Impact of F-18 Fluorodeoxyglucose PET/CT and PET/MRI on Initial Staging and Changes in Management of Pancreatic Ductal Adenocarcinoma: A Systemic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
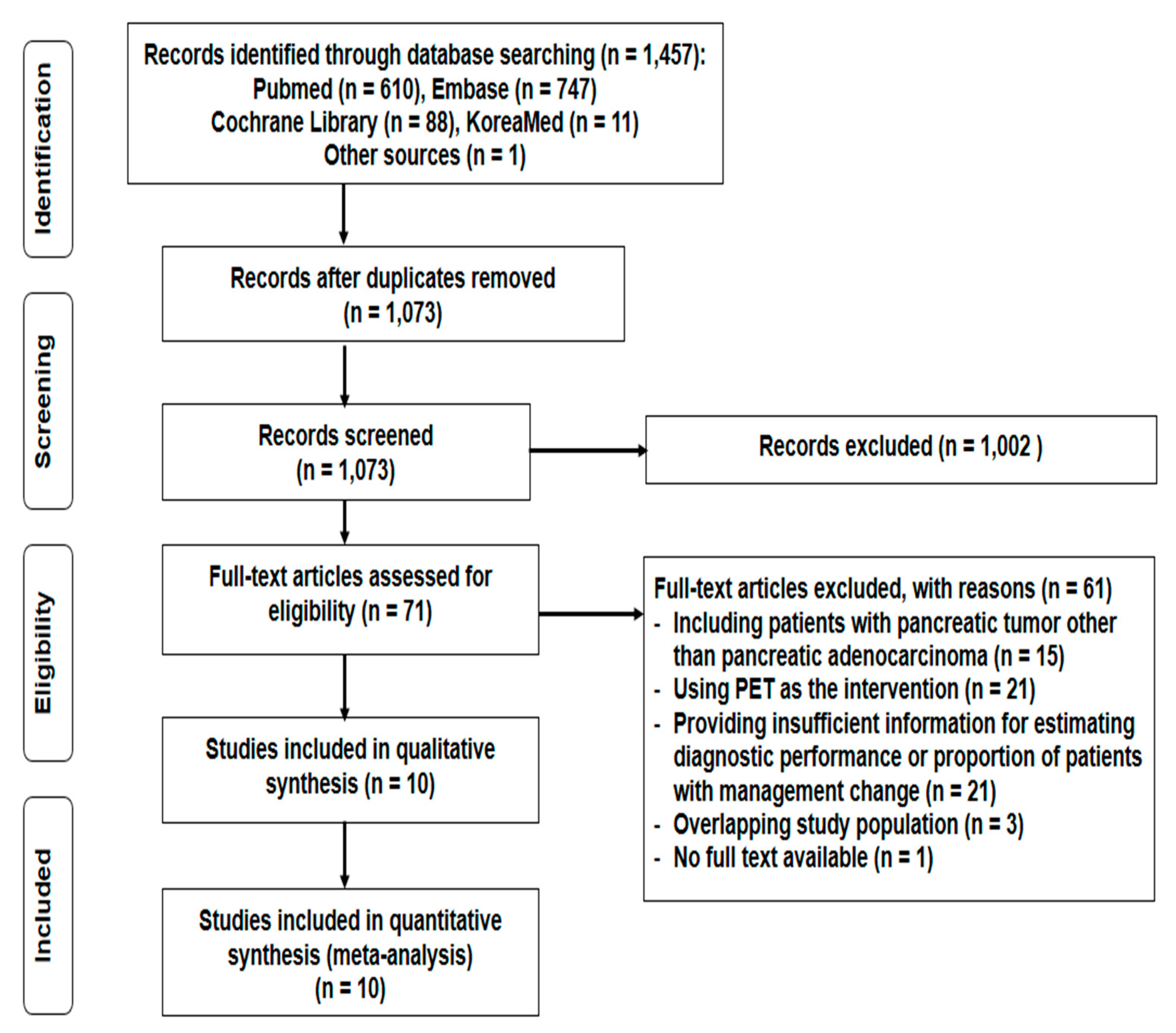
3.1. Study Selection and Characteristics
3.2. Quality Assessment
3.3. Diagnostic Performance of PET/CT and PET/MRI for Detection of Lymph Node and Distant Metastases
3.4. Management Changes Following PET/CT
3.5. Heterogeneity Analysis
3.6. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, S.C.; Cheung, W.Y. Evolving treatment landscape for early and advanced pancreatic cancer. World J. Gastrointest. Oncol. 2017, 9, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, J.Q.; Liu, J.L.; Qin, X.G.; Huang, Y. FDG-PET in diagnosis, staging and prognosis of pancreatic carcinoma: A meta-analysis. World J. Gastroenterol. 2013, 19, 4808–4817. [Google Scholar] [CrossRef] [PubMed]
- Pakzad, F.; Groves, A.M.; Ell, P.J. The role of positron emission tomography in the management of pancreatic cancer. Semin. Nucl. Med. 2006, 36, 248–256. [Google Scholar] [CrossRef]
- Santhosh, S.; Mittal, B.R.; Bhasin, D.K.; Rana, S.S.; Gupta, R.; Das, A.; Nada, R. Fluorodeoxyglucose-positron emission tomography/computed tomography performs better than contrast-enhanced computed tomography for metastasis evaluation in the initial staging of pancreatic adenocarcinoma. Ann. Nucl. Med. 2017, 31, 575–581. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, C.M.; Choi, H.J.; Lee, W.J.; Song, S.Y.; Lee, J.H.; Lee, J.D. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative ¹⁸F-FDG PET/CT in patients with pancreatic cancer. J. Nucl. Med. 2014, 55, 898–904. [Google Scholar] [CrossRef] [Green Version]
- Kitajima, K.; Murakami, K.; Yamasaki, E.; Kaji, Y.; Shimoda, M.; Kubota, K.; Suganuma, N.; Sugimura, K. Performance of integrated FDG-PET/contrast-enhanced CT in the diagnosis of recurrent pancreatic cancer: Comparison with integrated FDG-PET/non-contrast-enhanced CT and enhanced CT. Mol. Imaging Biol. 2010, 12, 452–459. [Google Scholar] [CrossRef]
- Kauhanen, S.P.; Komar, G.; Seppänen, M.P.; Dean, K.I.; Minn, H.R.; Kajander, S.A.; Rinta-Kiikka, I.; Alanen, K.; Borra, R.J.; Puolakkainen, P.A.; et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann. Surg. 2009, 250, 957–963. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, S.M. Radiomics in oncological PET/CT: Clinical applications. Nucl. Med. Mol. Imaging 2018, 52, 170–189. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, K.H.; Lee, K.T.; Lee, J.K.; Ku, B.H.; Oh, C.R.; Heo, J.S.; Choi, S.H.; Choi, D.W. The value of positron emission tomography/computed tomography for evaluating metastatic disease in patients with pancreatic cancer. Pancreas 2012, 41, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Salgarello, M.; Laiti, S.; Partelli, S.; Castelli, P.; Spinelli, A.E.; Tamburrino, D.; Zamboni, G.; Falconi, M. The role of (18)fluoro-deoxyglucose positron emission tomography/computed tomography in resectable pancreatic cancer. Dig. Liver Dis. 2014, 46, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Yang, F.; Jin, C.; Guan, Y.H.; Zhang, H.W.; Fu, D.L. The value of 18F-FDG positron emission tomography/computed tomography on the pre-operative staging and the management of patients with pancreatic carcinoma. Hepatogastroenterology 2014, 61, 2102–2109. [Google Scholar] [PubMed]
- Heinrich, S.; Goerres, G.W.; Schäfer, M.; Sagmeister, M.; Bauerfeind, P.; Pestalozzi, B.C.; Hany, T.F.; von Schulthess, G.K.; Clavien, P.A. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann. Surg. 2005, 242, 235–243. [Google Scholar] [CrossRef]
- Ghaneh, P.; Hanson, R.; Titman, A.; Lancaster, G.; Plumpton, C.; Lloyd-Williams, H.; Yeo, S.T.; Edwards, R.T.; Johnson, C.; Abu Hilal, M.; et al. PET-PANC: Multicentre prospective diagnostic accuracy and health economic analysis study of the impact of combined modality 18fluorine-2-fluoro-2-deoxy-d-glucose positron emission tomography with computed tomography scanning in the diagnosis and management of pancreatic cancer. Health Technol. Assess. 2018, 22, 1–114. [Google Scholar] [CrossRef]
- Ospina, M.; Horton, J.; Seida, J.; Vanermeer, B.; Liang, G. Positron emission tomography for nine cancers (bladder, brain, cervical, kidney, ovarian, pancreatic, prostate, small cell lung, testicular). In Database of Abstracts of Reviews of Effects (DARE): Quality-Assessed Reviews; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008. [Google Scholar]
- Yeh, R.; Dercle, L.; Garg, I.; Wang, Z.J.; Hough, D.M.; Goenka, A.H. The role of 18F-FDG PET/CT and PET/MRI in pancreatic ductal adenocarcinoma. Abdom. Radiol. 2018, 43, 415–434. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, B.S.; Lee, D.S.; Chung, J.K.; Lee, M.C.; Kim, S.; Kang, W.J. 18F-FDG PET/CT in mediastinal lymph node staging of non-small-cell lung cancer in a tuberculosis-endemic country: Consideration of lymph node calcification and distribution pattern to improve specificity. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1794–1802. [Google Scholar] [CrossRef]
- Frank, R.A.; Bossuyt, P.M.; McInnes, M.D.F. Systematic Reviews and Meta-Analyses of Diagnostic Test Accuracy: The PRISMA-DTA Statement. Radiology 2018, 289, 313–314. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, I.; Lee, J.M.; Lee, D.H.; Lee, E.S.; Paeng, J.C.; Lee, S.J.; Jang, J.Y.; Kim, S.W.; Ryu, J.K.; Lee, K.B. Preoperative Assessment of Pancreatic Cancer with FDG PET/MR Imaging versus FDG PET/CT Plus Contrast-enhanced Multidetector CT: A Prospective Preliminary Study. Radiology 2017, 282, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.R.; Seo, M.; Nah, Y.W.; Park, H.W.; Park, S.H. Clinical impact of fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography in patients with resectable pancreatic cancer: Diagnosing lymph node metastasis and predicting survival. Nucl. Med. Commun. 2018, 39, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Strobel, K.; Heinrich, S.; Bhure, U.; Soyka, J.; Veit-Haibach, P.; Pestalozzi, B.C.; Clavien, P.A.; Hany, T.F. Contrast-enhanced 18F-FDG PET/CT: 1-stop-shop imaging for assessing the resectability of pancreatic cancer. J. Nucl. Med. 2008, 49, 1408–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneyama, T.; Tateishi, U.; Endo, I.; Inoue, T. Staging accuracy of pancreatic cancer: Comparison between non-contrast-enhanced and contrast-enhanced PET/CT. Eur. J. Radiol. 2014, 83, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.; Park, M.S. The role of imaging in current treatment strategies for pancreatic adenocarcinoma. Korean J. Radiol. 2020. [Google Scholar] [CrossRef]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v56–v68. [Google Scholar] [CrossRef]
- Lai, J.P.; Yue, Y.; Zhang, W.; Zhou, Y.; Frishberg, D.; Jamil, L.H.; Mirocha, J.M.; Guindi, M.; Balzer, B.; Bose, S.; et al. Comparison of endoscopic ultrasound guided fine needle aspiration and PET/CT in preoperative diagnosis of pancreatic adenocarcinoma. Pancreatology 2017, 17, 617–622. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Okusaka, T.; Shimizu, K.; Furuse, J.; Ito, Y.; Hanada, K.; Shimosegawa, T.; Okazaki, K. Clinical Practice Guidelines for Pancreatic Cancer 2016 From the Japan Pancreas Society: A Synopsis. Pancreas 2017, 46, 595–604. [Google Scholar] [CrossRef]
- O’Reilly, D.; Fou, L.; Hasler, E.; Hawkins, J.; O’Connell, S.; Pelone, F.; Callaway, M.; Campbell, F.; Capel, M.; Charnley, R.; et al. Diagnosis and management of pancreatic cancer in adults: A summary of guidelines from the UK National Institute for Health and Care Excellence. Pancreatology 2018, 18, 962–970. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (Version 3. 2019). Available online: http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 6 November 2019).
- Balaban, E.P.; Mangu, P.B.; Khorana, A.A.; Shah, M.A.; Mukherjee, S.; Crane, C.H.; Javle, M.M.; Eads, J.R.; Allen, P.; Ko, A.H.; et al. Locally advanced, unresectable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 2654–2668. [Google Scholar] [CrossRef] [PubMed]
- Treadwell, J.R.; Mitchell, M.D.; Eatmon, K.; Jue, J.; Zafar, H.; Teitelbaum, U.; Schoelles, K. Imaging Tests for the Diagnosis and Staging of Pancreatic Adenocarcinoma. Comparative Effectiveness Review No. 141 (Prepared by the ECRI Institute-Penn Medicine Evidence-Based Practive Center under Contract No. 290-2012-00011-I). Available online: www.effectivehealthcare.ahrq.gov/reports/final.cfm (accessed on 6 November 2019).
- Gao, G.; Gong, B.; Shen, W. Meta-analysis of the additional value of integrated 18FDG PET-CT for tumor distant metastasis staging: Comparison with 18FDG PET alone and CT alone. Surg. Oncol. 2013, 22, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Lan, Y.; Zhang, K.; Ren, P.; Jia, Z. Comparison of 18F-FDG PET/CT and DWI for detection of mediastinal nodal metastasis in non-small cell lung cancer: A meta-analysis. PLoS ONE 2017, 12, e0173104. [Google Scholar] [CrossRef]
- Gormsen, L.C.; Vendelbo, M.H.; Pedersen, M.A.; Haraldsen, A.; Hjorthaug, K.; Bogsrud, T.V.; Petersen, L.J.; Jensen, K.J.; Brøndum, R.; El-Galaly, T.C. A comparative study of standardized quantitative and visual assessment for predicting tumor volume and outcome in newly diagnosed diffuse large B-cell lymphoma staged with 18F-FDG PET/CT. EJNMMI Res. 2019, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Chulroek, T.; Kordbacheh, H.; Wangcharoenrung, D.; Cattapan, K.; Heidari, P.; Harisinghani, M.G. Comparative accuracy of qualitative and quantitative 18F-FDG PET/CT analysis in detection of lymph node metastasis from anal cancer. Abdom. Radiol. 2019, 44, 828–835. [Google Scholar] [CrossRef]
- Wang, X.; Kattan, M.W. Cohort studies: Design, analysis, and reporting. Chest 2020, 158, S72–S78. [Google Scholar] [CrossRef]





| First Author/Year | Country | Study Design | No. of Patients | Median Age (Years) | Inclusion Criteria | Imaging Method | FDG Uptake Time (min) | Analytical Method | Reference Standard |
|---|---|---|---|---|---|---|---|---|---|
| Crippa/2014 | Italy | Retrospective | 72 | 65 | Potentially resectable pancreatic cancer | PET/CT | 60 | QN | Histopathological confirmation |
| Ghaneh/2018 | UK | Prospective | 261 | 66 | Staging work-up for suspected pancreatic cancer | PET/CT | 90 | QN | Histopathological confirmation and follow-up imaging studies |
| Heinrich/2005 | Switzerland | Prospective | 59 | 61 | Staging work-up for suspected pancreatic cancer | PET/CT | 60 | QL | Histopathological confirmation and imaging-based decisions |
| Joo/2017 | Korea | Prospective | 37 | 63 * | Potentially resectable pancreatic cancer | PET/MRI | 50–90 | QL | Histopathological confirmation and imaging-based decisions |
| Kim/2012 | Korea | Retrospective | 125 | 62 | Staging work-up for histopathologically proven pancreatic cancer | PET/CT | 45 | QN | Histopathological confirmation and follow-up imaging studies |
| Kim/2018 | Korea | Retrospective | 70 | 69 | Pancreatic cancer patients with radical surgery | PET/CT | 60–70 | QL | Histopathological confirmation |
| Santhosh/2017 | India | Retrospective | 54 | 58 * | Staging work-up for histopathologically proven pancreatic cancer | PET/CT | 60 ± 10 | QL | Histopathological confirmation and imaging-based decisions |
| Strobel/2008 | Switzerland | Retrospective | 50 | 64 * | Staging work-up for histopathologically proven pancreatic cancer | PET/CT | 60 | QL | Histopathological confirmation and follow-up imaging studies |
| Wang/2014 | China | Retrospective | 79 | 63 * | Staging work-up for histopathologically proven pancreatic cancer | PET/CT | NS | QN | Histopathological confirmation |
| Yoneyama/2014 | Japan | Retrospective | 45 | 67 * | Staging work-up for suspected pancreatic cancer | PET/CT | 63 ± 5 | QL | Histopathological confirmation and follow-up imaging studies |
| Factors | Lymph Node Metastasis | Distant Metastasis | Management Change | |||||
|---|---|---|---|---|---|---|---|---|
| No. of Studies | Sensitivity (95% CI) | Specificity (95% CI) | No. of Studies | Sensitivity (95% CI) | Specificity (95% CI) | No. of Studies | Proportion (95% CI) | |
| Country | ||||||||
| Asia | 4 | 0.78 (0.54–0.95) | 0.97 (0.87–1.00) | 3 | 9.8 * (2.8–20.4) | |||
| Non-Asia | 3 | 0.81 (0.69–0.91) | 0.99 (0.96–1.00) | 3 | 28.2 * (10.4–50.5) | |||
| Study design | ||||||||
| Prospective | 2 | 0.78 (0.58–0.92) | 0.99 (0.95–1.00) | 2 | 39.9 * (31.5–48.5) | |||
| Retrospective | 5 | 0.80 (0.63–0.93) | 0.97 (0.91–1.00) | 4 | 10.2 * (4.7–17.5) | |||
| Analytical method | ||||||||
| Qualitative | 4 | 0.64 * (0.45–0.81) | 0.91 (0.80–0.98) | 5 | 0.84 * (0.71–0.94) | 0.96 (0.89–1.00) | 2 | 25.5 * (13.3–40.1) |
| Quantitative | 2 | 0.40 * (0.18–0.65) | 0.96 (0.85–1.00) | 2 | 0.64 * (0.48–0.79) | 1.00 (0.98–1.00) | 4 | 14.8 * (1.6–37.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.W.; O, J.H.; Choi, M.; Choi, J.Y. Impact of F-18 Fluorodeoxyglucose PET/CT and PET/MRI on Initial Staging and Changes in Management of Pancreatic Ductal Adenocarcinoma: A Systemic Review and Meta-Analysis. Diagnostics 2020, 10, 952. https://doi.org/10.3390/diagnostics10110952
Lee JW, O JH, Choi M, Choi JY. Impact of F-18 Fluorodeoxyglucose PET/CT and PET/MRI on Initial Staging and Changes in Management of Pancreatic Ductal Adenocarcinoma: A Systemic Review and Meta-Analysis. Diagnostics. 2020; 10(11):952. https://doi.org/10.3390/diagnostics10110952
Chicago/Turabian StyleLee, Jeong Won, Joo Hyun O, Miyoung Choi, and Joon Young Choi. 2020. "Impact of F-18 Fluorodeoxyglucose PET/CT and PET/MRI on Initial Staging and Changes in Management of Pancreatic Ductal Adenocarcinoma: A Systemic Review and Meta-Analysis" Diagnostics 10, no. 11: 952. https://doi.org/10.3390/diagnostics10110952
APA StyleLee, J. W., O, J. H., Choi, M., & Choi, J. Y. (2020). Impact of F-18 Fluorodeoxyglucose PET/CT and PET/MRI on Initial Staging and Changes in Management of Pancreatic Ductal Adenocarcinoma: A Systemic Review and Meta-Analysis. Diagnostics, 10(11), 952. https://doi.org/10.3390/diagnostics10110952





