Implantation of a Dual-Chamber Automatic Cardioverter Defibrillator in a Patient with Persistent Left Superior Vena Cava: Case Report and Brief Literature Review
Abstract
:1. Introduction
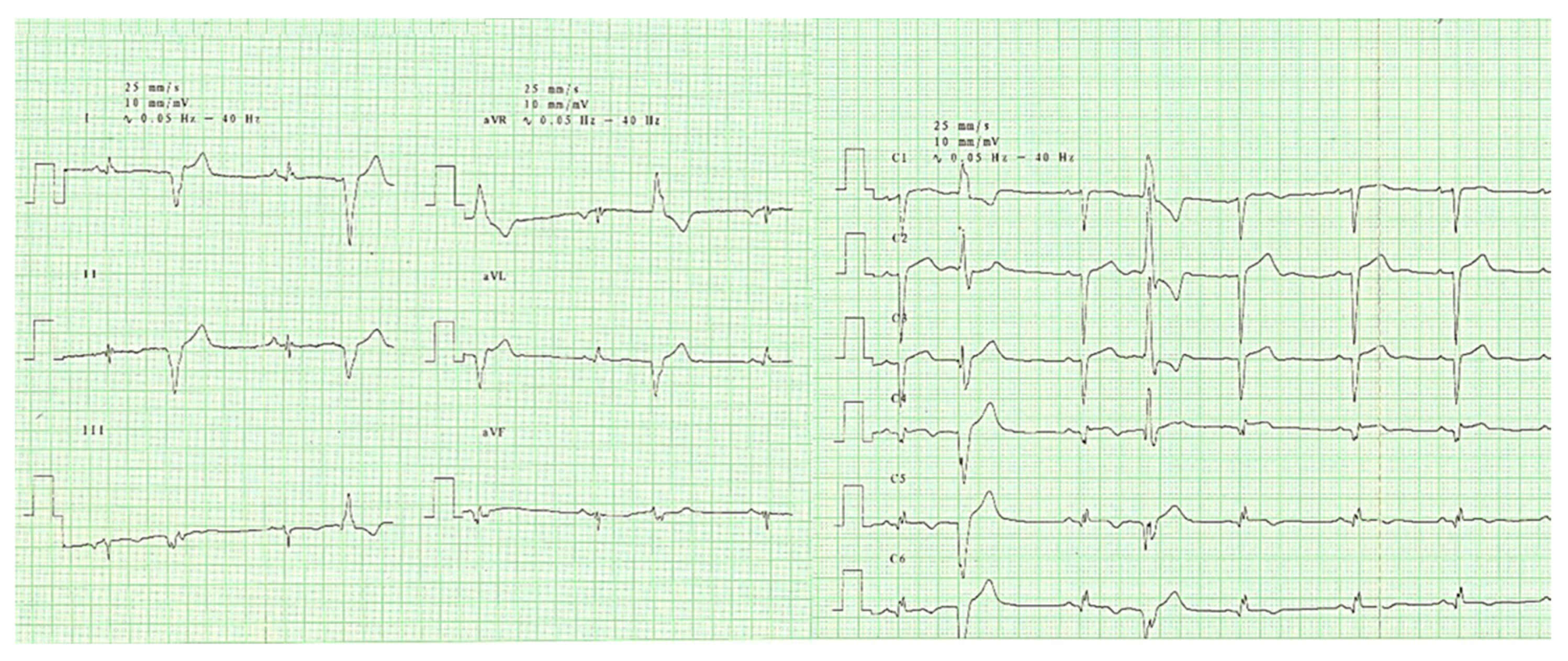
2. Case Report
3. Discussions
3.1. Epidemiology
3.2. Embryology and Anatomical Variants
3.3. Clinical and Technical Implications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Povoski, S.P.; Khabiri, H. Persistent left superior vena cava: Review of the literature, clinical implications, and relevance of alterations in thoracic central venous anatomy as pertaining to the general principles of central venous access device placement and venography in cancer patients. World J. Surg. Oncol. 2011, 9, 173. [Google Scholar] [PubMed] [Green Version]
- Sheikh, A.S.; Mazhar, S. Persistent left superior vena cava with absent right superior vena cava: Review of the literature and clinical implications. Echocardiography 2014, 31, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Ari, M.E.; Doğan, V.; Özgür, S.; Ceylan, Ö.; Ertuğrul, İ.; Kayalı, Ş.; Yoldaş, T.; Örün, U.A.; Kaya, Ö.; Karademir, S. Persistent left superior vena cava accompanying congenital heart disease in children: Experience of a tertiary care center. Echocardiography 2017, 34, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Biffi, M.; Boriani, G.; Frabetti, L.; Bronzetti, G.; Branzi, A. Left Superior Vena Cava Persistence in Patients Undergoing Pacemaker or Cardioverter-Defibrillator Implantation. Chest 2001, 120, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. ESC Scientific Document Group 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Marshall, J. On the development of the great anterior veins in man and mammalia; including an account of certain remnants of foetal structure found in the adult, a comparative view of these great veins in the different mammalia, and an analysis of their occasional peculiarities in the human subject. Philos. Trans. R. Soc. Lond. 1850, 140, 133–170. [Google Scholar]
- Demos, T.C.; Posniak, H.V.; Pierce, K.L.; Olson, M.C.; Muscato, M. Venous anomalies of the thorax. Am. J. Roentgenol. 2004, 182, 1139–1150. [Google Scholar] [CrossRef]
- Biffi, M.; Bertini, M.; Ziacchi, M.; Martignani, C.; Valzania, C.; Diemberger, I.; Branzi, A.; Boriani, G. Clinical implications of left superior vena cava persistence in candidates for pacemaker or cardioverter-defibrillator implantation. Heart Vessels 2009, 24, 142–146. [Google Scholar] [CrossRef]
- Demșa, I.; Crișu, D.; Haba, C.M.Ș.; Ursaru, A.M.; Afrăsânie, V.-A.; Costache, I.I.; Petriș, A.O.; Tesloianu, D.N. Persistent Left Superior Vena Cava with Absent Right Superior Vena Cava and Discrete Subaortic Stenosis Diagnosed in a Patient with Sick Sinus Syndrome: A Case Report and Brief Review of the Literature. Diagnostics 2020, 10, 847. [Google Scholar] [CrossRef]
- Schummer, W.; Schummer, C.; Fröber, R. Persistent left superior vena cava and central venous catheter position: Clinical impact illustrated by four cases. Surg. Radiol. Anat. 2003, 25, 315–321. [Google Scholar] [CrossRef]
- Lenox, C.C.; Zuberbuhler, J.R.; Park, S.C. Absent right superior vena cava with persistent left superior vena cava: Implications and management. Am. J. Cardiol. 1980, 45, 117–122. [Google Scholar] [CrossRef]
- Bartram, U.; Van Praagh, S.; Levine, J.C.; Hines, M.; Bensky, A.S.; Van Praagh, R. Absent right superior vena cava in visceroatrial situs solitus. Am. J. Cardiol. 1997, 80, 175–1834. [Google Scholar] [CrossRef]
- Campbell, M.; Deuchar, D.C. The left-sided superior vena cava. Br. Heart J. 1954, 16, 423–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arat, N.; Sokmen, Y.; Golbasi, Z. Persistent left superior vena cava with absent right superior vena cava and coronary sinus dilation mimicking a paracardiac mass. Tex. Heart Inst. J 2007, 34, 492–493. [Google Scholar]
- Aguilar, J.M.; Rodríguez-Serrano, F.; Ferreiro-Marzal, A.; Esteban-Molina, M.; Gabucio, A.; García, E.; Boni, L.; Garrido, J.M. Left superior vena cava draining into the left atrium: Clinical entities, diagnosis and surgical treatment. Arch. Cardiovasc. Dis. 2019, 112, 135–143. [Google Scholar] [CrossRef]
- Maki, R.; Miyajima, M.; Mishina, T.; Watanabe, A. Left upper pulmonary vein connected to the persistent left superior vena cava and the left atrium. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 723–725. [Google Scholar] [CrossRef]
- Blokland, D.; Lentjes, G.W.; Velthuis, B.K.; Chamuleau, S.A.J.; Rienks, R. Persistent left superior vena cava draining into the left superior pulmonary vein in a scuba diver: A case report and literature study. Scand. J. Med. Sci. Sports 2019, 29, 1265–1269. [Google Scholar] [CrossRef]
- Oliveira, J.D.; Martins, I. Congenital systemic venous return anomalies to the right atrium review. Insights Imaging 2019, 10, 115. [Google Scholar] [CrossRef]
- Uemura, M.; Suwa, F.; Takemura, A.; Toda, I.; Morishita, A. Classification of persistent left superior vena cava considering presence and development of both superior venae cavae, the anastomotic ramus between superior venae cavae, and the azygos venous system. Anat. Sci. Int. 2012, 87, 212–222. [Google Scholar] [CrossRef]
- Chen, X.; Yu, Z.; Bai, J.; Wang, W.; Qin, S.; Wang, J.; Sun, Z.; Han, F.; Su, Y.; Ge, J. Transvenous cardiac implantable electronic device implantation in patients with persistent left superior vena cava in a tertiary center. J. Interv. Card. Electrophysiol. 2018, 53, 255–262. [Google Scholar] [CrossRef]
- Yildiz, O. How to Safely Implant a Dual-Chamber Pacemaker for Right Ventricular Outflow Tract Pacing in a Patient with Persistent Left Superior Vena Cava: A Step by Step Guide. Acta Cardiol. Sin. 2019, 35, 430–432. [Google Scholar]
- Li, T.; Xu, Q.; Liao, H.-T.; Asvestas, D.; Letsas, K.P.; Li, Y. Transvenous dual-chamber pacemaker implantation in patients with persistent left superior vena cava. BMC Cardiovasc. Disord. 2019, 19, 100. [Google Scholar] [CrossRef] [Green Version]
- Keyser, A.; Hilker, M.K.; Ucer, E.; Wittmann, S.; Schmid, C.; Diez, C. Significance of intraoperative testing in right-sided implantable cardioverter-defibrillators. J. Cardiothorac. Surg. 2013, 8, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.; Adams, C.; Mechulan, A.; Leong-Sit, P.; Kiaii, B. Minimally invasive robotically assisted repair of atrial perforation from a pacemaker lead. Int. J. Med. Robot. 2012, 8, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Namazi, M.; Karbasi-Afshar, R.; Safi, M.; Serati, A. Diaphragmatic stimulation: A case of atrial lead dislodgement and right atrium perforation. Indian Pacing Electrophysiol. J. 2008, 8, 133–136. [Google Scholar] [PubMed]
- Jazayeri, M.-A.; Karim, R. What’s in a name? utilization of the innominate vein for pacemaker lead placement in the setting of persistent left superior vena cava. Cureus 2017, 9, e1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ejima, K.; Shoda, M.; Hagiwara, N. Placement of an ICD lead through a small innominate vein identified by a selective retrograde venogram in a case with a persistent left superior vena cava. Europace 2009, 11, 399. [Google Scholar] [CrossRef]
- Tauras, J.M.; Palma, E.C. Venoplasty of innominate bridge during implantation of single-chamber ICD in a patient with a persistent left-sided superior vena cava. Pacing Clin. Electrophysiol. 2008, 31, 1077–1078. [Google Scholar] [CrossRef]
- Palma, E.C.; Eugenio, P.L. Implantation of a biventricular ICD system in a patient with a persistent left superior vena cava. Clin. Cardiol. 2007, 30, 204. [Google Scholar] [CrossRef]
- Larsen, A.I.; Nilsen, D.W. Persistent left superior vena cava. Use of an innominate vein between left and right superior caval veins for the placement of a right ventricular lead during ICD/CRT implantation. Eur. Heart J. 2005, 26, 2178. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haba, M.C.; Ursaru, A.M.; Petriș, A.O.; Popescu, Ș.E.; Tesloianu, N.D. Implantation of a Dual-Chamber Automatic Cardioverter Defibrillator in a Patient with Persistent Left Superior Vena Cava: Case Report and Brief Literature Review. Diagnostics 2020, 10, 1071. https://doi.org/10.3390/diagnostics10121071
Haba MC, Ursaru AM, Petriș AO, Popescu ȘE, Tesloianu ND. Implantation of a Dual-Chamber Automatic Cardioverter Defibrillator in a Patient with Persistent Left Superior Vena Cava: Case Report and Brief Literature Review. Diagnostics. 2020; 10(12):1071. https://doi.org/10.3390/diagnostics10121071
Chicago/Turabian StyleHaba, Mihai Cristian, Andreea Maria Ursaru, Antoniu Octavian Petriș, Ștefan Eduard Popescu, and Nicolae Dan Tesloianu. 2020. "Implantation of a Dual-Chamber Automatic Cardioverter Defibrillator in a Patient with Persistent Left Superior Vena Cava: Case Report and Brief Literature Review" Diagnostics 10, no. 12: 1071. https://doi.org/10.3390/diagnostics10121071





