Failure of Lactate Clearance Predicts the Outcome of Critically Ill Septic Patients
Abstract
:1. Introduction
2. Methods
2.1. Study Subjects
2.2. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Fleischmann, C.; Thomas-Rueddel, D.O.; Hartmann, M.; Hartog, C.S.; Welte, T.; Heublein, S.; Dennler, U.; Reinhart, K. Hospital Incidence and Mortality Rates of Sepsis. Dtsch. Arztebl. Int. 2016, 113, 159–166. [Google Scholar] [PubMed] [Green Version]
- Vincent, J.L.; Marshall, J.C.; Namendys-Silva, S.A.; Francois, B.; Martin-Loeches, I.; Lipman, J.; Reinhart, K.; Antonelli, M.; Pickkers, P.; Njimi, H.; et al. Assessment of the worldwide burden of critical illness: The intensive care over nations (ICON) audit. Lancet Respir. Med. 2014, 2, 380–386. [Google Scholar] [CrossRef]
- Van Vught, L.A.; Klein Klouwenberg, P.M.; Spitoni, C.; Scicluna, B.P.; Wiewel, M.A.; Horn, J.; Schultz, M.J.; Nurnberg, P.; Bonten, M.J.; Cremer, O.L.; et al. Incidence, Risk Factors, and Attributable Mortality of Secondary Infections in the Intensive Care Unit After Admission for Sepsis. JAMA 2016, 315, 1469–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakr, Y.; Moreira, C.L.; Rhodes, A.; Ferguson, N.D.; Kleinpell, R.; Pickkers, P.; Kuiper, M.A.; Lipman, J.; Vincent, J.L. Extended Prevalence of Infection in Intensive Care Study Investigators. The impact of hospital and ICU organizational factors on outcome in critically ill patients: Results from the Extended Prevalence of Infection in Intensive Care study. Crit. Care Med. 2015, 43, 519–526. [Google Scholar] [CrossRef] [Green Version]
- Wernly, B.; Heramvand, N.; Masyuk, M.; Rezar, R.; Bruno, R.R.; Kelm, M.; Niederseer, D.; Lichtenauer, M.; Hoppe, U.C.; Bakker, J.; et al. Acidosis predicts mortality independently from hyperlactatemia in patients with sepsis. Eur. J. Intern. Med. 2020. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef]
- Nguyen, H.B.; Rivers, E.P.; Knoblich, B.P.; Jacobsen, G.; Muzzin, A.; Ressler, J.A.; Tomlanovich, M.C. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit. Care Med. 2004, 32, 1637–1642. [Google Scholar] [CrossRef]
- Wernly, B.; Bakker, J.; Jung, C. Venous blood lactate concentrations in patients with shock: Interesting but not really helpful. J. Crit. Care 2020, 58, 125–126. [Google Scholar] [CrossRef]
- Gattinoni, L.; Vasques, F.; Camporota, L.; Meessen, J.; Romitti, F.; Pasticci, I.; Duscio, E.; Vassalli, F.; Forni, L.G.; Payen, D.; et al. Understanding Lactatemia in Human Sepsis. Potential Impact for Early Management. Am. J. Respir. Crit. Care Med. 2019, 200, 582–589. [Google Scholar] [CrossRef]
- Brealey, D.; Brand, M.; Hargreaves, I.; Heales, S.; Land, J.; Smolenski, R.; Davies, N.A.; Cooper, C.E.; Singer, M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002, 360, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Ince, C.; Mik, E.G. Microcirculatory and mitochondrial hypoxia in sepsis, shock, and resuscitation. J. Appl. Physiol. (1985) 2016, 120, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, J.H.; Luchette, F.A.; McCarter, F.D.; Fischer, J.E. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet 1999, 354, 505–508. [Google Scholar] [CrossRef]
- Gutierrez, G.; Williams, J.D. The riddle of hyperlactatemia. Crit. Care 2009, 13, 176. [Google Scholar] [CrossRef] [Green Version]
- Monnet, X.; Delaney, A.; Barnato, A. Lactate-guided resuscitation saves lives: No. Intensive Care Med. 2016, 42, 470–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masyuk, M.; Wernly, B.; Lichtenauer, M.; Franz, M.; Kabisch, B.; Muessig, J.M.; Zimmermann, G.; Lauten, A.; Schulze, P.C.; Hoppe, U.C.; et al. Prognostic relevance of serum lactate kinetics in critically ill patients. Intensive Care Med. 2019, 45, 55–61. [Google Scholar] [CrossRef]
- Chertoff, J.; Chisum, M.; Garcia, B.; Lascano, J. Lactate kinetics in sepsis and septic shock: A review of the literature and rationale for further research. J. Intensive Care 2015, 3, 39. [Google Scholar] [CrossRef] [Green Version]
- Pollard, T.J.; Johnson, A.E.W.; Raffa, J.D.; Celi, L.A.; Mark, R.G.; Badawi, O. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci. Data 2018, 5, 180178. [Google Scholar] [CrossRef]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef]
- Broder, G.; Weil, M.H. Excess Lactate: An Index of Reversibility of Shock in Human Patients. Science 1964, 143, 1457–1459. [Google Scholar] [CrossRef]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [PubMed]
- Wernly, B.; Bruno, R.R.; Mamandipoor, B.; Jung, C.; Osmani, V. Sex-specific outcomes and management in critically ill septic patients. Eur. J. Intern. Med. 2020. [Google Scholar] [CrossRef]
- Ha, T.S.; Shin, T.G.; Jo, I.J.; Hwang, S.Y.; Chung, C.R.; Suh, G.Y.; Jeon, K. Lactate clearance and mortality in septic patients with hepatic dysfunction. Am. J. Emerg. Med. 2016, 34, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Hwang, S.Y.; Jo, I.J.; Jeon, K.; Suh, G.Y.; Lee, T.R.; Yoon, H.; Cha, W.C.; Sim, M.S.; Carriere, K.C.; et al. Impact of Metformin Use on Lactate Kinetics in Patients with Severe Sepsis and Septic Shock. Shock 2017, 47, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, X. Lactate clearance is a useful biomarker for the prediction of all-cause mortality in critically ill patients: A systematic review and meta-analysis. Crit. Care Med. 2014, 42, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, F.; Meo, F.; Giacomelli, I.; Tozzi, C.; Ralli, M.L.; Donnini, C.; Tassinari, I.; Caldi, F.; Zanobetti, M.; Pini, R. Prognostic value of serial lactate levels in septic patients with and without shock. Intern. Emerg. Med. 2019, 14, 1321–1330. [Google Scholar] [CrossRef]
- Arnold, R.C.; Shapiro, N.I.; Jones, A.E.; Schorr, C.; Pope, J.; Casner, E.; Parrillo, J.E.; Dellinger, R.P.; Trzeciak, S.; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Multicenter study of early lactate clearance as a determinant of survival in patients with presumed sepsis. Shock 2009, 32, 35–39. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Kumar, N. Validation of lactate clearance at 6 h for mortality prediction in critically ill children. Indian J. Crit. Care Med. 2016, 20, 570–574. [Google Scholar] [CrossRef]
- Kramer, A.; Urban, N.; Doll, S.; Hartwig, T.; Yahiaoui-Doktor, M.; Burkhardt, R.; Petros, S.; Gries, A.; Bernhard, M. Early Lactate Dynamics in Critically Ill Non-Traumatic Patients in a Resuscitation Room of a German Emergency Department (OBSERvE-Lactate-Study). J. Emerg. Med. 2019, 56, 135–144. [Google Scholar] [CrossRef]
- Fuernau, G.; Desch, S.; de Waha-Thiele, S.; Eitel, I.; Neumann, F.J.; Hennersdorf, M.; Felix, S.B.; Fach, A.; Bohm, M.; Poss, J.; et al. Arterial Lactate in Cardiogenic Shock: Prognostic Value of Clearance Versus Single Values. JACC Cardiovasc. Interv. 2020, 13, 2208–2216. [Google Scholar] [CrossRef]
- Jung, C.; Bueter, S.; Wernly, B.; Masyuk, M.; Saeed, D.; Albert, A.; Fuernau, G.; Kelm, M.; Westenfeld, R. Lactate Clearance Predicts Good Neurological Outcomes in Cardiac Arrest Patients Treated with Extracorporeal Cardiopulmonary Resuscitation. J. Clin. Med. 2019, 8, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizutani, T.; Umemoto, N.; Taniguchi, T.; Ishii, H.; Hiramatsu, Y.; Arata, K.; Takuya, H.; Inoue, S.; Sugiura, T.; Asai, T.; et al. The lactate clearance calculated using serum lactate level 6 h after is an important prognostic predictor after extracorporeal cardiopulmonary resuscitation: A single-center retrospective observational study. J. Intensive Care 2018, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.E.; Shapiro, N.I.; Trzeciak, S.; Arnold, R.C.; Claremont, H.A.; Kline, J.A.; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Lactate clearance versus central venous oxygen saturation as goals of early sepsis therapy: A randomized clinical trial. JAMA 2010, 303, 739–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, G.; Ospina-Tascon, G.A.; Damiani, L.P.; Estenssoro, E.; Dubin, A.; Hurtado, J.; Friedman, G.; Castro, R.; Alegria, L.; Teboul, J.L.; et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status versus Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA 2019, 321, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, F.G.; Damiani, L.P.; Bakker, J.; Ospina-Tascon, G.A.; Castro, R.; Cavalcanti, A.B.; Hernandez, G. Effects of a Resuscitation Strategy Targeting Peripheral Perfusion Status versus Serum Lactate Levels among Patients with Septic Shock. A Bayesian Reanalysis of the ANDROMEDA-SHOCK Trial. Am. J. Respir. Crit. Care Med. 2020, 201, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.R.; Reed, M.; Bimpong-Buta, N.Y.; Muessig, J.M.; Masyuk, M.; Binneboessel, S.; Franz, M.; Kelm, M.; Jung, C. Sublingual microcirculation in prehospital critical care medicine: A proof-of-concept study. Microcirculation 2020, 27, e12614. [Google Scholar] [CrossRef]
- Holley, A.D.; Dulhunty, J.; Udy, A.; Midwinter, M.; Lukin, B.; Stuart, J.; Boots, R.; Lassig-Smith, M.; Holley, R.B.; Paratz, J.; et al. Early Sequential Microcirculation Assessment in Shocked Patients as a Predictor of Outcome: A Prospective Observational Cohort Study. Shock 2020. [Google Scholar] [CrossRef]
- Leligdowicz, A.; Dodek, P.M.; Norena, M.; Wong, H.; Kumar, A.; Kumar, A.; Co-operative Antimicrobial Therapy of Septic Shock Database Research Group. Association between source of infection and hospital mortality in patients who have septic shock. Am. J. Respir. Crit. Care Med. 2014, 189, 1204–1213. [Google Scholar] [CrossRef]



| Lactate Clearance ≤ 0% | Lactate Clearance > 0% | ||
|---|---|---|---|
| n = 1528 | n = 1771 | p-Value | |
| Male sex | 753 (49) | 947 (54) | 0.02 |
| BMI | 27 (10) | 27 (10) | 0.27 |
| Age (years) | 67 (21) | 66 (21) | 0.56 |
| Age > 65 years | 802 (53) | 923 (52) | 0.83 |
| SOFA score | 9 (6) | 7 (6) | <0.001 |
| SOFA > 10 | 595 (39) | 436 (25) | <0.001 |
| Heart rate >110 bpm | 567 (39) | 620 (37) | 0.20 |
| Body temperature >38 °C | 203 (14) | 228 (14) | 0.76 |
| Creatinine (mg/dL) | 1.80 (1.67) | 1.53 (1.49) | <0.001 |
| Creatinine > 2.0 mg/dL | 655 (44) | 632 (36) | 0.001 |
| Lactate | |||
| Baseline (mmol/L) | 3.40 (2.90) | 3.60 (2.60) | 0.11 |
| at 6 hours (mmol/L) | 4.00 (4.00) | 2.20 (1.80) | <0.001 |
| Focus | |||
| UTI | 273 (18) | 369 (21) | 0.03 |
| Pulmonary | 555 (36) | 607 (34) | 0.22 |
| GI | 268 (18) | 306 (17) | 0.84 |
| Cutaneous | 90 (6) | 122 (7) | 0.24 |
| Unknown | 215 (14) | 248 (14) | 0.96 |
| Other | 123 (8) | 113 (6) | 0.06 |
| Gynecologic | 4 (<1) | 6 (<1) | 0.67 |
| Ethnic | |||
| Caucasian | 1165 (76) | 1376 (78) | 0.32 |
| AfricanAmerican | 172 (11) | 180 (10) | 0.31 |
| Hispanic | 59 (4) | 66 (4) | 0.84 |
| Asian | 28 (2) | 34 (2) | 0.85 |
| Native American | 16 (1) | 19 (1) | 0.94 |
| Other | 88 (6) | 96 (5) | 0.67 |
| Length of stay (h) | 66 (115) | 67 (95) | 0.48 |
| Fluid management in first 24 h | |||
| Total amount of fluids (mL); median (IQR) | 3358 (3860) | 3375 (3377) | 0.90 |
| Amount of fluid per kg bodyweight; median (IQR) | 43 (59) | 42 (50) | 0.77 |
| Amount of fluid per kg BW >30 mL/kg/h; n (%) | 454 (64) | 493 (63) | 0.73 |
| Crude Events | |||||
|---|---|---|---|---|---|
| Lactate Clearance ≤ 0% (n = 1528) | Lactate Clearance > 0% (n = 1771) | Model 1 | Model 2 | Model 3 | |
| n (%) | n (%) | aOR (95% CI, p-value) | aOR (95% CI, p-value) | aOR (95% CI, p-value) | |
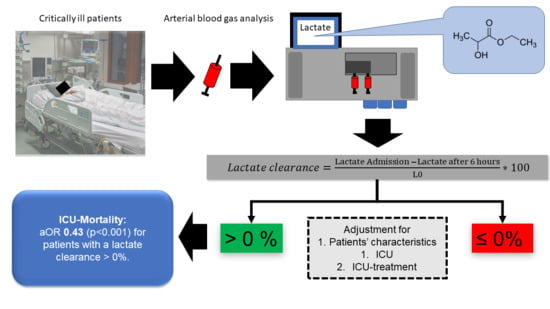
| ICU mortality | 488 (32) | 242 (14) | 0.34 (0.28–0.40; <0.001) | 0.42 (0.34–0.52; <0.001) | 0.43 (0.36–0.53; <0.001) |
| Management | |||||
| Mechanical ventilation | 617 (40) | 503 (28) | 0.51 (0.44–0.60; <0.001) | 0.85 (0.70–1.03; 0.01) | |
| Vasopressor use | 817 (54) | 653 (37) | 0.58 (0.50–0.69; <0.001) | 0.69 (0.57–0.83; <0.001) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, R.R.; Wernly, B.; Binneboessel, S.; Baldia, P.; Duse, D.A.; Erkens, R.; Kelm, M.; Mamandipoor, B.; Osmani, V.; Jung, C. Failure of Lactate Clearance Predicts the Outcome of Critically Ill Septic Patients. Diagnostics 2020, 10, 1105. https://doi.org/10.3390/diagnostics10121105
Bruno RR, Wernly B, Binneboessel S, Baldia P, Duse DA, Erkens R, Kelm M, Mamandipoor B, Osmani V, Jung C. Failure of Lactate Clearance Predicts the Outcome of Critically Ill Septic Patients. Diagnostics. 2020; 10(12):1105. https://doi.org/10.3390/diagnostics10121105
Chicago/Turabian StyleBruno, Raphael Romano, Bernhard Wernly, Stephan Binneboessel, Philipp Baldia, Dragos Andrei Duse, Ralf Erkens, Malte Kelm, Behrooz Mamandipoor, Venet Osmani, and Christian Jung. 2020. "Failure of Lactate Clearance Predicts the Outcome of Critically Ill Septic Patients" Diagnostics 10, no. 12: 1105. https://doi.org/10.3390/diagnostics10121105
APA StyleBruno, R. R., Wernly, B., Binneboessel, S., Baldia, P., Duse, D. A., Erkens, R., Kelm, M., Mamandipoor, B., Osmani, V., & Jung, C. (2020). Failure of Lactate Clearance Predicts the Outcome of Critically Ill Septic Patients. Diagnostics, 10(12), 1105. https://doi.org/10.3390/diagnostics10121105