Diagnostic Problems in Diffuse Axonal Injury
Abstract
:1. Introduction
2. Problem 1: The Shortage of Scientific Evidence for the 6-Hour LOC Diagnostic Criterion Separating Concussion and DAI
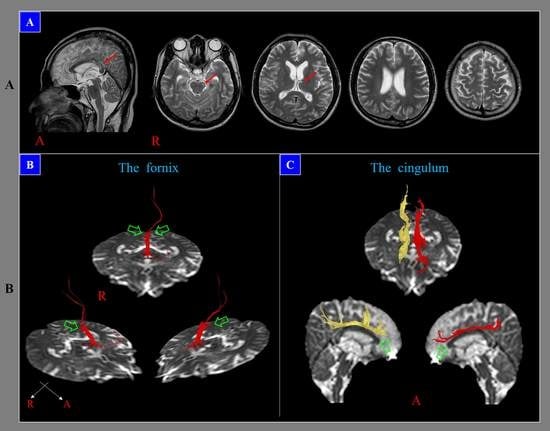
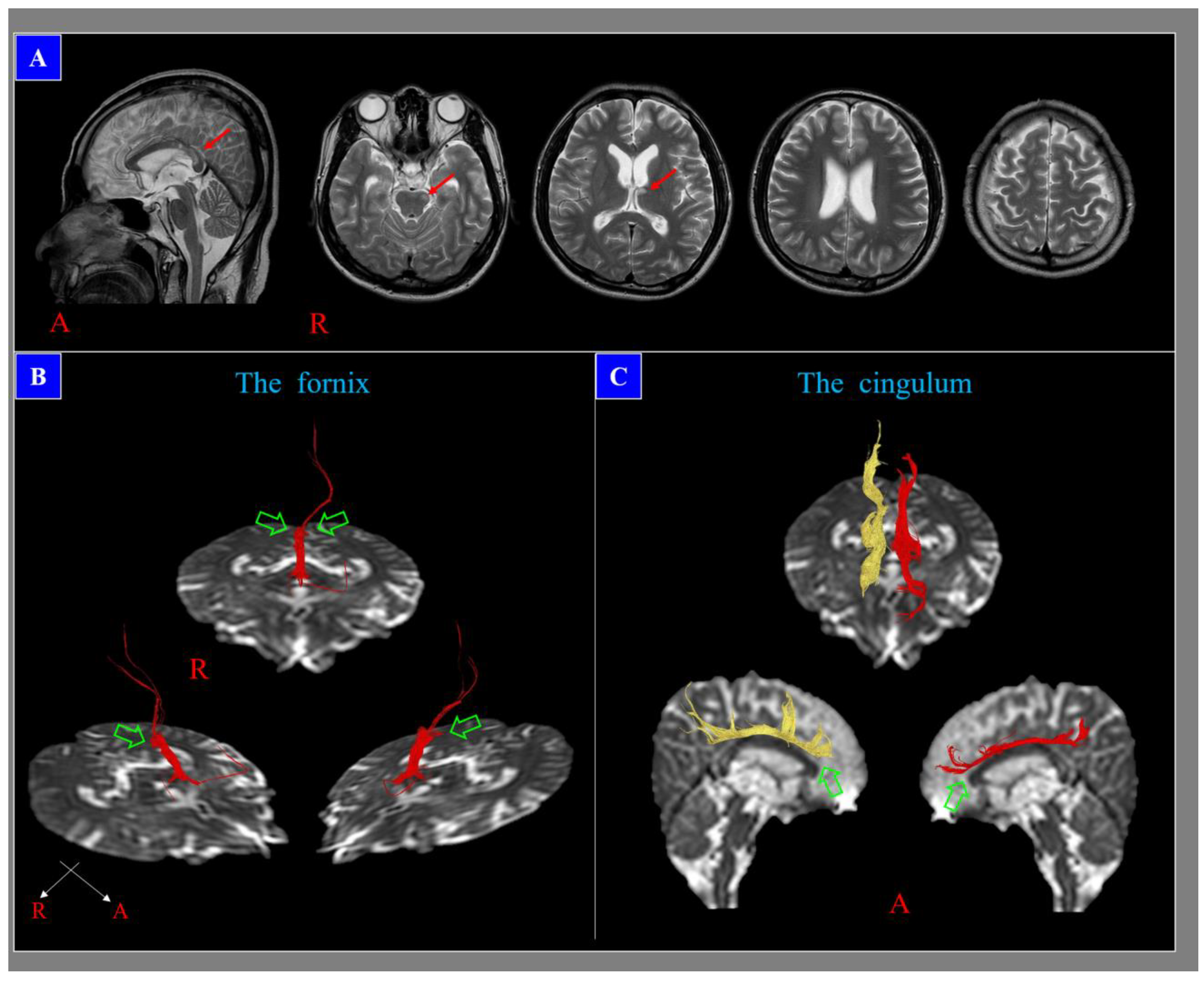
3. Problem 2: The Low Sensitivity of Conventional Brain MRI in the Detection of DAI Lesions
4. Problem 3. The Inappropriateness of the Term Diffuse in DAI
5. Conclusions
Funding
Conflicts of Interest
References
- Adams, J.H.; Graham, D.I.; Murray, L.S.; Scott, G. Diffuse axonal injury due to nonmissile head injury in humans: An analysis of 45 cases. Ann. Neurol. 1982, 12, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.H.; Doyle, D.; Ford, I.; Gennarelli, T.A.; Graham,, D.I.; McLellan, D.R. Diffuse axonal injury in head injury: Definition, diagnosis and grading. Histopathology 1989, 15, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Park, Y.S.; Nam, T.K.; Kwon, J.T.; Min, B.K.; Hwang, S.N. Locations and clinical significance of nonhemorrhagic brain lesions in diffuse axonal injuries. J. Korean Neurosurg Soc. 2012, 52, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.H.; Meaney, D.F.; Shull, W.H. Diffuse axonal injury in head trauma. J. Head Trauma. Rehabil. 2003, 18, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Povlishock, J.T.; Christman, C.W. The pathobiology of traumatically induced axonal injury in animals and humans: A review of current thoughts. J. Neurotrauma 1995, 12, 555–564. [Google Scholar] [CrossRef]
- Buki, A.; Povlishock, J.T. All roads lead to disconnection?--traumatic axonal injury revisited. Acta. Neurochir (Wien) 2006, 148, 181–193. [Google Scholar] [CrossRef]
- Maxwell, W.L.; Povlishock, J.T.; Graham, D.L. A mechanistic analysis of nondisruptive axonal injury: A review. J. Neurotrauma 1997, 14, 419–440. [Google Scholar] [CrossRef]
- Meythaler, J.M.; Peduzzi, J.D.; Eleftheriou, E.; Novack, T.A. Current concepts: Diffuse axonal injury-associated traumatic brain injury. Arch. Phys. Med. Rehabil. 2001, 82, 1461–1471. [Google Scholar] [CrossRef]
- Grassi, D.C.; Conceicao, D.M.D.; Leite, C.D.C.; Andrade, C.S. Current contribution of diffusion tensor imaging in the evaluation of diffuse axonal injury. Arq. Neuropsiquiatr 2018, 76, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Jing, S.; Ju, Y.; He, Y.; He, M.; Mao, B. Clinical features of diffuse axonal injury. Chin. J. Traumatol. 2001, 4, 204–207. [Google Scholar]
- Gennarelli, T.A. Cerebral concussion and diffuse brain injuries, 3rd ed.; Williams & Wilkins: Baltimore, Maryland, 1987; pp. 137–158. [Google Scholar]
- Humble, S.S.; Wilson, L.D.; Wang, L.; Long, D.A.; Smith, M.A.; Siktberg, J.C.; Mirhoseini, M.F.; Bhatia, A.; Pruthi, S.; Day, M.A.; et al. Prognosis of diffuse axonal injury with traumatic brain injury. J. Trauma Acute Care Surg. 2018, 85, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, D.R. Microscopic lesions in the brain following head injury. J. Neurol. Neurosurg Psychiatry 1968, 31, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumbergs, P.C.; Scott, G.; Manavis, J.; Wainwright, H.; Simpson, D.A.; McLean, A.J. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet 1994, 344, 1055–1056. [Google Scholar] [CrossRef]
- Mittl, R.L.; Grossman, R.I.; Hiehle, J.F.; Hurst, R.W.; Kauder, D.R.; Gennarelli, T.A.; Alburger, G.W. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. AJNR Am. J. Neuroradiol. 1994, 15, 1583–1589. [Google Scholar]
- Topal, N.B.; Hakyemez, B.; Erdogan, C.; Bulut, M.; Koksal, O.; Akkose, S.; Dogan, S.; Parlak, M.; Ozguc, H.; Korfali, E. MR imaging in the detection of diffuse axonal injury with mild traumatic brain injury. Neurol. Res. 2008, 30, 974–978. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Axonal pathology in traumatic brain injury. Exp. Neurol. 2013, 246, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Gennarelli, T.A.; Thibault, L.E.; Adams, J.H.; Graham, D.I.; Thompson, C.J.; Marcincin, R.P. Diffuse axonal injury and traumatic coma in the primate. Ann. Neurol. 1982, 12, 564–574. [Google Scholar] [CrossRef]
- Shenton, M.E.; Hamoda, H.M.; Schneiderman, J.S.; Bouix, S.; Pasternak, O.; Rathi, Y.; Vu, M.A.; Purohit, M.P.; Helmer, K.; Koerte, I.; et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012, 6, 137–192. [Google Scholar] [CrossRef]
- D’Souza, M.M.; Trivedi, R.; Singh, K.; Grover, H.; Choudhury, A.; Kaur, P.; Kumar, P.; Tripathi, R.P. Traumatic brain injury and the post-concussion syndrome: A diffusion tensor tractography study. Indian J. Radiol Imaging 2015, 25, 404–414. [Google Scholar] [CrossRef]
- Lee, S.J.; Bae, C.H.; Seo, J.P.; Jang, S.H. Diagnosis of Tinnitus Due to Auditory Radiation Injury Following Whiplash Injury: A Case Study. Diagnostics (Basel) 2019, 30, 10. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.H.; Lee, H.D. Diagnostic Approach to Traumatic Axonal Injury of the Spinothalamic Tract in Individual Patients with Mild Traumatic Brain Injury. Diagnostics (Basel) 2019, 21, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, S.H. Diagnostic history of traumatic axonal injury in patients with cerebral concussion and mild traumatic brain injury. Brain NeuroRehabil. 2016, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bigler, E.D. Neuropsychological results and neuropathological findings at autopsy in a case of mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2004, 10, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H. Traumatic axonal injury in mild traumatic brain injury. In Traumatic Brain Injury, 1st ed.; Gorbunoy, N., Ed.; In Tech: London, UK, 2018; pp. 137–154. [Google Scholar]
- Hill, C.S.; Coleman, M.P.; Menon, D.K. Traumatic axonal injury: Mechanisms and translational opportunities. Trends Neurosci. 2016, 39, 311–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentry, L.R.; Godersky, J.C.; Thompson, B. MR imaging of head trauma: Review of the distribution and radiopathologic features of traumatic lesions. AJR Am. J. Roentgenol. 1988, 150, 663–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katzman, G.L. Trauma; Diffuse axonal injury. In Diagnostic Imaging: Brain, 2nd ed.; Osborn, A.G., Salzman, K.L., Barkovich, A.J., Eds.; Amirsys: Manitoba, ON, Canada, 2010; pp. 36–39. [Google Scholar]
- Jang, S.H.; Kim, S.H.; Lim, H.W.; Yeo, S.S. Injury of the lower ascending reticular activating system in patients with hypoxic-ischemic brain injury: Diffusion tensor imaging study. Neuroradiology 2014, 56, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.F.; Chiang, F.L.; Stephens, N.; Huang, S.Y.; Bilgic, B.; Tantiwongkosi, B.; Romero, R. Characterization of normal-appearing white matter in multiple sclerosis using quantitative susceptibility mapping in conjunction with diffusion tensor imaging. Neuroradiology 2019, 61, 71–79. [Google Scholar] [CrossRef]
- Basser, P.J.; Mattiello, J.; LeBihan, D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. 1994, 103, 247–254. [Google Scholar] [CrossRef]
- Mori, S.; Crain, B.J.; Chacko, V.P.; van Zijl, P.C. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 1999, 45, 265–269. [Google Scholar] [CrossRef]
- Huisman, T.A.; Schwamm, L.H.; Schaefer, P.W.; Koroshetz, W.J.; Shetty-Alva, N.; Ozsunar, Y.; Wu, O.; Sorensen, A.G. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am. J. Neuroradiol. 2004, 25, 370–376. [Google Scholar]
- Brandstack, N.; Kurki, T.; Tenovuo, O. Quantitative diffusion-tensor tractography of long association tracts in patients with traumatic brain injury without associated findings at routine MR imaging. Radiology 2013, 267, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Choi, C.G.; Chun, M.H. Usefulness of diffusion tensor imaging for evaluation of motor function in patients with traumatic brain injury: Three case studies. J. Head Trauma Rehabil. 2006, 21, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Bakhadirov, K.; Devous, M.D., Sr.; Abdi, H.; McColl, R.; Moore, C.; Marquez de la Plata, C.D.; Ding, K.; Whittemore, A. Diffusion tensor tractography of traumatic diffuse axonal injury. Arch. Neurol. 2008, 65, 619–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, S.H.; Seo, Y.S. Difference between injuries of the corticospinal tract and corticoreticulospinal tract in patients with diffuse axonal injury: A diffusion tensor tractography study. Int. J. Neurosci. 2020, 130, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.S.; Kim, O.L.; Kim, S.H.; Ahn, S.H.; Cho, Y.W.; Son, S.M.; Jang, S.H. Classification of cause of motor weakness in traumatic brain injury using diffusion tensor imaging. Arch. Neurol. 2012, 69, 363–367. [Google Scholar] [CrossRef]
- Saatman, K.E.; Duhaime, A.C.; Bullock, R.; Maas, A.I.; Valadka, A.; Manley, G.T.; Workshop Scientific, T.; Advisory Panel, M. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma 2008, 25, 719–738. [Google Scholar] [CrossRef] [Green Version]
- Povlishock, J.T.; Erb, D.E.; Astruc, J. Axonal response to traumatic brain injury: Reactive axonal change, deafferentation, and neuroplasticity. J. Neurotrauma 1992, 9, S189–S200. [Google Scholar]
- Wallace, E.J.; Mathias, J.L.; Ward, L. Diffusion tensor imaging changes following mild, moderate and severe adult traumatic brain injury: A meta-analysis. Brain Imaging Behav. 2018, 12, 1607–1621. [Google Scholar] [CrossRef]

| Patho-Anatomy | |||
| Diffuse | Focal | ||
| Concussion | Contusion | ||
| Traumatic axonal injury/diffuse axonal injury | Penetrating | ||
| Explosion | Hematoma | ||
| Abusive head trauma | - Epidural | ||
| - Subarachnoid | |||
| - Subdural | |||
| - Intraventricular | |||
| - Intracerebral | |||
| Severity of Head Trauma | |||
| LOC | PTA | GCS | |
| Mild: | ≤30 min | ≤24 h | 13–15 |
| Moderate: | >30 min, ≤24 h | >24 h, ≤7 days | 9–12 |
| Severe: | >24 h | >7 days | 3–8 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.H. Diagnostic Problems in Diffuse Axonal Injury. Diagnostics 2020, 10, 117. https://doi.org/10.3390/diagnostics10020117
Jang SH. Diagnostic Problems in Diffuse Axonal Injury. Diagnostics. 2020; 10(2):117. https://doi.org/10.3390/diagnostics10020117
Chicago/Turabian StyleJang, Sung Ho. 2020. "Diagnostic Problems in Diffuse Axonal Injury" Diagnostics 10, no. 2: 117. https://doi.org/10.3390/diagnostics10020117