Impaired Ratio of Unsaturated to Saturated Non-Esterified Fatty Acids in Saliva from Patients with Cystic Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Samples
2.3. Salivary Non-Esterified Lipid Analysis
2.4. Salivary IL-6 Analysis
2.5. Statistical Analysis
3. Results
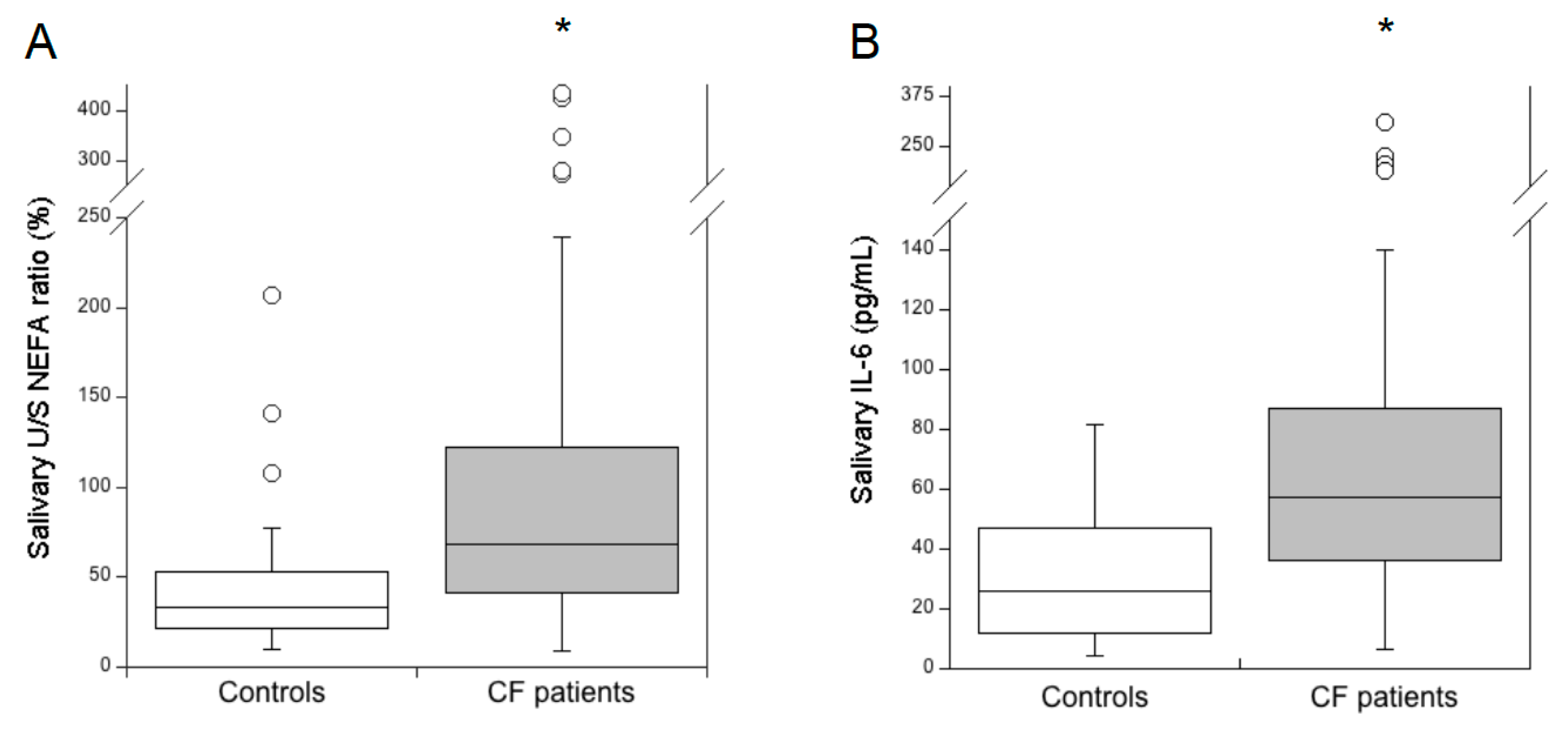
3.1. Salivary Lipid Composition and IL-6 Levels in Healthy Subjects and Patients with CF
3.2. Salivary Lipids versus Serum Lipids and Pancreatic Status in CF Patients
3.3. Salivary Lipids versus Lung Disease Severity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Klimova, B.; Kuca, K.; Novotny, M.; Maresova, P. Cystic fibrosis revisited—a review study. Med. Chem. 2017, 13, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.R.; Tullis, E.; Castaños, C.; Castellani, C.; et al. The future of cystic fibrosis care: A global perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef]
- Di Lullo, A.M.; Scorza, M.; Amato, F.; Comegna, M.; Raia, V.; Maiuri, L.; Ilardi, G.; Cantone, E.; Castaldo, G.; Iengo, M. An “ex vivo model” contributing to the diagnosis and evaluation of new drugs in cystic fibrosis. Acta Otorhinolaryngol. Ital. 2017, 37, 207–213. [Google Scholar] [PubMed]
- Amato, F.; Scudieri, P.; Musante, I.; Tomati, V.; Caci, E.; Comegna, M.; Maietta, S.; Manzoni, F.; Di Lullo, A.M.; De Wachter, E.; et al. Two CFTR mutations within codon 970 differently impact on the chloride channel functionality. Hum. Mutat. 2019, 40, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, E.; Gambazza, S.; Pradal, U.; Braggion, C. Lung clearance index in subjects with cystic fibrosis in Italy. Ital. J. Pediatr. 2019, 45, 56. [Google Scholar] [CrossRef]
- Forton, J.T. Detecting respiratory infection in children with cystic fibrosis: Cough swab, sputum induction or bronchoalveolar lavage. Paediatr. Respir. Rev. 2019, 31, 28–31. [Google Scholar] [CrossRef]
- Eyns, H.; Piérard, D.; De Wachter, E.; Eeckhout, L.; Vaes, P.; Malfroot, A. Respiratory bacterial culture sampling in expectorating and non-expectorating patients with cystic fibrosis. Front. Pediatr. 2018, 6, 403. [Google Scholar] [CrossRef]
- Nunes, L.A.S.; Mussavira, S.; Bindhu, O.S. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: A systematic review. Biochem. Med. 2015, 25, 177–192. [Google Scholar] [CrossRef]
- Courtney, J.M.; Ennis, M.; Elborn, J.S. Cytokines and inflammatory mediators in cystic fibrosis. J. Cyst. Fibros. 2004, 3, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Zhang, H.; Mayer, K.M.; Oppenheim, F.G.; Little, F.F.; Greenberg, J.; Uluer, A.Z.; Walt, D.R. Correlations of salivary biomarkers with clinical assessments in patients with cystic fibrosis. PLos ONE 2015, 10, e0135237. [Google Scholar] [CrossRef]
- Reeves, E.P.; Williamson, M.; O’Neill, S.J.; Greally, P.; McElvaney, N.G. Nebulized hypertonic saline decreases IL-8 in sputum of patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2011, 183, 1517–1523. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Elce, A.; Amato, F.; Zarrilli, F.; Calignano, A.; Troncone, R.; Castaldo, G.; Canani, R.B. Butyrate modulating effects on pro-inflammatory pathways in human intestinal epithelial cells. Benef. Microbes. 2017, 8, 841–847. [Google Scholar] [CrossRef]
- Canani, R.B.; Terrin, G.; Elce, A.; Pezzella, V.; Heinz-Erian, P.; Pedrolli, A.; Centenari, C.; Amato, F.; Tomaiuolo, R.; Calignano, A.; et al. Genotype-dependency of butyrate efficacy in children with congenital chloride diarrhea. Orphanet J. Rare Dis. 2013, 8, 194. [Google Scholar] [CrossRef]
- Grunnet, L.; Poulsen, P.; Klarlund Pedersen, B.; Mandrup-Poulsen, T.; Vaag, A. Plasma cytokine levels in young and elderly twins: Genes versus environment and relation to in vivo insulin action. Diabetologia 2006, 49, 343–350. [Google Scholar] [CrossRef][Green Version]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [CrossRef]
- Peretti, N.; Marcil, V.; Drouin, E.; Levy, E. Mechanisms of lipid malabsorption in cystic fibrosis: The impact of essential fatty acids deficiency. Nutr. Metab. 2005, 2, 11. [Google Scholar] [CrossRef]
- Freedman, S.D.; Blanco, P.G.; Zaman, M.M.; Shea, J.C.; Ollero, M.; Hopper, I.K.; Weed, D.A.; Gelrud, A.; Regan, M.M.; Laposata, M.; et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N. Engl. J. Med. 2004, 350, 560–569. [Google Scholar] [CrossRef]
- Farrell, P.M.; Mischler, E.H.; Engle, M.J.; Brown, D.J.; Lau, S.M. Fatty acid abnormalities in cystic fibrosis. Pediatr. Res. 1985, 19, 104–109. [Google Scholar] [CrossRef]
- Matczuk, J.; Żendzian-Piotrowska, M.; Maciejczyk, M.; Kurek, K. Salivary lipids: A review. Adv. Clin. Exp. Med. 2017, 26, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Slomiany, B.L.; Slomiany, A.; Mandel, I.D. Lipid composition of human submandibular gland secretion from light and heavy calculus formers. Arch. Oral Biol. 1980, 25, 749–751. [Google Scholar] [CrossRef]
- Van Biervliet, S.; Vanbillemont, G.; Van Biervliet, J.P.; Declercq, D.; Robberecht, E.; Christophe, A. Relation between fatty acid composition and clinical status or genotype in cystic fibrosis patients. Ann. Nutr. Metab. 2007, 51, 541–549. [Google Scholar] [CrossRef]
- Castellani, C.; Assael, B.M. Cystic fibrosis: A clinical view. Cell. Mol. Life Sci. 2017, 74, 129–140. [Google Scholar] [CrossRef]
- Tomaiuolo, R.; Sangiuolo, F.; Bombieri, C.; Bonizzato, A.; Cardillo, G.; Raia, V.; D’Apice, M.R.; Bettin, M.D.; Pignatti, P.F.; Castaldo, G.; et al. Epidemiology and a novel procedure for large scale analysis of CFTR rearrangements in classic and atypical CF patients: A multicentric Italian study. J. Cyst. Fibros. 2008, 7, 347–351. [Google Scholar] [CrossRef]
- Amato, F.; Bellia, C.; Cardillo, G.; Castaldo, G.; Ciaccio, M.; Elce, A.; Lembo, F.; Tomaiuolo, R. Extensive molecular analysis of patients bearing CFTR-Related disorders. J. Mol. Diagn. 2012, 14, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Terlizzi, V.; Lucarelli, M.; Salvatore, D.; Angioni, A.; Bisogno, A.; Braggion, C.; Buzzetti, R.; Carnovale, V.; Casciaro, R.; Castaldo, G.; et al. Clinical expression of cystic fibrosis in a large cohort of Italian siblings. BMC Pulm. Med. 2018, 18, 196. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. ERS global lung function initiative. Multi-ethnic reference values for spirometry for the 3–95 year age range: The global lung function 2012 equations. Eur. Respir. J 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Gelzo, M.; Dello Russo, A.; Corso, G. Stability study of dehydrocholesterols in dried spot of blood from patients with Smith-Lemli-Opitz syndrome, using filter-paper treated with butylated hydroxytoluene. Clin. Chim. Acta 2012, 413, 525–526. [Google Scholar] [CrossRef][Green Version]
- Di Taranto, M.D.; Gelzo, M.; Giacobbe, C.; Gentile, M.; Marotta, G.; Savastano, S.; Dello Russo, A.; Fortunato, G.; Corso, G. Cerebrotendinous xanthomatosis, a metabolic disease with different neurological signs: Two case reports. Metab. Brain Dis. 2016, 31, 1185–1188. [Google Scholar] [CrossRef]
- Itaya, K.; Ui, M. Colorimetric determination of free fatty acids in biological fluids. J. Lipid Res. 1965, 6, 16–20. [Google Scholar]
- Corso, G.; Gelzo, M.; Sanges, C.; Chambery, A.; Di Maro, A.; Severino, V.; Dello Russo, A.; Piccioli, C.; Arcari, P. Polar and non-polar organic binder characterization in Pompeian wall paintings: Comparison to a simulated painting mimicking an “a secco” technique. Anal. Bioanal. Chem. 2012, 402, 3011–3016. [Google Scholar] [CrossRef][Green Version]
- Gelzo, M.; Sica, C.; Elce, A.; Dello Russo, A.; Iacotucci, P.; Carnovale, V.; Raia, V.; Salvatore, D.; Corso, G.; Castaldo, G. Reduced absorption and enhanced synthesis of cholesterol in patients with cystic fibrosis: A preliminary study of plasma sterols. Clin. Chem. Lab. Med. 2016, 54, 1461–1466. [Google Scholar] [CrossRef]
- White, N.M.; Jiang, D.; Burgess, J.D.; Bederman, I.R.; Previs, S.F.; Kelley, T.J. Altered cholesterol homeostasis in cultured and in vivo models of cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L476–L486. [Google Scholar] [CrossRef]
- Tomezsko, J.L.; Stallings, V.A.; Kawchak, D.A.; Goin, J.E.; Diamond, G.; Scanlin, T.F. Energy Expenditure and Genotype of Children With Cystic Fibrosis. Pediatr. Res. 1994, 35, 451–460. [Google Scholar] [CrossRef][Green Version]
- Ionescu, A.A.; Nixon, L.S.; Luzio, S.; Lewis-Jenkins, V.; Evans, W.D.; Stone, M.D.; Owens, D.R.; Routledge, P.A.; Shale, D.J. Pulmonary function, body composition, and protein catabolism in adults with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2002, 165, 495–500. [Google Scholar] [CrossRef]
- Kulkarni, B.V.; Wood, K.V.; Mattes, R.D. Quantitative and qualitative analyses of human salivary NEFA with gas-chromatography and mass spectrometry. Front. Physiol. 2012, 3, 328. [Google Scholar] [CrossRef]
- Thomsen, K.F.; Laposata, M.; Njoroge, S.W.; Umunakwe, O.C.; Katrangi, W.; Seegmiller, A.C. Increased Elongase 6 and Δ9-desaturase Activity Are Associated With n-7 and n-9 Fatty Acid Changes in Cystic Fibrosis. Lipids 2011, 46, 669–677. [Google Scholar] [CrossRef]
- Hallows, K.R.; Fitch, A.C.; Richardson, C.A.; Reynolds, P.R.; Clancy, J.P.; Dagher, P.C.; Witters, L.A.; Kolls, J.K.; Pilewski, J.M. Up-regulation of AMP-activated kinase by dysfunctional cystic fibrosis transmembrane conductance regulator in cystic fibrosis airway epithelial cells mitigates excessive inflammation. J. Biol. Chem. 2006, 281, 4231–4241. [Google Scholar] [CrossRef]
- Umunakwe, O.C.; Seegmiller, A.C. Abnormal n-6 fatty acid metabolism in cystic fibrosis is caused by activation of AMP-activated protein kinase. J. Lipid Res. 2014, 55, 1489–1497. [Google Scholar] [CrossRef]
- Owens, J.M.; Shroyer, K.R.; Kingdom, T.T. Expression of cyclooxygenase and lipoxygenase enzymes in sinonasal mucosa of patients with cystic fibrosis. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 825–831. [Google Scholar] [CrossRef]
- Spencer, D.A.; Sampson, A.P.; Green, C.P.; Costello, J.F.; Piper, P.J.; Price, J.F. Sputum cysteinyl-leukotriene levels correlate with the severity of pulmonary disease in children with cystic fibrosis. Pediatr. Pulmonol. 1992, 12, 90–94. [Google Scholar] [CrossRef]
- Reid, D.W.; Misso, N.; Aggarwal, S.; Thompson, P.J.; Walters, E.H. Oxidative stress and lipid-derived inflammatory mediators during acute exacerbations of cystic fibrosis. Respirology 2007, 12, 63–69. [Google Scholar] [CrossRef]
- Bhura-Bandali, F.N.; Suh, M.; Man, S.F.; Clandinin, M.T. The deltaF508 mutation in the cystic fibrosis transmembrane conductance regulator alters control of essential fatty acid utilization in epithelial cells. J. Nutr. 2000, 130, 2870–2875. [Google Scholar] [CrossRef]
- Galanti, N.; Baserga, R. Glycolipid synthesis in the early prereplicative phase of isoproterenol-stimulated salivary glands of mice. J. Biol. Chem. 1971, 246, 6814–6821. [Google Scholar]
- Pritchard, E.T. Sulpholipid formation in rat submandibular gland. Biochem. J. 1977, 166, 141–144. [Google Scholar] [CrossRef]
- Castaldo, A.; Iacotucci, P.; Carnovale, V.; Cimino, R.; Liguori, R.; Comegna, M.; Raia, V.; Corso, G.; Castaldo, G.; Gelzo, M. Salivary Cytokines and Airways Disease Severity in Patients with Cystic Fibrosis. Diagn. (Basel) 2020, 10, 222. [Google Scholar]


| Controls (n = 48) | CF Patients (n = 66) | |
|---|---|---|
| Age (years) | 27 (23–37) | 23 (18–39) |
| Males, n (%) | 26 (54.2) | 37 (56.1) |
| Lung disease severity, n (%): | ||
| severe | - | 15 (22.7) |
| moderate | - | 20 (30.3) |
| mild | - | 31 (47.0) |
| Pancreatic insufficiency, n (%) | - | 37 (56.1) |
| Salivary Lipids (mg/L) | Controls (n = 48) | CF Patients (n = 66) | Δ (%) a | p-Value b |
|---|---|---|---|---|
| Free cholesterol | 0.38 (0.22–0.65) | 0.58 (0.36–1.09) | +52.6 | 0.01 |
| Total NEFA | 2.26 (1.20–3.70) | 3.29 (2.24–5.49) | +45.6 | 0.001 |
| C16:0 | 0.78 (0.38–1.10) | 0.95 (0.69–1.55) | +21.8 | 0.01 |
| C18:0 | 0.67 (0.32–1.30) | 0.82 (0.58–1.26) | +22.4 | 0.139 |
| cis-C18:1 | 0.35 (0.17–0.62) | 0.75 (0.49–1.34) | +114.3 | 3.5 × 10−7 |
| C18:2 | 0.16 (0.08–0.32) | 0.42 (0.25–0.95) | +162.5 | 2.3 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelzo, M.; Iacotucci, P.; Carnovale, V.; Castaldo, A.; Comegna, M.; Cernera, G.; Corso, G.; Castaldo, G. Impaired Ratio of Unsaturated to Saturated Non-Esterified Fatty Acids in Saliva from Patients with Cystic Fibrosis. Diagnostics 2020, 10, 915. https://doi.org/10.3390/diagnostics10110915
Gelzo M, Iacotucci P, Carnovale V, Castaldo A, Comegna M, Cernera G, Corso G, Castaldo G. Impaired Ratio of Unsaturated to Saturated Non-Esterified Fatty Acids in Saliva from Patients with Cystic Fibrosis. Diagnostics. 2020; 10(11):915. https://doi.org/10.3390/diagnostics10110915
Chicago/Turabian StyleGelzo, Monica, Paola Iacotucci, Vincenzo Carnovale, Alice Castaldo, Marika Comegna, Gustavo Cernera, Gaetano Corso, and Giuseppe Castaldo. 2020. "Impaired Ratio of Unsaturated to Saturated Non-Esterified Fatty Acids in Saliva from Patients with Cystic Fibrosis" Diagnostics 10, no. 11: 915. https://doi.org/10.3390/diagnostics10110915
APA StyleGelzo, M., Iacotucci, P., Carnovale, V., Castaldo, A., Comegna, M., Cernera, G., Corso, G., & Castaldo, G. (2020). Impaired Ratio of Unsaturated to Saturated Non-Esterified Fatty Acids in Saliva from Patients with Cystic Fibrosis. Diagnostics, 10(11), 915. https://doi.org/10.3390/diagnostics10110915





