High-Density Mineralized Protrusions and Central Osteophytes: Associated Osteochondral Junction Abnormalities in Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Surgical Tissues
2.2. Computed Tomography (CT) Imaging and Analysis
2.3. Fluorescent Labelling
2.4. Confocal Imaging and Histologic Analysis
2.5. Statistical Analysis
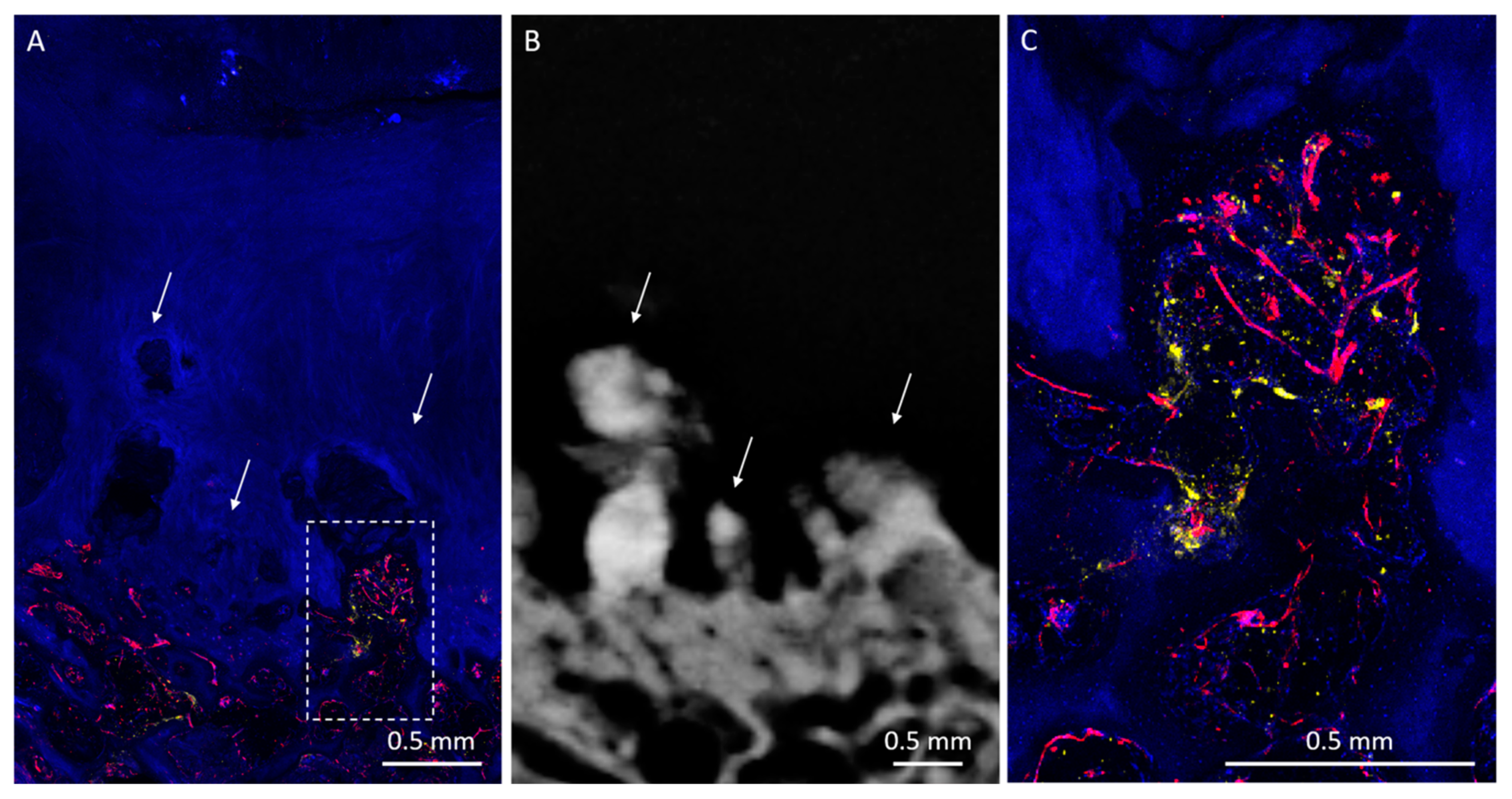
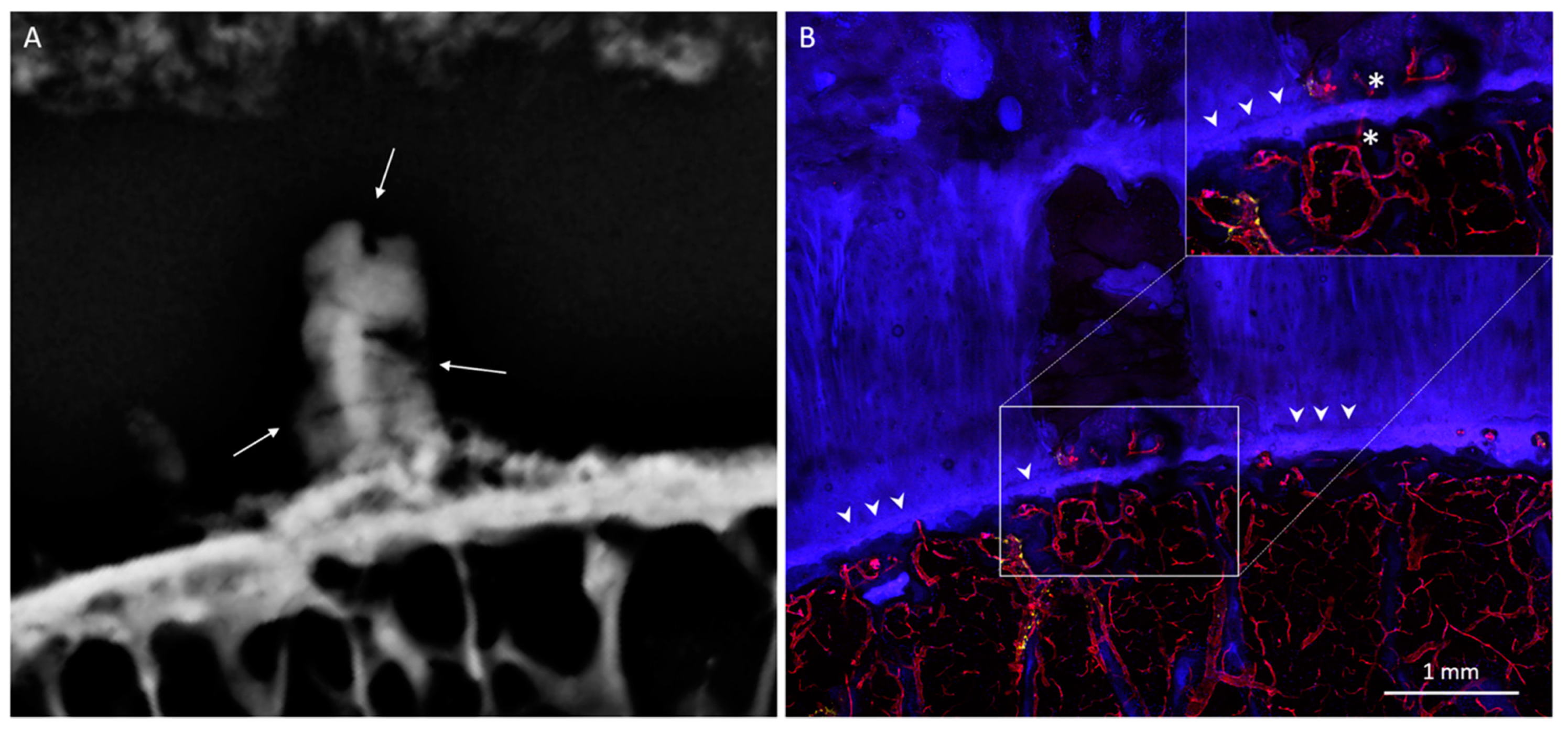
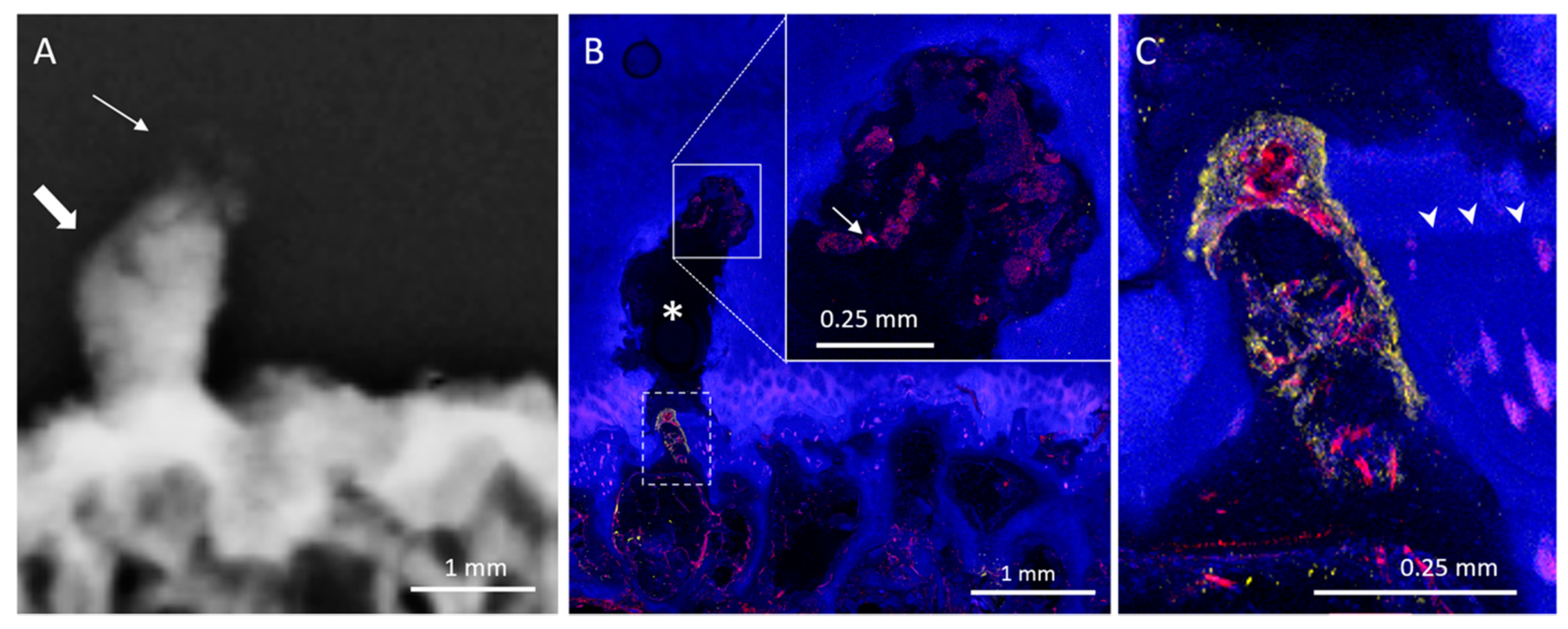
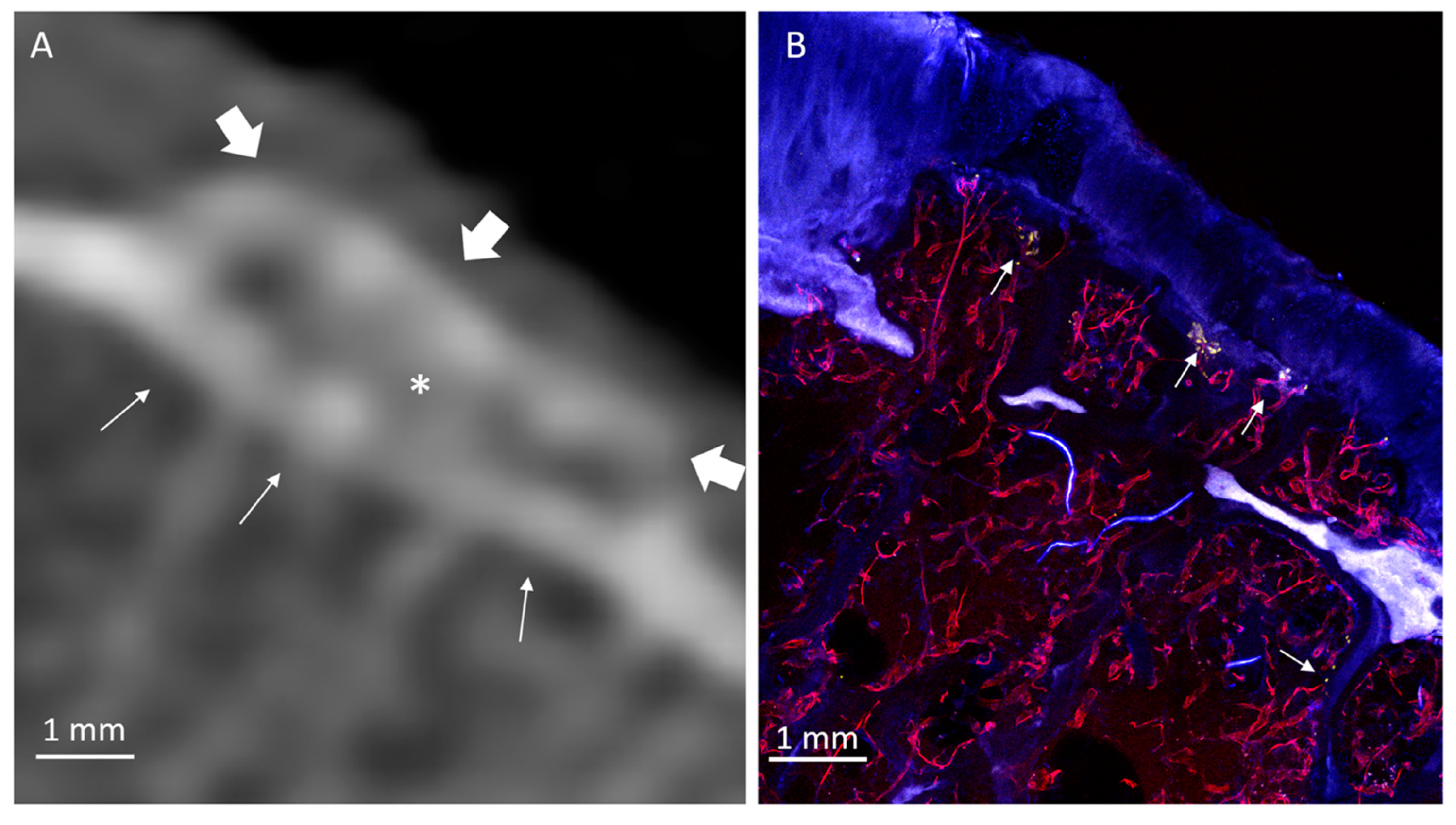
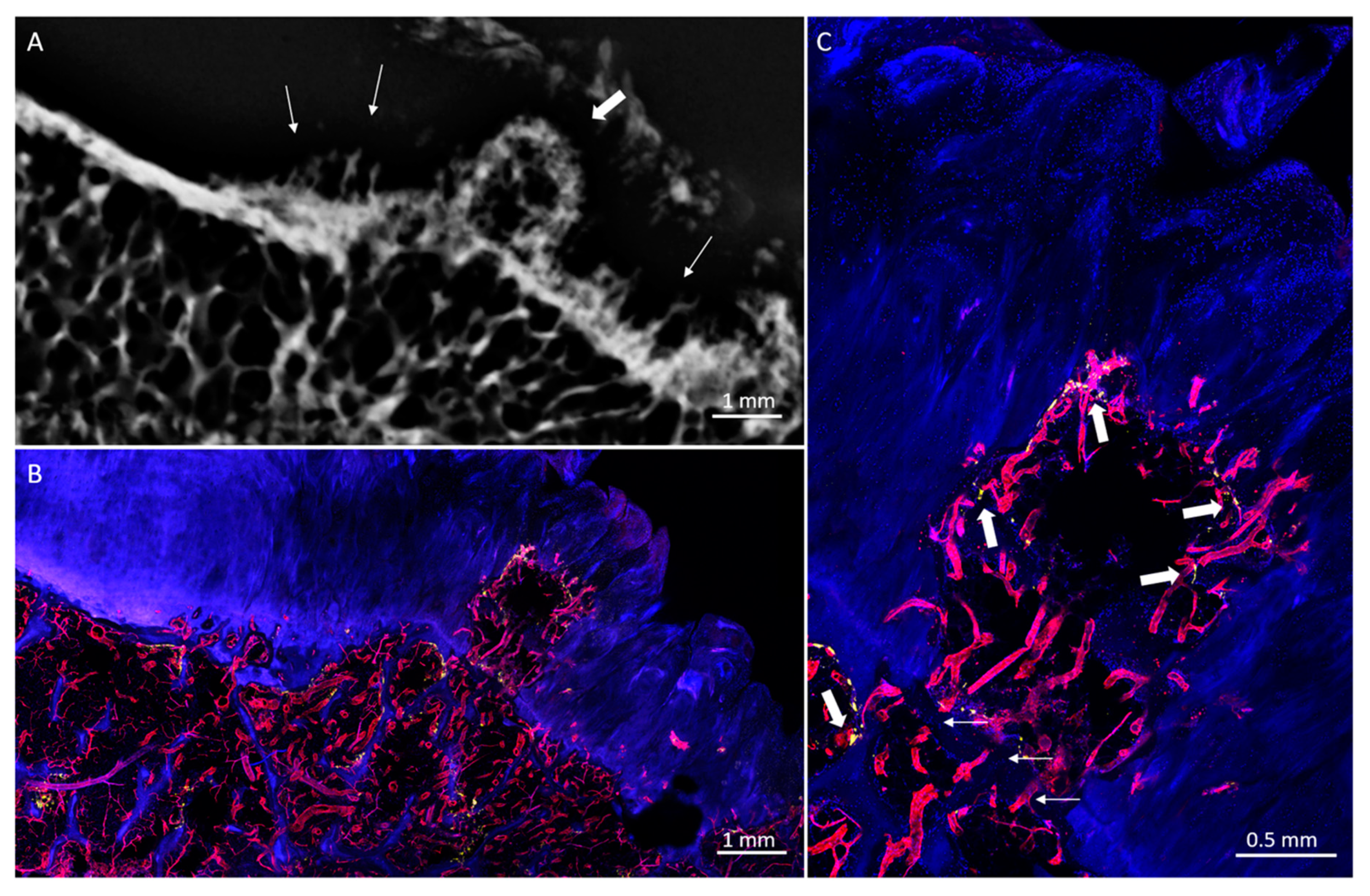
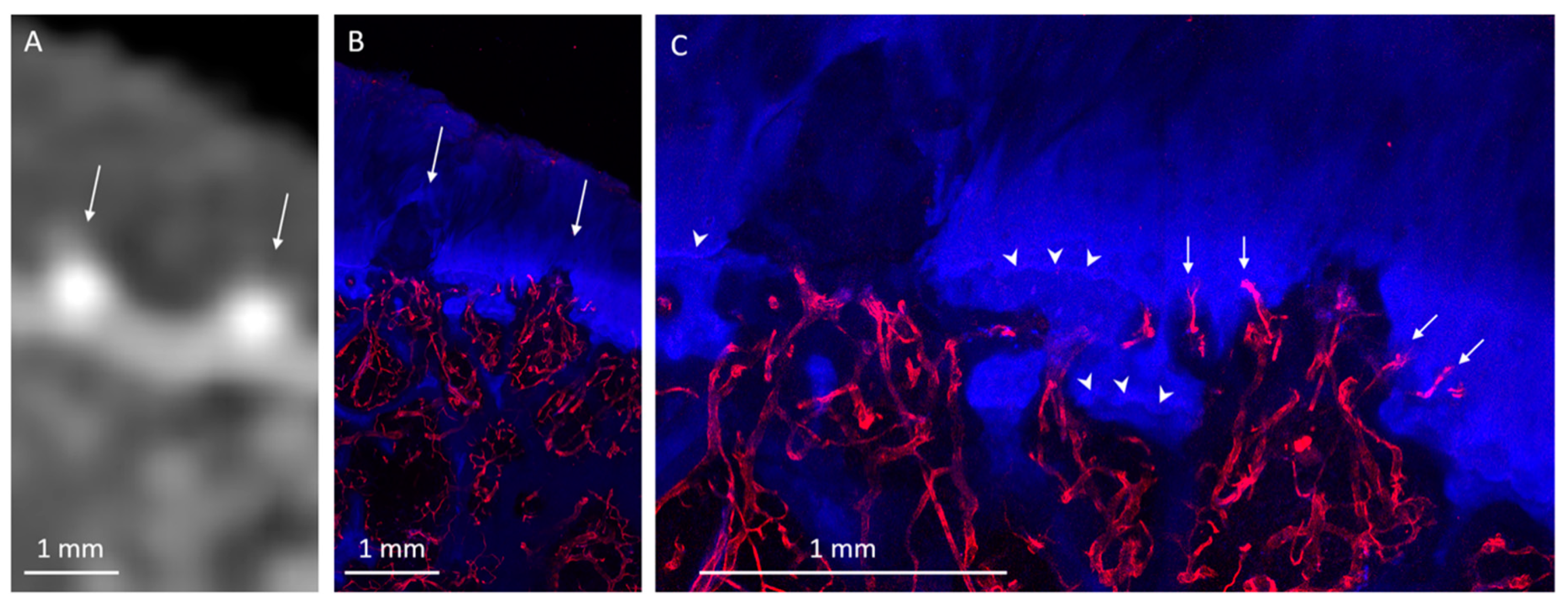
3. Results
3.1. Radiologic Analysis
3.2. Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HDMP | High-Density Mineralized Protrusion |
| CO | Central Osteophyte |
| OCJ | Osteochondral Junction |
| DAPI | 4′, 6-diamidino-2-phenylindole |
| ELF | Enzyme labeled fluorescence |
| UEA | Ulex europaeus agglutinin 1 |
| TRAP | Tartrate-resistant acid phosphatase activity type 5 |
| iDISCO | Immunolabeling-enabled imaging of solvent-cleared organs |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| BMP-2 | Bone morphogenetic protein 2 |
References
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef]
- Hernández-Molina, G.; Neogi, T.; Hunter, D.J.; Niu, J.; Guermazi, A.; Reichenbach, S.; Roemer, F.W.; McLennan, C.E.; Felson, D.T. The association of bone attrition with knee pain and other MRI features of osteoarthritis. Ann. Rheum. Dis. 2008, 67, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Englund, M.; Guermazi, A.; Roemer, F.W.; Aliabadi, P.; Yang, M.; Lewis, C.E.; Torner, J.; Nevitt, M.C.; Sack, B.; Felson, D.T. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The multicenter osteoarthritis study. Arthritis Rheum. 2009, 60, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.L.; Seo, G.S.; Gale, D.; Totterman, S.; Gale, M.E.; Felson, D.T. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum. 2005, 52, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Clockaerts, S.; Bastiaansen-Jenniskens, Y.M.; Runhaar, J.; Van Osch, G.J.V.M.; Van Offel, J.F.; Verhaar, J.A.N.; De Clerck, L.S.; Somville, J. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: A narrative review. Osteoarthr. Cartil. 2010, 18, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Roemer, F.W.; Guermazi, A.; Felson, D.T.; Niu, J.; Nevitt, M.C.; Crema, M.D.; Lynch, J.A.; Lewis, C.E.; Torner, J.; Zhang, Y. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: The MOST study. Ann. Rheum. Dis. 2011, 70, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Ayral, X.; Pickering, E.H.; Woodworth, T.G.; Mackillop, N.; Dougados, M. Synovitis: A potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis—Results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthr. Cartil. 2005, 13, 361–367. [Google Scholar] [CrossRef]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef]
- Suri, S.; Walsh, D.A. Osteochondral alterations in osteoarthritis. Bone 2012, 51, 204–211. [Google Scholar] [CrossRef]
- Mori, S.; Harruff, R.; Burr, D. Microcracks in articular calcified cartilage of human femoral heads. Arch. Pathol. Lab. Med. 1993, 117, 196–198. [Google Scholar]
- Mapp, P.I.; Walsh, D.A. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 390. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B.; Radin, E.L. Microfractures and microcracks in subchondral bone: Are they relevant to osteoarthrosis? Rheum. Dis. Clin. 2003, 29, 675–685. [Google Scholar] [CrossRef]
- Hashimoto, S.; Creighton-Achermann, L.; Takahashi, K.; Amiel, D.; Coutts, R.; Lotz, M. Development and regulation of osteophyte formation during experimental osteoarthritis. Osteoarthr. Cartil. 2002, 10, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, M.; Heilmeier, U.; Foreman, S.C.; Joseph, G.B.; McCulloch, C.E.; Nevitt, M.C.; Link, T.M. Central osteophytes develop in cartilage with abnormal structure and composition: Data from the Osteoarthritis Initiative cohort. Skelet. Radiol. 2019, 48, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- McCauley, T.R.; Kornaat, P.R.; Jee, W.-H. Central osteophytes in the knee: Prevalence and association with cartilage defects on MR imaging. Am. J. Roentgenol. 2001, 176, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, H.J. Degenerative Joint Disease. In Metabolic, Degenerative, and Inflammatory Diseases of Bones and Joints; Lea & Febiger: Philadelphia, PA, USA, 1972; pp. 754–756. [Google Scholar]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.-P.; Revell, P.; Salter, D.; Van den Berg, W. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [PubMed]
- van der Kraan, P.M.; van den Berg, W.B. Osteophytes: Relevance and biology. Osteoarthr. Cartil. 2007, 15, 237–244. [Google Scholar] [CrossRef]
- Ferguson, V.L.; Bushby, A.J.; Boyde, A. Nanomechanical properties and mineral concentration in articular calcified cartilage and subchondral bone. J. Anat. 2003, 203, 191–202. [Google Scholar] [CrossRef]
- Boyde, A.; Davis, G.; Mills, D.; Zikmund, T.; Cox, T.; Adams, V.; Niker, A.; Wilson, P.; Dillon, J.; Ranganath, L. On fragmenting, densely mineralised acellular protrusions into articular cartilage and their possible role in osteoarthritis. J. Anat. 2014, 225, 436–446. [Google Scholar] [CrossRef]
- Boyde, A.; Riggs, C.; Bushby, A.; McDermott, B.; Pinchbeck, G.; Clegg, P. Cartilage damage involving extrusion of mineralisable matrix from the articular calcified cartilage and subchondral bone. Eur. Cell Mater. 2011, 21, 470–478. [Google Scholar] [CrossRef]
- Boyde, A.; Firth, E.C. High resolution microscopic survey of third metacarpal articular calcified cartilage and subchondral bone in the juvenile horse: Possible implications in chondro-osseous disease. Microsc. Res. Tech. 2008, 71, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Laverty, S.; Lacourt, M.; Gao, C.; Henderson, J.E.; Boyde, A. High density infill in cracks and protrusions from the articular calcified cartilage in osteoarthritis in standardbred horse carpal bones. Int. J. Mol. Sci. 2015, 16, 9600–9611. [Google Scholar] [CrossRef] [PubMed]
- Klose-Jensen, R.; Nielsen, A.W.; Hartlev, L.B.; Thomsen, J.S.; Boel, L.W.T.; Laursen, M.; Keller, K.K.; Hauge, E.-M. Histomorphometric case-control study of subarticular osteophytes in patients with osteoarthritis of the hip. BMC Musculoskelet. Disord. 2020, 21, 653. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X.; Guo, J.; Jin, D.; Letuchy, E.M.; Burns, T.L.; Levy, S.M.; Hoffman, E.A.; Saha, P.K. Quantitative imaging of peripheral trabecular bone microarchitecture using MDCT. Med. Phys. 2018, 45, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Bjelle, A. Morphological study of articular cartilage in pyrophosphate arthropathy. (Chondrocalcinosis articularis or calcium pyrophosphate dihydrate crystal deposition diseases). Ann. Rheum. Dis. 1972, 31, 449. [Google Scholar] [CrossRef] [PubMed]
- Abrahim-Zadeh, R.; Yu, J.S.; Resnick, D. Central (interior) osteophytes of the distal femur. Imaging and pathologic findings. Investig. Radiol. 1994, 29, 1001–1005. [Google Scholar] [CrossRef]
- Filgueira, L. Fluorescence-based staining for tartrate-resistant acidic phosphatase (TRAP) in osteoclasts combined with other fluorescent dyes and protocols. J. Histochem. Cytochem. 2004, 52, 411–414. [Google Scholar] [CrossRef]
- Kindynis, P.; Haller, J.; Kang, H.; Resnick, D.; Sartoris, D.; Trudell, D.; Tyson, R. Osteophytosis of the knee: Anatomic, radiologic, and pathologic investigation. Radiology 1990, 174, 841–846. [Google Scholar] [CrossRef]
- Boyde, A. The real response of bone to exercise. J. Anat. 2003, 203, 173–189. [Google Scholar] [CrossRef]
- Thomas, N.; Jeffery, N.; van’t Hof, R.; Adams, V.; Saers, J.; Ranganath, L.; Boyde, A.; Gallagher, J. An Investigation of High Density Mineralised Protrusions in Human Cadaveric Knees by MRI and Microtomography. Osteoarthr. Cartil. 2017, 25, S253–S254. [Google Scholar] [CrossRef][Green Version]
- Erben, R.G. Embedding of bone samples in methylmethacrylate: An improved method suitable for bone histomorphometry, histochemistry, and immunohistochemistry. J. Histochem. Cytochem. 1997, 45, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Savi, F.M.; Brierly, G.I.; Baldwin, J.; Theodoropoulos, C.; Woodruff, M.A. Comparison of different decalcification methods using rat mandibles as a model. J. Histochem. Cytochem. 2017, 65, 705–722. [Google Scholar] [CrossRef] [PubMed]
- Renier, N.; Wu, Z.; Simon, D.J.; Yang, J.; Ariel, P.; Tessier-Lavigne, M. iDISCO: A simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 2014, 159, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.A.; McWilliams, D.F.; Turley, M.J.; Dixon, M.R.; Fransès, R.E.; Mapp, P.I.; Wilson, D. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology 2010, 49, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M. The structure of vascular channels in the subchondral plate. J. Anat. 1990, 171, 105. [Google Scholar] [PubMed]
- Buckwalter, J.A.; Kuettner, K.E.; Thonar, E.J.M. Age-related changes in articular cartilage proteoglycans: Electron microscopic studies. J. Orthop. Res. 1985, 3, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.G.; Alawi, K.M.; Rodrigues, J.; Singh, A.; Kusumbe, A.P.; Ramasamy, S.K. Endothelial proteolytic activity and interaction with non-resorbing osteoclasts mediate bone elongation. Nat. Cell Biol. 2019, 21, 430–441. [Google Scholar] [CrossRef]
- Slevin, M.; Kumar, S.; Gaffney, J. Angiogenic oligosaccharides of hyaluronan induce multiple signaling pathways affecting vascular endothelial cell mitogenic and wound healing responses. J. Biol. Chem. 2002, 277, 41046–41059. [Google Scholar] [CrossRef]
- Bastow, E.; Byers, S.; Golub, S.; Clarkin, C.; Pitsillides, A.; Fosang, A. Hyaluronan synthesis and degradation in cartilage and bone. Cell. Mol. Life Sci. 2008, 65, 395–413. [Google Scholar] [CrossRef]
- Kong, Y.; Liang, Q.; Chen, Y.; Yang, P.; Liu, X.; Li, Y.; Feng, S.; Wu, J.; Liu, W.; Tang, J. Hyaluronan negatively regulates vascular calcification involving BMP2 signaling. Lab. Investig. 2018, 98, 1320–1332. [Google Scholar] [CrossRef]
- Derwall, M.; Malhotra, R.; Lai, C.S.; Beppu, Y.; Aikawa, E.; Seehra, J.S.; Zapol, W.M.; Bloch, K.D.; Yu, P.B. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, H.-Y.; Giachelli, C.M. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis 2008, 199, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Ikeda, K.; Akakabe, Y.; Koide, M.; Uraoka, M.; Yutaka, K.-t.; Kurimoto-Nakano, R.; Takahashi, T.; Matoba, S.; Yamada, H.; et al. Paracrine Osteogenic Signals via Bone Morphogenetic Protein-2 Accelerate the Atherosclerotic Intimal Calcification In Vivo. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1908–1915. [Google Scholar] [CrossRef]
- Shu, B.; Zhang, M.; Xie, R.; Wang, M.; Jin, H.; Hou, W.; Tang, D.; Harris, S.E.; Mishina, Y.; O’keefe, R.J. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J. Cell Sci. 2011, 124, 3428–3440. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Hu, N.; Liao, J.-Y.; Lin, L.-B.; Zhao, C.; Si, W.-K.; Yang, Z.; Yi, S.-X.; Fan, T.-X.; Bao, W. HIF-1α as a regulator of BMP2-induced chondrogenic differentiation, osteogenic differentiation, and endochondral ossification in stem cells. Cell. Physiol. Biochem. 2015, 36, 44–60. [Google Scholar] [CrossRef]
- Schmal, H.; Niemeyer, P.; Zwingmann, J.; Stoffel, F.; Südkamp, N.P.; Mehlhorn, A.T. Association between expression of the bone morphogenetic proteins 2 and 7 in the repair of circumscribed cartilage lesions with clinical outcome. BMC Musculoskelet. Disord. 2010, 11, 170. [Google Scholar] [CrossRef][Green Version]
- Claus, S.; Aubert-Foucher, E.; Demoor, M.; Camuzeaux, B.; Paumier, A.; Piperno, M.; Damour, O.; Duterque-Coquillaud, M.; Galera, P.; Mallein-Gerin, F. Chronic exposure of bone morphogenetic protein-2 favors chondrogenic expression in human articular chondrocytes amplified in monolayer cultures. J. Cell. Biochem. 2010, 111, 1642–1651. [Google Scholar] [CrossRef]
- Deng, Z.; Li, Y.; Gao, X.; Lei, G.; Huard, J. Bone morphogenetic proteins for articular cartilage regeneration. Osteoarthr. Cartil. 2018, 26, 1153–1161. [Google Scholar] [CrossRef]
- Gooch, K.; Blunk, T.; Courter, D.; Sieminski, A.; Vunjak-Novakovic, G.; Freed, L. Bone morphogenetic proteins-2,-12, and-13 modulate in vitro development of engineered cartilage. Tissue Eng. 2002, 8, 591–601. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, R.; Yin, R.; Yin, W. Correlation of bone morphogenetic protein-2 levels in serum and synovial fluid with disease severity of knee osteoarthritis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 363. [Google Scholar]
- Fukui, N.; Zhu, Y.; Maloney, W.J.; Clohisy, J.; Sandell, L.J. Stimulation of BMP-2 expression by pro-inflammatory cytokines IL-1 and TNF-α in normal and osteoarthritic chondrocytes. JBJS 2003, 85, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Nakase, T.; Miyaji, T.; Tomita, T.; Kaneko, M.; Kuriyama, K.; Myoui, A.; Sugamoto, K.; Ochi, T.; Yoshikawa, H. Localization of bone morphogenetic protein-2 in human osteoarthritic cartilage and osteophyte. Osteoarthr. Cartil. 2003, 11, 278–284. [Google Scholar] [CrossRef]
- Schmal, H.; Mehlhorn, A.T.; Pilz, I.H.; Dovi-Akue, D.; Kirchhoff, C.; Südkamp, N.P.; Gerlach, U.; Lohrmann, C.; Niemeyer, P. Immunohistological localization of BMP-2, BMP-7, and their receptors in knee joints with focal cartilage lesions. Sci. World J. 2012, 2012, 467892. [Google Scholar] [CrossRef]
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical principles and clinical prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef]
- Eidelman, N.; Boyde, A.; Bushby, A.J.; Howell, P.G.; Sun, J.; Newbury, D.E.; Miller, F.W.; Robey, P.G.; Rider, L.G. Microstructure and mineral composition of dystrophic calcification associated with the idiopathic inflammatory myopathies. Arthritis Res. Ther. 2009, 11, R159. [Google Scholar] [CrossRef]


| Patient | Age | Gender | Side | BMI | Hypertensive | Smoker |
|---|---|---|---|---|---|---|
| 1 | 68 | M | L | 32.2 | + | + |
| 2 | 84 | M | L | 28.0 | + | - |
| 3 | 73 | M | R | 23.8 | - | - |
| 4 | 66 | M | L | 32.4 | + | - |
| 5 | 66 | M | L | 26.3 | - | - |
| 6 | 67 | M | R | 29.6 | + | + |
| 7 | 67 | M | L | 31.3 | - | - |
| 8 | 70 | M | L | 26.4 | + | - |
| 9 | 72 | M | R | 24.4 | + | - |
| 10 | 65 | M | L | 37.6 | + | - |
| 11 | 77 | M | L | 35.4 | + | - |
| 12 | 72 | M | R | 28.9 | - | + |
| 13 | 71 | M | L | 25.7 | - | - |
| 14 | 58 | M | R | 27.6 | + | - |
| 15 | 63 | M | L | 24.4 | + | - |
| 16 | 66 | M | R | 26.9 | + | - |
| 17 | 50 | M | L | 27.7 | - | - |
| Mean (±SD) | 67.9 ± 7.4 | 28.7 ± 3.9 |
| Patient Number | HDMP | Central Osteophyte |
|---|---|---|
| 1 | - | - |
| 2 | + | + |
| 3 | + | + |
| 4 | - | - |
| 5 | - | - |
| 6 | - | - |
| 7 | - | - |
| 8 | - | - |
| 9 | + | - |
| 10 | + | + |
| 11 | + | + |
| 12 | - | - |
| 13 | + | + |
| 14 | + | + |
| 15 | - | + |
| 16 | - | - |
| 17 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardi, A.F.; Tang, Q.; Wong, J.H.; Williams, J.L.; Jerban, S.; Ma, Y.; Jang, H.; Du, J.; Chang, E.Y. High-Density Mineralized Protrusions and Central Osteophytes: Associated Osteochondral Junction Abnormalities in Osteoarthritis. Diagnostics 2020, 10, 1051. https://doi.org/10.3390/diagnostics10121051
Lombardi AF, Tang Q, Wong JH, Williams JL, Jerban S, Ma Y, Jang H, Du J, Chang EY. High-Density Mineralized Protrusions and Central Osteophytes: Associated Osteochondral Junction Abnormalities in Osteoarthritis. Diagnostics. 2020; 10(12):1051. https://doi.org/10.3390/diagnostics10121051
Chicago/Turabian StyleLombardi, Alecio F., Qingbo Tang, Jonathan H. Wong, Judith L. Williams, Saeed Jerban, Yajun Ma, Hyungseok Jang, Jiang Du, and Eric Y. Chang. 2020. "High-Density Mineralized Protrusions and Central Osteophytes: Associated Osteochondral Junction Abnormalities in Osteoarthritis" Diagnostics 10, no. 12: 1051. https://doi.org/10.3390/diagnostics10121051
APA StyleLombardi, A. F., Tang, Q., Wong, J. H., Williams, J. L., Jerban, S., Ma, Y., Jang, H., Du, J., & Chang, E. Y. (2020). High-Density Mineralized Protrusions and Central Osteophytes: Associated Osteochondral Junction Abnormalities in Osteoarthritis. Diagnostics, 10(12), 1051. https://doi.org/10.3390/diagnostics10121051






