]
- Regner, K.R.; Zuk, A.; Van Why, S.K.; Shames, B.D.; Ryan, R.P.; Falck, J.R.; Manthati, V.L.; McMullen, M.E.; Ledbetter, S.R.; Roman, R.J. Protective effect of 20-HETE analogues in experimental renal ischemia reperfusion injury. Kidney Int. 2009, 75, 511–517. [Google Scholar] [CrossRef]
- Chawla, L.S.; Amdur, R.L.; Amodeo, S.; Kimmel, P.L.; Palant, C.E. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011, 79, 1361–1369. [Google Scholar] [CrossRef]
- Wang, H.-J.; Varner, A.; AbouShwareb, T.; Atala, A.; Yoo, J.J. Ischemia/Reperfusion-Induced Renal Failure in Rats as a Model for Evaluating Cell Therapies. Ren. Fail. 2012, 34, 1324–1332. [Google Scholar] [CrossRef][Green Version]
- Scurt, F.G.; Menne, J.; Brandt, S.; Bernhardt, A.; Mertens, P.R.; Haller, H.; Chatzikyrkou, C.; Ito, S.; Izzo, J.L.; Januszewicz, A.; et al. Systemic Inflammation Precedes Microalbuminuria in Diabetes. Kidney Int. Rep. 2019, 4, 1373–1386. [Google Scholar] [CrossRef]
- Kasoji, S.K.; Rivera, J.N.; Gessner, R.C.; Chang, S.X.; Dayton, P.A. Early assessment of tumor response to radiation therapy using high-resolution quantitative microvascular ultrasound imaging. Theranostics 2018, 8, 156–168. [Google Scholar] [CrossRef]
- Hlushchuk, R.; Zubler, C.; Barré, S.; Correa Shokiche, C.; Schaad, L.; Röthlisberger, R.; Wnuk, M.; Daniel, C.; Khoma, O.; Tschanz, S.A.; et al. Cutting-edge microangio-CT: New dimensions in vascular imaging and kidney morphometry. Am. J. Physiol. Physiol. 2018, 314, F493–F499. [Google Scholar] [CrossRef] [PubMed]
- Perrien, D.S.; Saleh, M.A.; Takahashi, K.; Madhur, M.S.; Harrison, D.G.; Harris, R.C.; Takahashi, T. Novel methods for microCT-based analyses of vasculature in the renal cortex reveal a loss of perfusable arterioles and glomeruli in eNOS-/- mice. BMC Nephrol. 2016, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Morikawa, T. The effect of formalin fixation on the size of pelvic sidewall lymph nodes. Int. J. Colorectal Dis. 2018, 33, 1493–1495. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.; Jan, N.J.; Hu, D.; Voorhees, A.; Schuman, J.S.; Smith, M.A.; Wollstein, G.; Sigal, I.A. Formalin Fixation and Cryosectioning Cause only Minimal Changes in Shape or Size of Ocular Tissues. Sci. Rep. 2017, 7, 12065. [Google Scholar] [CrossRef]
- Pöschinger, T.; Renner, A.; Eisa, F.; Dobosz, M.; Strobel, S.; Weber, T.G.; Brauweiler, R.; Kalender, W.A.; Scheuer, W. Dynamic Contrast-Enhanced Micro–Computed Tomography Correlates With 3-Dimensional Fluorescence Ultramicroscopy in Antiangiogenic Therapy of Breast Cancer Xenografts. Investig. Radiol. 2014, 49, 445–456. [Google Scholar] [CrossRef]
- Pohlmann, A.; Hentschel, J.; Fechner, M.; Hoff, U.; Bubalo, G.; Arakelyan, K.; Cantow, K.; Seeliger, E.; Flemming, B.; Waiczies, H.; et al. High Temporal Resolution Parametric MRI Monitoring of the Initial Ischemia/Reperfusion Phase in Experimental Acute Kidney Injury. PLoS ONE 2013, 8, e57411. [Google Scholar] [CrossRef]
- Starosolski, Z.; Villamizar, C.A.; Rendon, D.; Paldino, M.J.; Milewicz, D.M.; Ghaghada, K.B.; Annapragada, A.V. Ultra High-Resolution In vivo Computed Tomography Imaging of Mouse Cerebrovasculature Using a Long Circulating Blood Pool Contrast Agent. Sci. Rep. 2015, 5, 10178. [Google Scholar] [CrossRef]
- Miller, D.L.; Averkiou, M.A.; Brayman, A.A.; Everbach, E.C.; Holland, C.K.; Wible, J.H.; Wu, J. Bioeffects Considerations for Diagnostic Ultrasound Contrast Agents. J. Ultrasound Med. 2008, 27, 611–632. [Google Scholar] [CrossRef]
- Tang, M.X.; Mulvana, H.; Gauthier, T.; Lim, A.K.P.; Cosgrove, D.O.; Eckersley, R.J.; Stride, E. Quantitative contrast-enhanced ultrasound imaging: A review of sources of variability. Interface Focus 2011, 1, 520–539. [Google Scholar] [CrossRef]
- Demené, C.; Payen, T.; Dizeux, A.; Barrois, G.; Gennisson, J.-L.; Bridal, L.; Tanter, M. 3-D Longitudinal Imaging of Tumor Angiogenesis in Mice in Vivo Using Ultrafast Doppler Tomography. Ultrasound Med. Biol. 2019, 45, 1284–1296. [Google Scholar] [CrossRef]
- Huang, C.; Lowerison, M.R.; Lucien, F.; Gong, P.; Wang, D.; Song, P.; Chen, S. Noninvasive Contrast-Free 3D Evaluation of Tumor Angiogenesis with Ultrasensitive Ultrasound Microvessel Imaging. Sci. Rep. 2019, 9, 4907. [Google Scholar] [CrossRef]
- Jensen, J.A.; Tomov, B.G.; Ommen, M.L.; Øygard, S.H.; Schou, M.; Sams, T.; Stuart, M.B.; Beers, C.; Thomsen, E.V.; Larsen, N.B. Three-Dimensional Super-Resolution Imaging Using a Row-Column Array. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Heiles, B.; Correia, M.; Hingot, V.; Pernot, M.; Provost, J.; Tanter, M.; Couture, O. Ultrafast 3D Ultrasound Localization Microscopy Using a 32 × 32 Matrix Array. IEEE Trans. Med. Imaging 2019, 38, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Harput, S.; Tortoli, P.; Eckersley, R.J.; Dunsby, C.; Tang, M.X.; Christensen-Jeffries, K.; Ramalli, A.; Brown, J.; Zhu, J.; Zhang, G.; et al. 3-D Super-Resolution Ultrasound Imaging with a 2-D Sparse Array. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 269–277. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, S.B.; Taghavi, I.; Hoyos, C.A.V.; Søgaard, S.B.; Gran, F.; Lönn, L.; Hansen, K.L.; Jensen, J.A.; Nielsen, M.B.; Sørensen, C.M. Super-Resolution Imaging with Ultrasound for Visualization of the Renal Microvasculature in Rats Before and After Renal Ischemia: A Pilot Study. Diagnostics 2020, 10, 862. https://doi.org/10.3390/diagnostics10110862
Andersen SB, Taghavi I, Hoyos CAV, Søgaard SB, Gran F, Lönn L, Hansen KL, Jensen JA, Nielsen MB, Sørensen CM. Super-Resolution Imaging with Ultrasound for Visualization of the Renal Microvasculature in Rats Before and After Renal Ischemia: A Pilot Study. Diagnostics. 2020; 10(11):862. https://doi.org/10.3390/diagnostics10110862
Chicago/Turabian StyleAndersen, Sofie Bech, Iman Taghavi, Carlos Armando Villagómez Hoyos, Stinne Byrholdt Søgaard, Fredrik Gran, Lars Lönn, Kristoffer Lindskov Hansen, Jørgen Arendt Jensen, Michael Bachmann Nielsen, and Charlotte Mehlin Sørensen. 2020. "Super-Resolution Imaging with Ultrasound for Visualization of the Renal Microvasculature in Rats Before and After Renal Ischemia: A Pilot Study" Diagnostics 10, no. 11: 862. https://doi.org/10.3390/diagnostics10110862
APA StyleAndersen, S. B., Taghavi, I., Hoyos, C. A. V., Søgaard, S. B., Gran, F., Lönn, L., Hansen, K. L., Jensen, J. A., Nielsen, M. B., & Sørensen, C. M. (2020). Super-Resolution Imaging with Ultrasound for Visualization of the Renal Microvasculature in Rats Before and After Renal Ischemia: A Pilot Study. Diagnostics, 10(11), 862. https://doi.org/10.3390/diagnostics10110862