Analysis of the Gene Expression Profile of Stromal Pro-Tumor Factors in Cancer-Associated Fibroblasts from Luminal Breast Carcinomas
Abstract
:1. Introduction
2. Material and Methods
2.1. Patient Selection and Their Characteristics, and Tissue Specimen Handling
2.2. Cell Lines
2.3. Primary Cell Culture
2.4. Co-Culture Experiments
2.5. Real Time-PCR
2.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results
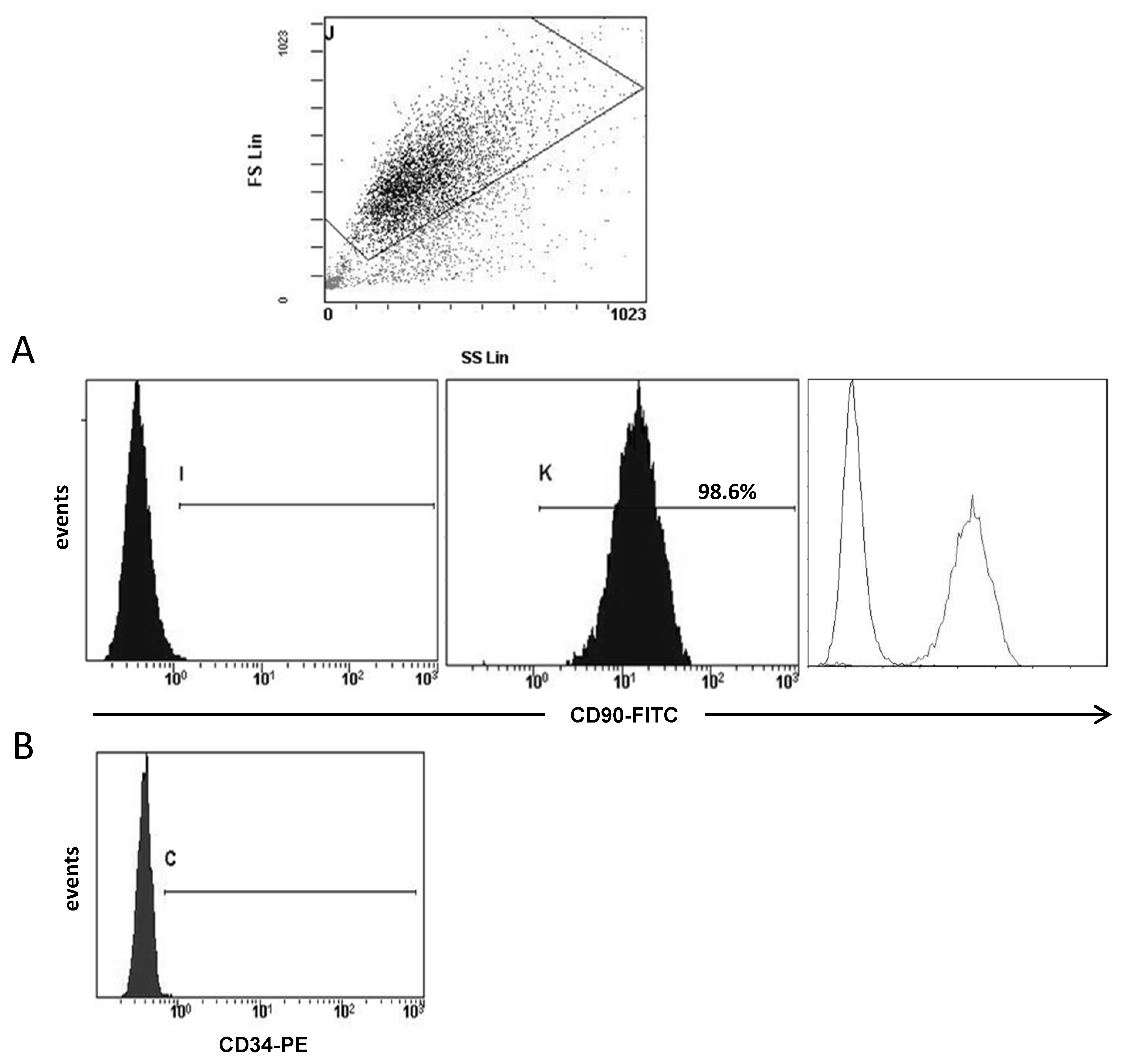
3.1. Molecular Profile of CAFs
3.2. Molecular Profile of CAFs Co-Culture with MCF-7 and MDA-MB-231 Cancer Cell Lines
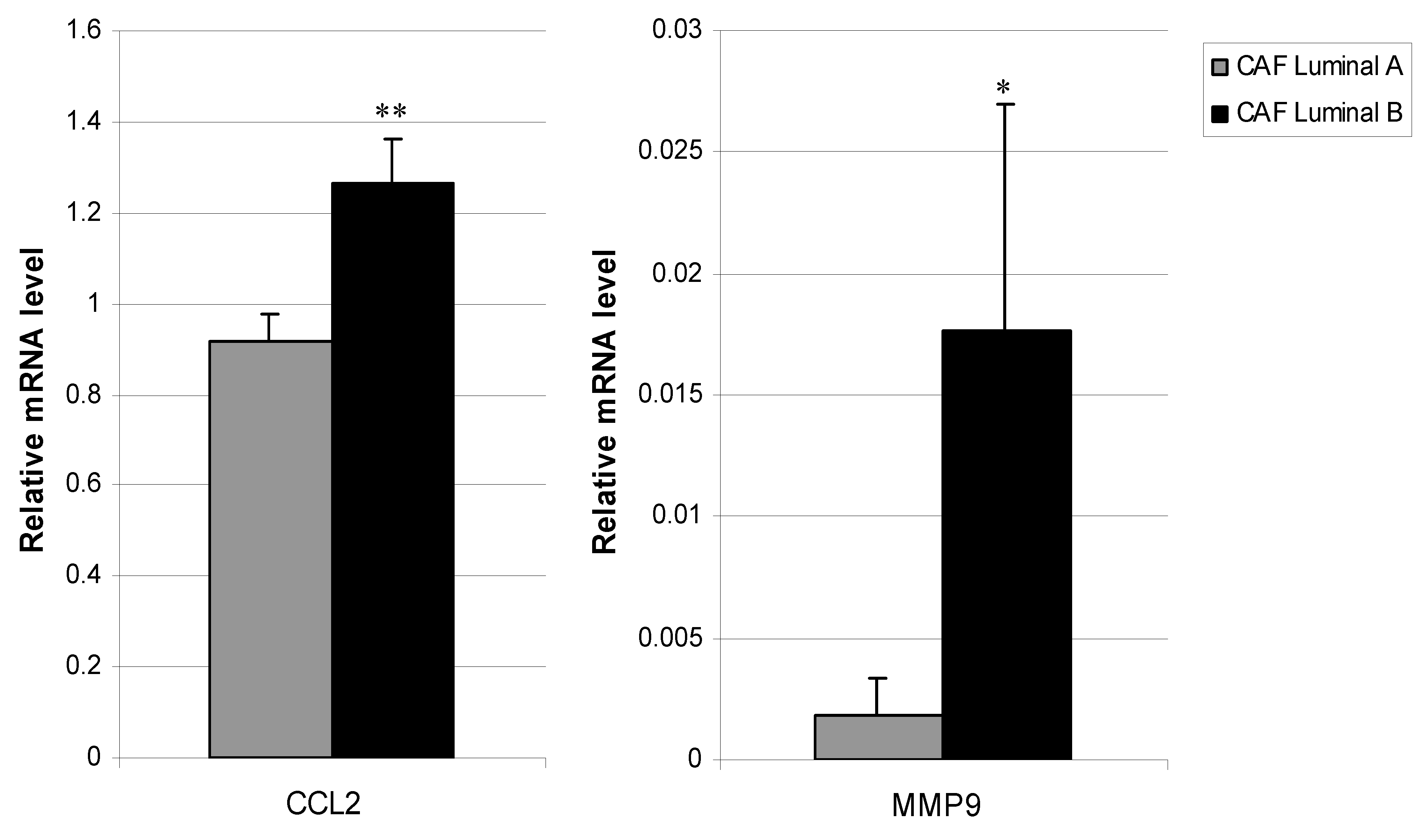
3.3. Relationship between CAFs’ Molecular Profile and Development of Distant Metastasis
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thurlimann, B.; Senn, H.J.; Panel, m. Strategies for subtypes--dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Montagna, E.; Bagnardi, V.; Rotmensz, N.; Viale, G.; Cancello, G.; Mazza, M.; Cardillo, A.; Ghisini, R.; Galimberti, V.; Veronesi, P.; et al. Immunohistochemically defined subtypes and outcome in occult breast carcinoma with axillary presentation. Breast Cancer Res. Treat. 2011, 129, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thurlimann, B.; Senn, H.J.; Panel, m. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Breast Cancer Facts & Figures 2013–2014; American Cancer Society: Atlanta, GA, USA, 2013. [Google Scholar]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006, 295, 2492–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finak, G.; Bertos, N.; Pepin, F.; Sadekova, S.; Souleimanova, M.; Zhao, H.; Chen, H.; Omeroglu, G.; Meterissian, S.; Omeroglu, A.; et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 2008, 14, 518–527. [Google Scholar] [CrossRef]
- Farmer, P.; Bonnefoi, H.; Anderle, P.; Cameron, D.; Wirapati, P.; Becette, V.; Andre, S.; Piccart, M.; Campone, M.; Brain, E.; et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat. Med. 2009, 15, 68–74. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Brennan, D.J.; Rexhepaj, E.; Ruffell, B.; Shiao, S.L.; Madden, S.F.; Gallagher, W.M.; Wadhwani, N.; Keil, S.D.; Junaid, S.A.; et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011, 1, 54–67. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.; Cho, J.; Sung, J.; Lee, J.E.; Nam, S.J.; Kim, K.M.; Cho, E.Y. The prognostic significance of tumor-associated stroma in invasive breast carcinoma. Tumour Biol. 2012, 33, 1573–1580. [Google Scholar] [CrossRef]
- Koontongkaew, S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J. Cancer 2013, 4, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Madar, S.; Goldstein, I.; Rotter, V. ‘Cancer associated fibroblasts’--more than meets the eye. Trends Mol. Med. 2013, 19, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Erez, N.; Truitt, M.; Olson, P.; Arron, S.T.; Hanahan, D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell 2010, 17, 135–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soon, P.S.; Kim, E.; Pon, C.K.; Gill, A.J.; Moore, K.; Spillane, A.J.; Benn, D.E.; Baxter, R.C. Breast cancer-associated fibroblasts induce epithelial-to-mesenchymal transition in breast cancer cells. Endocr. Relat. Cancer 2013, 20, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Allen, M.; Louise Jones, J. Jekyll and Hyde: The role of the microenvironment on the progression of cancer. J. Pathol. 2011, 223, 162–176. [Google Scholar] [CrossRef]
- Mao, Y.; Keller, E.T.; Garfield, D.H.; Shen, K.; Wang, J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013, 32, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, L.; Eiro, N.; Fernandez-Garcia, B.; Gonzalez, L.O.; Dominguez, F.; Vizoso, F.J. Gene expression profile of normal and cancer-associated fibroblasts according to intratumoral inflammatory cells phenotype from breast cancer tissue. Mol. Carcinog. 2016, 55, 1489–1502. [Google Scholar] [CrossRef]
- Kogan-Sakin, I.; Cohen, M.; Paland, N.; Madar, S.; Solomon, H.; Molchadsky, A.; Brosh, R.; Buganim, Y.; Goldfinger, N.; Klocker, H.; et al. Prostate stromal cells produce CXCL-1, CXCL-2, CXCL-3 and IL-8 in response to epithelia-secreted IL-1. Carcinogenesis 2009, 30, 698–705. [Google Scholar] [CrossRef] [Green Version]
- Protti, M.P.; De Monte, L. Cross-talk within the tumor microenvironment mediates Th2-type inflammation in pancreatic cancer. Oncoimmunology 2012, 1, 89–91. [Google Scholar] [CrossRef] [Green Version]
- Sotiriou, C.; Neo, S.Y.; McShane, L.M.; Korn, E.L.; Long, P.M.; Jazaeri, A.; Martiat, P.; Fox, S.B.; Harris, A.L.; Liu, E.T. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc. Natl. Acad. Sci. USA 2003, 100, 10393–10398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voduc, K.D.; Cheang, M.C.; Tyldesley, S.; Gelmon, K.; Nielsen, T.O.; Kennecke, H. Breast cancer subtypes and the risk of local and regional relapse. J. Clin. Oncol. 2010, 28, 1684–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gyorffy, B.; Schafer, R. Meta-analysis of gene expression profiles related to relapse-free survival in 1,079 breast cancer patients. Breast Cancer Res. Treat 2009, 118, 433–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, B.; Bedard, P.L. Luminal-B breast cancer and novel therapeutic targets. Breast Cancer Res. 2011, 13, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Prat, A.; Cheang, M.C.; Martin, M.; Parker, J.S.; Carrasco, E.; Caballero, R.; Tyldesley, S.; Gelmon, K.; Bernard, P.S.; Nielsen, T.O.; et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J. Clin. Oncol. 2013, 31, 203–209. [Google Scholar] [CrossRef]
- Metzger-Filho, O.; Sun, Z.; Viale, G.; Price, K.N.; Crivellari, D.; Snyder, R.D.; Gelber, R.D.; Castiglione-Gertsch, M.; Coates, A.S.; Goldhirsch, A.; et al. Patterns of Recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: Results from international breast cancer study group trials VIII and IX. J. Clin. Oncol. 2013, 31, 3083–3090. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.L.; Walker, R.A. Control of matrix metalloproteinase activity in cancer. J. Pathol. 1997, 183, 377–379. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Chantrain, C.F.; Shimada, H.; Jodele, S.; Groshen, S.; Ye, W.; Shalinsky, D.R.; Werb, Z.; Coussens, L.M.; DeClerck, Y.A. Stromal matrix metalloproteinase-9 regulates the vascular architecture in neuroblastoma by promoting pericyte recruitment. Cancer Res. 2004, 64, 1675–1686. [Google Scholar] [CrossRef] [Green Version]
- Pellikainen, J.M.; Ropponen, K.M.; Kataja, V.V.; Kellokoski, J.K.; Eskelinen, M.J.; Kosma, V.M. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin. Cancer Res. 2004, 10, 7621–7628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizoso, F.J.; Gonzalez, L.O.; Corte, M.D.; Rodriguez, J.C.; Vazquez, J.; Lamelas, M.L.; Junquera, S.; Merino, A.M.; Garcia-Muniz, J.L. Study of matrix metalloproteinases and their inhibitors in breast cancer. Br. J. Cancer 2007, 96, 903–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Frangogiannis, N.G. MCP-1/CCL2 as a therapeutic target in myocardial infarction and ischemic cardiomyopathy. Inflamm. Allergy Drug Targets 2007, 6, 101–107. [Google Scholar] [PubMed]
- Ueno, T.; Toi, M.; Saji, H.; Muta, M.; Bando, H.; Kuroi, K.; Koike, M.; Inadera, H.; Matsushima, K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin. Cancer Res. 2000, 6, 3282–3289. [Google Scholar] [PubMed]
- Lebrecht, A.; Grimm, C.; Lantzsch, T.; Ludwig, E.; Hefler, L.; Ulbrich, E.; Koelbl, H. Monocyte chemoattractant protein-1 serum levels in patients with breast cancer. Tumour Biol. 2004, 25, 14–17. [Google Scholar] [CrossRef]
- Youngs, S.J.; Ali, S.A.; Taub, D.D.; Rees, R.C. Chemokines induce migrational responses in human breast carcinoma cell lines. Int. J. Cancer 1997, 71, 257–266. [Google Scholar] [CrossRef]
- Qian, B.Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.A.; Lee, Y.H.; Schiemann, W.P. Role of TGF-beta and the tumor microenvironment during mammary tumorigenesis. Gene Expr. 2011, 15, 117–132. [Google Scholar] [CrossRef]
- Bierie, B.; Moses, H.L. Tumour microenvironment: TGFbeta: The molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer 2006, 6, 506–520. [Google Scholar] [CrossRef]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Fernandis, A.Z.; Prasad, A.; Band, H.; Klosel, R.; Ganju, R.K. Regulation of CXCR4-mediated chemotaxis and chemoinvasion of breast cancer cells. Oncogene 2004, 23, 157–167. [Google Scholar] [CrossRef]
- Lee, B.C.; Lee, T.H.; Avraham, S.; Avraham, H.K. Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1alpha in breast cancer cell migration through human brain microvascular endothelial cells. Mol. Cancer Res. 2004, 2, 327–338. [Google Scholar] [PubMed]
- Kishimoto, H.; Wang, Z.; Bhat-Nakshatri, P.; Chang, D.; Clarke, R.; Nakshatri, H. The p160 family coactivators regulate breast cancer cell proliferation and invasion through autocrine/paracrine activity of SDF-1alpha/CXCL12. Carcinogenesis 2005, 26, 1706–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.; Watkins, G.; Parr, C.; Douglas-Jones, A.; Mansel, R.E.; Jiang, W.G. Stromal cell derived factor-1: Its influence on invasiveness and migration of breast cancer cells in vitro, and its association with prognosis and survival in human breast cancer. Breast Cancer Res. 2005, 7, R402–R410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.C.; Luker, K.E.; Garbow, J.R.; Prior, J.L.; Jackson, E.; Piwnica-Worms, D.; Luker, G.D. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004, 64, 8604–8612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamamura, H.; Hori, A.; Kanzaki, N.; Hiramatsu, K.; Mizumoto, M.; Nakashima, H.; Yamamoto, N.; Otaka, A.; Fujii, N. T140 analogs as CXCR4 antagonists identified as anti-metastatic agents in the treatment of breast cancer. FEBS Lett. 2003, 550, 79–83. [Google Scholar] [CrossRef]
- Liang, Z.; Yoon, Y.; Votaw, J.; Goodman, M.M.; Williams, L.; Shim, H. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res. 2005, 65, 967–971. [Google Scholar]
- Liotta, L.A.; Kohn, E.C. The microenvironment of the tumour-host interface. Nature 2001, 411, 375–379. [Google Scholar] [CrossRef]
- Talvensaari-Mattila, A.; Paakko, P.; Turpeenniemi-Hujanen, T. Matrix metalloproteinase-2 (MMP-2) is associated with survival in breast carcinoma. Br. J. Cancer 2003, 89, 1270–1275. [Google Scholar] [CrossRef]
- Obermair, A.; Kucera, E.; Mayerhofer, K.; Speiser, P.; Seifert, M.; Czerwenka, K.; Kaider, A.; Leodolter, S.; Kainz, C.; Zeillinger, R. Vascular endothelial growth factor (VEGF) in human breast cancer: Correlation with disease-free survival. Int. J. Cancer 1997, 74, 455–458. [Google Scholar] [CrossRef]
- Salven, P.; Perhoniemi, V.; Tykka, H.; Maenpaa, H.; Joensuu, H. Serum VEGF levels in women with a benign breast tumor or breast cancer. Breast Cancer Res. Treat. 1999, 53, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Manders, P.; Beex, L.V.; Tjan-Heijnen, V.C.; Geurts-Moespot, J.; Van Tienoven, T.H.; Foekens, J.A.; Sweep, C.G. The prognostic value of vascular endothelial growth factor in 574 node-negative breast cancer patients who did not receive adjuvant systemic therapy. Br. J. Cancer 2002, 87, 772–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt-Hansen, B.; Ornas, D.; Grigorian, M.; Klingelhofer, J.; Tulchinsky, E.; Lukanidin, E.; Ambartsumian, N. Extracellular S100A4(mts1) stimulates invasive growth of mouse endothelial cells and modulates MMP-13 matrix metalloproteinase activity. Oncogene 2004, 23, 5487–5495. [Google Scholar] [CrossRef] [Green Version]
- Cabezon, T.; Celis, J.E.; Skibshoj, I.; Klingelhofer, J.; Grigorian, M.; Gromov, P.; Rank, F.; Myklebust, J.H.; Maelandsmo, G.M.; Lukanidin, E.; et al. Expression of S100A4 by a variety of cell types present in the tumor microenvironment of human breast cancer. Int. J. Cancer 2007, 121, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Hansen, B.; Klingelhofer, J.; Grum-Schwensen, B.; Christensen, A.; Andresen, S.; Kruse, C.; Hansen, T.; Ambartsumian, N.; Lukanidin, E.; Grigorian, M. Functional significance of metastasis-inducing S100A4(Mts1) in tumor-stroma interplay. J. Biol. Chem. 2004, 279, 24498–24504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, S.R.; Barraclough, R.; West, C.R.; Rudland, P.S. S100A4 regulates cell motility and invasion in an in vitro model for breast cancer metastasis. Br. J. Cancer 2004, 90, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Ismail, N.I.; Kaur, G.; Hashim, H.; Hassan, M.S. S100A4 overexpression proves to be independent marker for breast cancer progression. Cancer Cell Int. 2008, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Montero, P.; Londono-Vallejo, A.; Vernot, J.P. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun. Signal 2017, 15, 17. [Google Scholar] [CrossRef] [Green Version]
- Salgado, R.; Junius, S.; Benoy, I.; Van Dam, P.; Vermeulen, P.; Van Marck, E.; Huget, P.; Dirix, L.Y. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int. J. Cancer 2003, 103, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, T.; Ray-Coquard, I.; Menetrier-Caux, C.; Rastkha, M.; Duc, A.; Blay, J.Y. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br. J. Cancer 2003, 88, 1721–1726. [Google Scholar] [CrossRef] [Green Version]
- Benoy, I.H.; Salgado, R.; Van Dam, P.; Geboers, K.; Van Marck, E.; Scharpe, S.; Vermeulen, P.B.; Dirix, L.Y. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin. Cancer Res. 2004, 10, 7157–7162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.A.; Sung, M.K.; Yeon, J.Y.; Ro, J.; Kim, J. Prognostic role of interleukin-6, interleukin-8, and leptin levels according to breast cancer subtype. Cancer Res. Treat. 2013, 45, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chow, S.O.; Boernert, K.; Basel, D.; Mikuscheva, A.; Kim, S.; Fong-Yee, C.; Trivedi, T.; Buttgereit, F.; Sutherland, R.L.; et al. Direct crosstalk between cancer and osteoblast lineage cells fuels metastatic growth in bone via auto-amplification of IL-6 and RANKL signaling pathways. J. Bone Miner. Res. 2014, 29, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, F.; Wang, H.; Wang, X.; Jiang, Y.; Li, D. Wortmannin reduces metastasis and angiogenesis of human breast cancer cells via nuclear factor-kappaB-dependent matrix metalloproteinase-9 and interleukin-8 pathways. J. Int. Med. Res. 2012, 40, 867–876. [Google Scholar] [CrossRef] [Green Version]
- Pravtcheva, D.D.; Wise, T.L. Metastasizing mammary carcinomas in H19 enhancers-Igf2 transgenic mice. J. Exp. Zool. 1998, 281, 43–57. [Google Scholar] [CrossRef]
- Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 2008, 8, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tsao, S.W.; Chan, K.W.; Ludwig, D.L.; Novosyadlyy, R.; Li, Y.Y.; He, Q.Y.; Cheung, A.L. Id1-induced IGF-II and its autocrine/endocrine promotion of esophageal cancer progression and chemoresistance--implications for IGF-II and IGF-IR-targeted therapy. Clin. Cancer Res. 2014, 20, 2651–2662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, E.J.; LeRoith, D. Minireview: IGF, Insulin, and Cancer. Endocrinology 2011, 152, 2546–2551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salisbury, T.B.; Morris, G.Z.; Tomblin, J.K.; Chaudhry, A.R.; Cook, C.R.; Santanam, N. Aryl hydrocarbon receptor ligands inhibit igf-ii and adipokine stimulated breast cancer cell proliferation. ISRN Endocrinol. 2013, 2013, 104850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomblin, J.K.; Salisbury, T.B. Insulin like growth factor 2 regulation of aryl hydrocarbon receptor in MCF-7 breast cancer cells. Biochem. Biophys. Res. Commun. 2014, 443, 1092–1096. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, N.; Marquez-Garban, D.; Mah, V.; Fernando, G.; Elshimali, Y.; Garban, H.; Elashoff, D.; Vadgama, J.; Goodglick, L.; Pietras, R. Biologic roles of estrogen receptor-beta and insulin-like growth factor-2 in triple-negative breast cancer. Biomed. Res. Int. 2015, 2015, 925703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tominaga, K.; Shimamura, T.; Kimura, N.; Murayama, T.; Matsubara, D.; Kanauchi, H.; Niida, A.; Shimizu, S.; Nishioka, K.; Tsuji, E.I.; et al. Addiction to the IGF2-ID1-IGF2 circuit for maintenance of the breast cancer stem-like cells. Oncogene 2017, 36, 1276–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gui, Y.; Aguilar-Mahecha, A.; Krzemien, U.; Hosein, A.; Buchanan, M.; Lafleur, J.; Pollak, M.; Ferrario, C.; Basik, M. Metastatic Breast Carcinoma-Associated Fibroblasts Have Enhanced Protumorigenic Properties Related to Increased IGF2 Expression. Clin. Cancer Res. 2019, 25, 7229–7242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronbaek, H.; Flyvbjerg, A.; Mellemkjaer, L.; Tjonneland, A.; Christensen, J.; Sorensen, H.T.; Overvad, K. Serum insulin-like growth factors, insulin-like growth factor binding proteins, and breast cancer risk in postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1759–1764. [Google Scholar]
- Peiro, G.; Adrover, E.; Sanchez-Tejada, L.; Lerma, E.; Planelles, M.; Sanchez-Paya, J.; Aranda, F.I.; Giner, D.; Gutierrez-Avino, F.J. Increased insulin-like growth factor-1 receptor mRNA expression predicts poor survival in immunophenotypes of early breast carcinoma. Mod. Pathol. 2011, 24, 201–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gee, J.M.; Robertson, J.F.; Gutteridge, E.; Ellis, I.O.; Pinder, S.E.; Rubini, M.; Nicholson, R.I. Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signalling and oestrogen receptor activity in clinical breast cancer. Endocr Relat Cancer 2005, 12 (Suppl. 1), S99–S111. [Google Scholar] [CrossRef] [Green Version]
- Chan, T.W.; Pollak, M.; Huynh, H. Inhibition of insulin-like growth factor signaling pathways in mammary gland by pure antiestrogen ICI 182,780. Clin. Cancer Res. 2001, 7, 2545–2554. [Google Scholar]
- Singer, C.F.; Rasmussen, A.; Lippman, M.E.; Cullen, K.J. Coexpression of stromelysin-3 and insulin-like growth factor II in tumors of ectodermal, mesodermal, and endodermal origin: Indicator of a fetal cell phenotype. J. Clin. Endocrinol. Metab. 1997, 82, 1917–1922. [Google Scholar] [CrossRef] [Green Version]



| Characteristics | Luminal A | Luminal B | |
|---|---|---|---|
| Nº (%) | Nº (%) | p Value | |
| All patients | 7 | 12 | |
| Median age (years) | 0.515 | ||
| <62 | 4 (57.1%) | 5 (41.7%) | |
| >62 | 3 (42.9%) | 7 (58.3%) | |
| Tumor size | |||
| T1 | 3 (42.9%) | 1 (8.3%) | |
| T2 | 4 (57.1%) | 10 (83.4%) | |
| T3 | 0 (0.0%) | 1 (8.3%) | |
| Histological grade | 0.082 | ||
| Well differentiated (I) | 2 (28.6%) | 0 (0%) | |
| Moderately differentiated (II) | 4 (57.1%) | 6 (50.0%) | |
| Poorly differentiated (III) | 1 (14.3%) | 6 (50.0%) | |
| Nodal status | 0.960 | ||
| Negative | 3 (42.9%) | 5 (41.7%) | |
| Positive | 4 (57.1%) | 7 (58.3%) | |
| Estrogen receptors | - | ||
| Negative | 0 (0%) | 0 (0%) | |
| Positive | 7 (100%) | 12 (100%) | |
| Progesterone receptors | 0.253 | ||
| Negative | 0 (0%) | 2 (16.7%) | |
| Positive | 7 (100%) | 10 (83.3%) | |
| HER2 | 0.149 | ||
| Negative | 7 (100%) | 9 (75.0%) | |
| Positive | 0 (0%) | 3 (25.0%) | |
| Ki67 | 0.020 | ||
| <20% | 4 (57.1%) | 1 (8.3%) | |
| ≥20% | 3 (42.9%) | 11 (91.7%) |
| Gene Symbol | References | Gene Name | Main Role |
|---|---|---|---|
| S100A4 | 110779 | S100 calcium binding protein A4 | Invasion |
| TGFβ | 101210 | Transforming growth factor beta | Inflammation |
| HGF | 108357 | Hepatocyte growth factor | Cell growth/Invasion |
| FGF2 | 118274 | Fibroblast growth factor 2 (basic) | Angiogenesis |
| FGF7 | 113109 | Fibroblast growth factor 7 | Cell growth/Invasion |
| PDGFA | 110648 | Platelet-derived growth factor alpha | Angiogenesis |
| PDGFB | 110713 | Platelet-derived growth factor beta | Angiogenesis |
| uPA | 109571 | Urokinase-type plasminogen activator | ECM remodelling |
| IL6 | 113614 | Interleukin 6 | Inflammation |
| IL8 | 103136 | Interleukin 8 | Inflammation |
| CXCL12 | 110618 | Chemokine (C-X-C motif) ligand 12 | Inflammation |
| CCL2 | 141156 | Chemokine (C-C motif) ligand 2 | Inflammation |
| NFkB | 100646 | Nuclear factor kappa B | Inflammation/Tumor growth |
| MMP2 | 103899 | Matrix metalloproteases 2 | ECM remodelling |
| MMP9 | 139820 | Matrix metalloproteases 9 | ECM remodelling |
| MMP11 | 103163 | Matrix metalloproteases 11 | ECM remodelling |
| TIMP1 | 103847 | Tissue inhibitor of metalloproteases 1 | ECM remodelling |
| VEGFA | 140392 | Vascular endothelial growth factor A | Angiogenesis |
| IGF2 | 113548 | Insulin-like growth factor 2 | Cell growth |
| ACTB | 101125 | Actin, beta | - |
| SDHA | 102136 | Succinate dehydrogenase complex, subunit A, flavoprotein | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eiro, N.; Cid, S.; Fraile, M.; Cabrera, J.R.; Gonzalez, L.O.; Vizoso, F.J. Analysis of the Gene Expression Profile of Stromal Pro-Tumor Factors in Cancer-Associated Fibroblasts from Luminal Breast Carcinomas. Diagnostics 2020, 10, 865. https://doi.org/10.3390/diagnostics10110865
Eiro N, Cid S, Fraile M, Cabrera JR, Gonzalez LO, Vizoso FJ. Analysis of the Gene Expression Profile of Stromal Pro-Tumor Factors in Cancer-Associated Fibroblasts from Luminal Breast Carcinomas. Diagnostics. 2020; 10(11):865. https://doi.org/10.3390/diagnostics10110865
Chicago/Turabian StyleEiro, Noemi, Sandra Cid, María Fraile, Jorge Ruben Cabrera, Luis O. Gonzalez, and Francisco J. Vizoso. 2020. "Analysis of the Gene Expression Profile of Stromal Pro-Tumor Factors in Cancer-Associated Fibroblasts from Luminal Breast Carcinomas" Diagnostics 10, no. 11: 865. https://doi.org/10.3390/diagnostics10110865
APA StyleEiro, N., Cid, S., Fraile, M., Cabrera, J. R., Gonzalez, L. O., & Vizoso, F. J. (2020). Analysis of the Gene Expression Profile of Stromal Pro-Tumor Factors in Cancer-Associated Fibroblasts from Luminal Breast Carcinomas. Diagnostics, 10(11), 865. https://doi.org/10.3390/diagnostics10110865





