Prognostic Implications of Epilepsy Onset Age According to Relapse Pattern in Patients with Four-Year Remission
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Registration and Inclusion Criteria
2.2. Patient Grouping and Follow-Up
2.3. AED Treatment and Policy Regarding AED Withdrawal
2.4. Standard Protocol Approvals and Patient Consent
2.5. Data Analysis
2.6. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Statistical Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reynolds, E.H. Early treatment and prognosis of epilepsy. Epilepsia 1987, 28, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.H.; Elwes, R.D.C.; Shorvon, S.D. Why does epilepsy become intractable? Lancet 1983, 322, 952–954. [Google Scholar] [CrossRef]
- Shorvon, S.D.; Reynolds, E.H. Early prognosis of epilepsy. BMJ 1982, 285, 1699–1701. [Google Scholar] [CrossRef] [PubMed]
- French, J.A. Response to early AED therapy and its prognostic implications. Epilepsy Curr. 2002, 2, 69–71. [Google Scholar] [CrossRef][Green Version]
- Schiller, Y. Seizure relapse and development of drug resistance following long-term seizure remission. Arch. Neurol. 2009, 66, 1233–1239. [Google Scholar] [CrossRef]
- Brodie, M.J.; Barry, S.J.; Bamagous, G.A.; Norrie, J.D.; Kwan, P. Patterns of treatment response in newly diagnosed epilepsy. Neurology 2012, 78, 1548–1554. [Google Scholar] [CrossRef]
- Shinnar, S.; Berg, A.T. Does antiepileptic drug therapy prevent the development of “chronic” epilepsy? Epilepsia 1996, 37, 701–708. [Google Scholar] [CrossRef]
- Chadwick, D. Do anticonvulsants alter the natural course of epilepsy? Case for early treatment is not established. BMJ 1995, 310, 177–178. [Google Scholar] [CrossRef]
- Braathen, G.; Theorell, K. Is one-year treatment enough in uncomplicated epilepsy. Epilepsia 1995, 36, S163. [Google Scholar]
- Park, S.; Lee, D.H.; Kim, S.W.; Roh, Y.H. Prognostic analysis of patients with epilepsy according to time of relapse after withdrawal of antiepileptic drugs following four seizure-free years. Epilepsia 2017, 58, 60–67. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshe, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE commission for classification and terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, R.; Brodie, M.J. Diagnosing refractory epilepsy: Response to sequential treatment schedules. Eur. J. Neurol. 2006, 13, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Sillanpaa, M. Remission of seizures and predictors of intractability in long-term follow-up. Epilepsia 1993, 34, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Brodie, M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000, 342, 314–319. [Google Scholar] [CrossRef]
- Dlugos, D.J.; Sammel, M.D.; Strom, B.L.; Farrar, J.T. Response to first drug trial predicts outcome in childhood temporal lobe epilepsy. Neurology 2001, 57, 2259–2264. [Google Scholar] [CrossRef]
- Schiller, Y.; Najjar, Y. Quantifying the response to antiepileptic drugs: Effect of past treatment history. Neurology 2008, 70, 54–65. [Google Scholar] [CrossRef]
- Bonnett, L.J.; Tudur Smith, C.; Donegan, S.; Marson, A.G. Treatment outcome after failure of a first antiepileptic drug. Neurology 2014, 83, 552–560. [Google Scholar] [CrossRef]
- Hitiris, N.; Mohanraj, R.; Norrie, J.; Sills, G.J.; Brodie, M.J. Predictors of pharmacoresistant epilepsy. Epilepsy Res. 2007, 75, 192–196. [Google Scholar] [CrossRef]
- Chen, Z.; Brodie, M.J.; Liew, D.; Kwan, P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs. JAMA Neurol. 2018, 75, 279–286. [Google Scholar] [CrossRef]
- Beghi, E.; Tognoni, G. Prognosis of epilepsy in newly referred patients: A multicenter prospective study. Collaborative group for the study of epilepsy. Epilepsia 1988, 29, 236–243. [Google Scholar] [CrossRef]
- Feksi, A.T.; Kaamugisha, J.; Sander, J.W.; Gatiti, S.; Shorvon, S.D. Comprehensive primary health care antiepileptic drug treatment programme in rural and semi-urban Kenya. ICBERG (International community-based epilepsy research group). Lancet 1991, 337, 406–409. [Google Scholar] [CrossRef]
- Camfield, C.; Camfield, P.; Gordon, K.; Dooley, J. Does the number of seizures before treatment influence ease of control or remission of childhood epilepsy? Not if the number is 10 or less. Neurology 1996, 46, 41–44. [Google Scholar] [CrossRef] [PubMed]

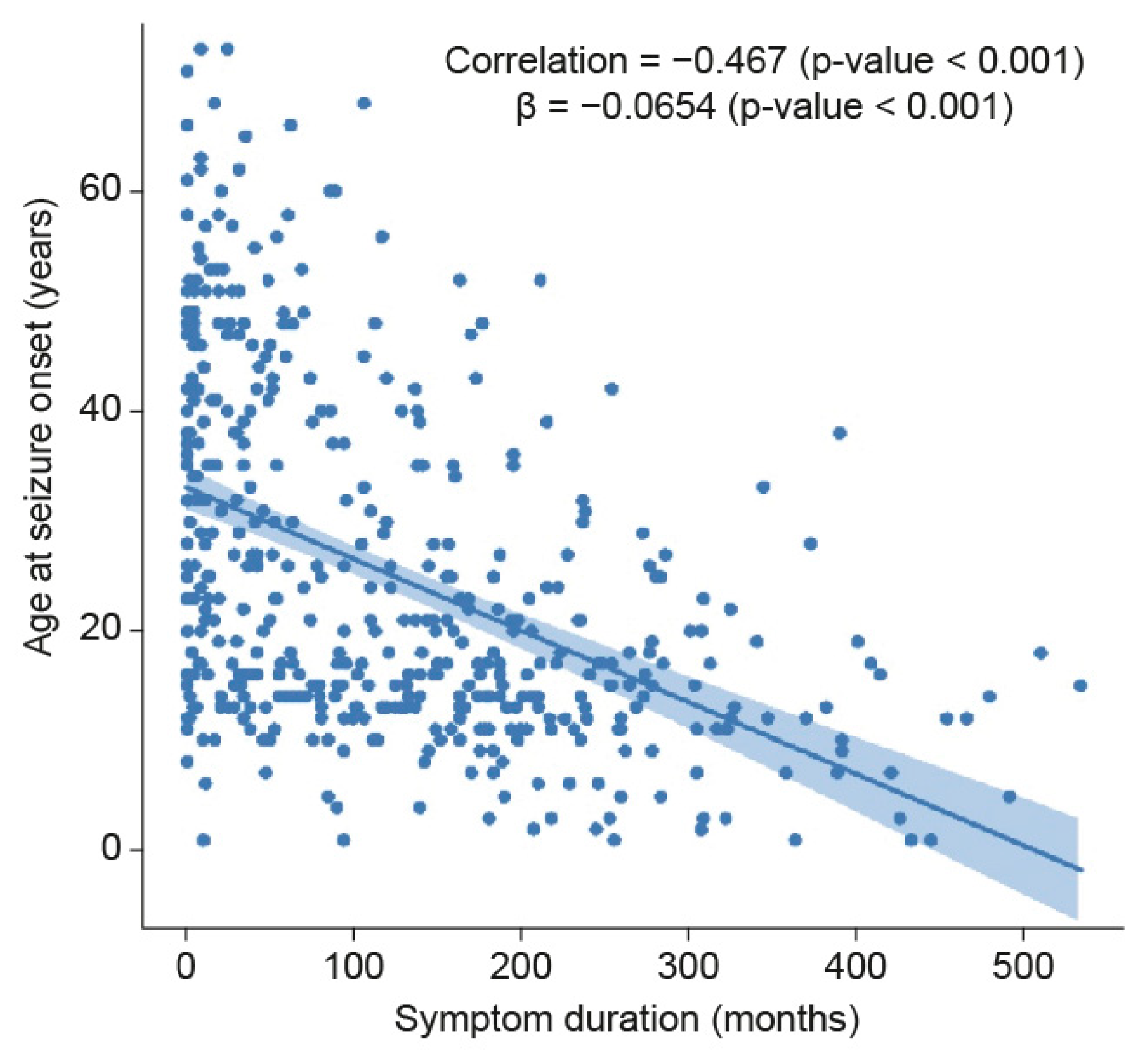
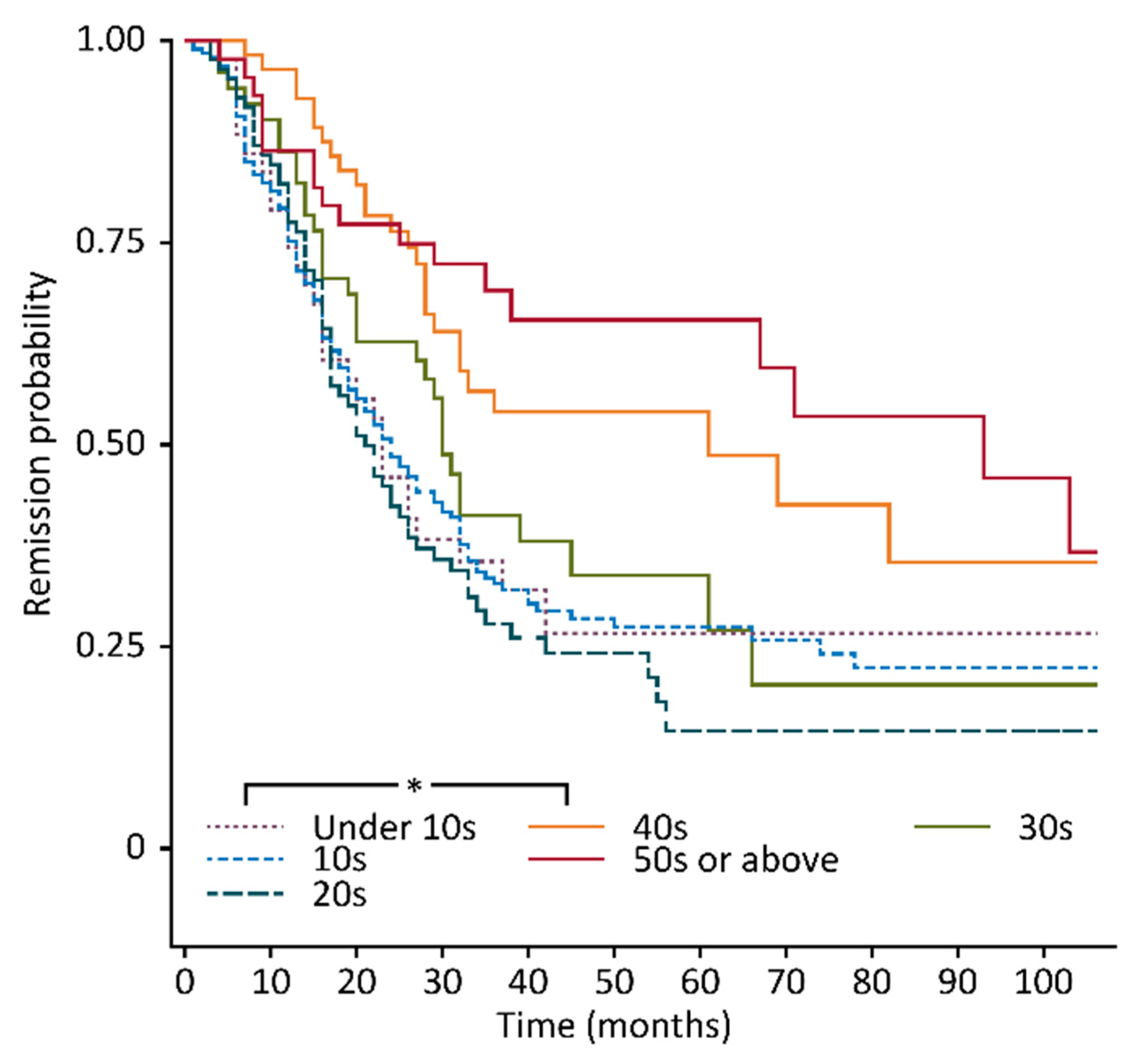
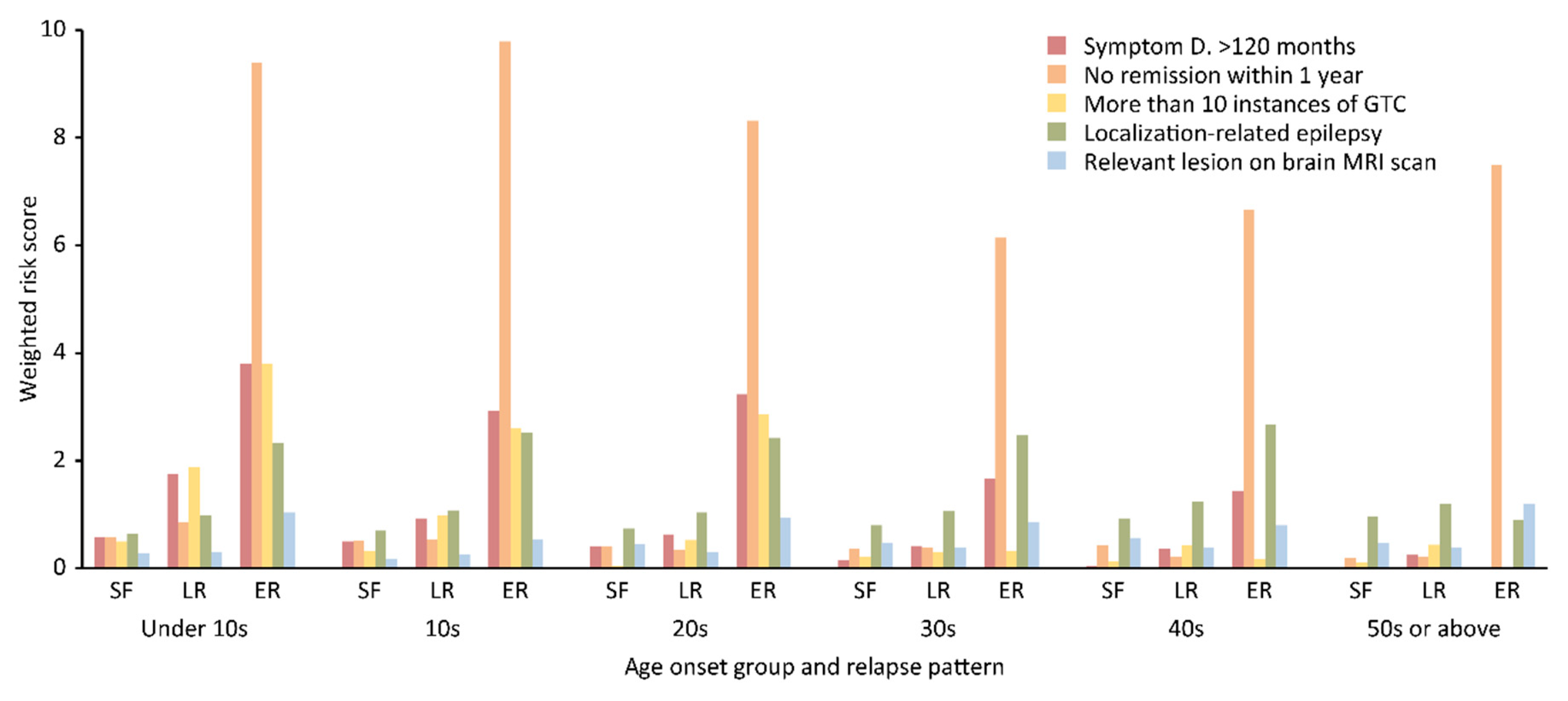

| Age of Onset Group, n, (%) † | Under 10 Years Old, n = 43 (9.1) | 10–19 Years Old, n = 192 (40.7) | 20–29 Years Old, n = 85 (18.0) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Relapse Pattern | SF | LR | ER | SF | LR | ER | SF | LR | ER |
| n, (%) †† | 14 (32.6) | 12 (27.9) | 17 (39.5) | 62 (32.3) | 77 (40.1) | 53 (27.6) | 22 (25.9) | 39 (45.9) | 24 (28.2) |
| Demographic data | |||||||||
| M: F | 9:5 | 7:5 | 10:7 | 35:27 | 40:37 | 31:22 | 15:7 | 28:11 | 17:7 |
| Mean age at seizure onset a ± SD, y | 5.0 ± 2.6 | 5.0 ± 2.7 | 5.1 ± 3.1 | 14.1 ± 2.4 | 14.5 ± 2.6 | 13.8 ± 2.2 | 23.8 ± 2.9 | 23.9 ± 2.9 | 23.7 ± 2.9 |
| Mean duration of symptoms b ± SD, m | 164.8 ± 141.8 | 286.7 ± 90.7 | 252.9 ± 108.0 | 138.9 ± 117.7 | 145.9 ± 114.2 | 183.3 ± 104.3 | 100.2 ± 95.8 | 95.6 ± 80.8 | 179.7 ± 106.6 |
| Prognostic variables, (%) ††† | |||||||||
| Antecedents of epilepsy | 7 (50.0) | 7 (58.3) | 10 (58.8) | 20 (32.3) | 28 (36.4) | 23 (43.4) | 9 (40.9) | 14 (35.9) | 12 (50.0) |
| Symptom duration > 120 m | 8 (57.1) | 12 (100.0) | 15 (88.2) | 30 (48.4) | 41 (53.2) | 36 (67.9) | 9 (40.9) | 14 (35.9) | 18 (75.0) |
| No remission within 1 y | 8 (57.1) | 10 (83.3) | 16 (94.1) | 32 (51.6) | 40 (51.9) | 52 (98.1) | 9 (39.9) | 13 (33.3) | 20 (83.3) |
| More than 10 GTC seizures | 7 (50.0) | 11 (91.7) | 15 (88.2) | 20 (32.3) | 37 (48.1) | 32 (60.4) | 1 (4.5) | 10 (25.6) | 16 (86.7) |
| Localization-related epilepsy | 9 (64.3) | 9 (75.0) | 13 (76.5) | 39 (62.9) | 60 (77.9) | 48 (90.6) | 14 (63.6) | 31 (79.5) | 19 (79.2) |
| Relevant lesions on brain MRI scan | 4 (28.6) | 4 (25.0) | 11 (64.7) | 11 (17.7) | 22 (28.6) | 18 (34.0) | 10 (45.5) | 13 (33.3) | 14 (58.3) |
| Nocturnal preponderance | 3 (21.4) | 6 (50.0) | 5 (29.4) | 21 (33.9) | 24 (31.2) | 13 (24.5) | 10 (45.5) | 14 (35.9) | 8 (33.3) |
| Age of Onset Group, n, (%) † | 30–39 Years Old, n = 53 | 40–49 Years Old, n = 55 | 50 Years Old or Older, n = 44 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Relapse Pattern | SF | LR | ER | SF | LR | ER | SF | LR | ER |
| n (%) †† | 19 (35.8) | 21 (39.6) | 13 (24.5) | 30 (54.5) | 19 (34.5) | 6 (10.9) | 26 (59.3) | 14 (33.3) | 4 (7.4) |
| Demographic data | |||||||||
| M: F | 11:8 | 13:8 | 6:7 | 19:11 | 5:14 | 4:6 | 15:11 | 6:8 | 3:1 |
| Mean age at epilepsy onset a ± SD, y. | 34.6 ± 2.8 | 34.8 ± 3.3 | 34.1 ± 2.5 | 44.7 ± 3.5 | 45.3 ± 2.7 | 42.5 ± 2.2 | 58.1 ± 6.3 | 57.8 ± 7.1 | 56.8 ± 6.9 |
| Mean duration of symptoms b ± SD, m. | 58.7 ± 72.9 | 66.6 ± 74.6 | 124.1 ± 128.4 | 43.6 ± 52.4 | 61.7 ± 61.3 | 61.3 ± 57.4 | 24.3 ± 27.6 | 56.2 ± 64.1 | 39.0 ± 34.6 |
| Prognostic variables (%) ††† | |||||||||
| Antecedents of epilepsy | 6 (31.6) | 9 (42.9) | 5 (38.5) | 13 (43.3) | 6 (31.6) | 4 (66.7) | 14 (53.8) | 6 (42.9) | 1 (25.0) |
| Symptom duration > 120 m | 3 (15.8) | 5 (23.8) | 5 (38.5) | 1 (3.3) | 4 (21.1) | 2 (33.3) | 0 (0) | 2 (14.3) | 0 (0) |
| No remission within 1 y | 7 (26.8) | 8 (38.1) | 8(61.5) | 13 (43.3) | 4 (21.1) | 4 (66.7) | 5(19.3) | 3 (11.4) | 3 (75.0) |
| More than 10 GTC seizures | 4 (21.1) | 3 (14.3) | 1 (7.7) | 4 (13.3) | 4 (21.1) | 1 (16.7) | 3 (11.5) | 3 (21.4) | 0 (0) |
| Localization-related epilepsy | 12 (63.2) | 17 (81.0) | 12 (92.3) | 25 (83.3) | 16 (84.2) | 6 (100) | 23 (88.5) | 12 (85.7) | 1 (25.0) |
| Relevant lesions on brain MRI scan | 9 (47.4) | 9 (42.9) | 7 (53.8) | 17 (56.7) | 8 (42.1) | 3 (50.0) | 12 (46.2) | 6 (42.9) | 3 (75.0) |
| Nocturnal preponderance | 7 (36.8) | 5 (23.8) | 3 (23.1) | 1 (3.3) | 8 (42.1) | 0 (0.0) | 3 (11.5) | 3 (21.4) | 1 (25.0) |
| Variable | Level | VIF | SF Patients (Ref.) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LR Patients | ER Patients | ||||||||
| OR | 95% CI | p-Value | OR | 95% CI | p-Value a | ||||
| Age of onset group | Under 10 years old | 2.17 | 1.59 | 0.58–4.36 | 0.366 | 7.89 | 2.22–28.05 | 0.001 | |
| 10–19 years old | 3.70 | 2.31 | 1.11–4.79 | 0.025 | 5.56 | 1.82–16.93 | 0.003 | ||
| 20–29 years old | 2.58 | 3.29 | 1.43–7.58 | 0.005 | 7.09 | 2.13–23.56 | 0.001 | ||
| 30–39 years old | 1.99 | 2.05 | 0.84–5.04 | 0.117 | 4.45 | 1.25–15.79 | 0.021 | ||
| 40–49 years old | 2.00 | 1.18 | 0.49–2.80 | 0.714 | 1.30 | 0.33–5.11 | 0.708 | ||
| 50 years old or older | Ref. | ||||||||
| Antecedents of epilepsy | Yes | 1.72 | 0.94 | 0.62–1.44 | 0.780 | 1.34 | 0.83–2.15 | 0.229 | |
| No | Ref. | ||||||||
| Symptom duration: 120 months | >120 | 1.18 | 1.76 | 1.13–2.70 | 0.013 | 4.31 | 2.62–7.11 | <0.0001 | |
| ≤120 | Ref. | Ref. | |||||||
| No remission within 1 year | Yes | 1.18 | 1.02 | 0.67–1.55 | 0.9371 | 9.977 | 5.288–18.826 | <0.0001 | |
| No | Ref. | Ref. | |||||||
| More than 10 GTC seizures | Yes | 1.37 | 2.05 | 1.29–3.67 | 0.003 | 4.30 | 2.58–7.15 | <0.0001 | |
| No | |||||||||
| Localization-related epilepsy | Yes | 1.44 | 1.31 | 0.75–2.27 | 0.340 | 2.68 | 1.26–5.70 | 0.011 | |
| No | Ref. | Ref. | |||||||
| Relevant lesions on brain MRI scan | Yes | 1.12 | 0.9 | 0.58–1.39 | 0.643 | 1.60 | 0.99–2.58 | 0.053 | |
| No | |||||||||
| Covariate | Level | SF Patients (Ref.) | |||||
|---|---|---|---|---|---|---|---|
| LR Patients | ER Patients | ||||||
| OR (95%, CI) | p-Value | Relative Risk a | OR (95%, CI) | p-Value | Relative Risk a | ||
| Age of onset group | Under 10 years old | 1.17 (0.38–3.63) | 0.782 | 2.18 | 1.53 (0.34–6.78) | 0.580 | 7.58 |
| 10–19 years old | 1.91 (0.86–4.25) | 0.112 | 1.79 | 1.92 (0.55–6.73) | 0.310 | 7.91 | |
| 20–29 years old | 3.11 (1.30–7.4) | 0.011 | 3.22 | 3.42 (0.90–12.94) | 0.070 | 19.44 | |
| 30–39 years old | 2.12 (0.85–5.32) | 0.108 | 1.44 | 3.12 (0.79–12.35) | 0.106 | 6.73 | |
| 40–49 years old | 1.17 (0.48–2.83) | 0.732 | 3.37 | 0.83 (0.19–3.59) | 0.804 | 12.99 | |
| 50 years old or older | Ref. | 1.41 | Ref. | 8.50 | |||
| Symptom duration: 120 months | >120 | 1.26 (0.73–2.17) | 0.408 | 1.60 (0.85–3.04) | 0.146 | ||
| ≤120 | Ref. | Ref. | |||||
| No remission within 1 year | Yes | 0.80 (0.50–1.28) | 0.347 | 7.18 (3.63–14.22) | <0.001 | ||
| No | Ref. | Ref. | |||||
| More than 10 GTC seizures | Yes | 1.88 (1.09–3.25) | 0.024 | 2.82 (1.49–5.33) | 0.001 | ||
| No | Ref. | Ref. | |||||
| Localization-related epilepsy | Yes | 1.60 (0.89–2.87) | 0.119 | 2.64 (1.14–6.13) | 0.023 | ||
| No | Ref. | Ref. | |||||
| Relevant lesions on brain MRI scan | Yes | 0.87 (0.54–1.40) | 0.561 | 1.83 (1.03–3.27) | 0.040 | ||
| No | Ref. | Ref. | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Lee, M. Prognostic Implications of Epilepsy Onset Age According to Relapse Pattern in Patients with Four-Year Remission. Diagnostics 2020, 10, 1089. https://doi.org/10.3390/diagnostics10121089
Park S, Lee M. Prognostic Implications of Epilepsy Onset Age According to Relapse Pattern in Patients with Four-Year Remission. Diagnostics. 2020; 10(12):1089. https://doi.org/10.3390/diagnostics10121089
Chicago/Turabian StylePark, Soochul, and Myeongjee Lee. 2020. "Prognostic Implications of Epilepsy Onset Age According to Relapse Pattern in Patients with Four-Year Remission" Diagnostics 10, no. 12: 1089. https://doi.org/10.3390/diagnostics10121089
APA StylePark, S., & Lee, M. (2020). Prognostic Implications of Epilepsy Onset Age According to Relapse Pattern in Patients with Four-Year Remission. Diagnostics, 10(12), 1089. https://doi.org/10.3390/diagnostics10121089





