Cardiac Evaluation Using Two-Dimensional Speckle-Tracking Echocardiography and Conventional Echocardiography in Taiwanese Patients with Mucopolysaccharidoses
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurements of Echocardiographic Parameters
2.2.1. 2D STE
2.2.2. Conventional Echocardiography
2.3. Data Analysis and Statistics
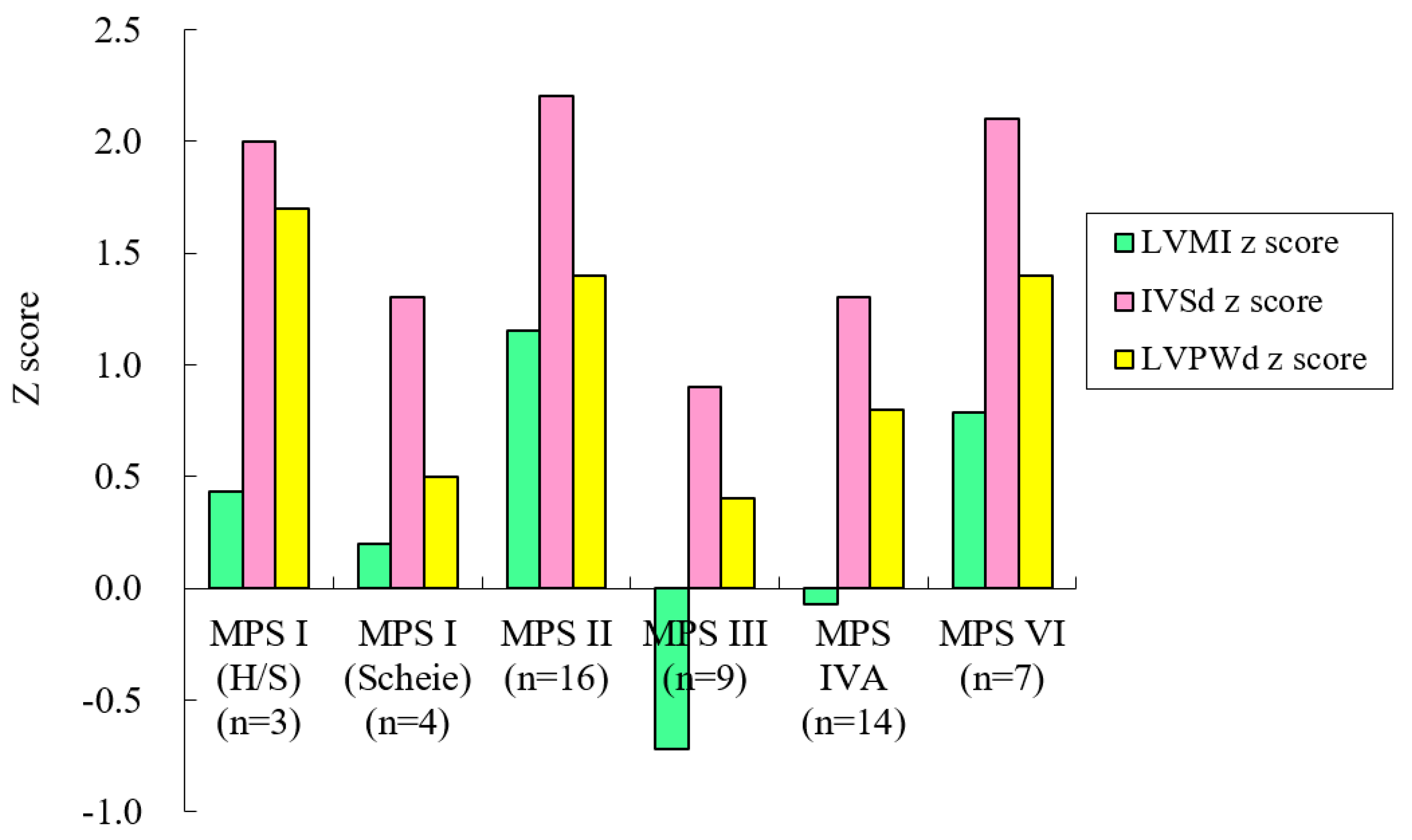
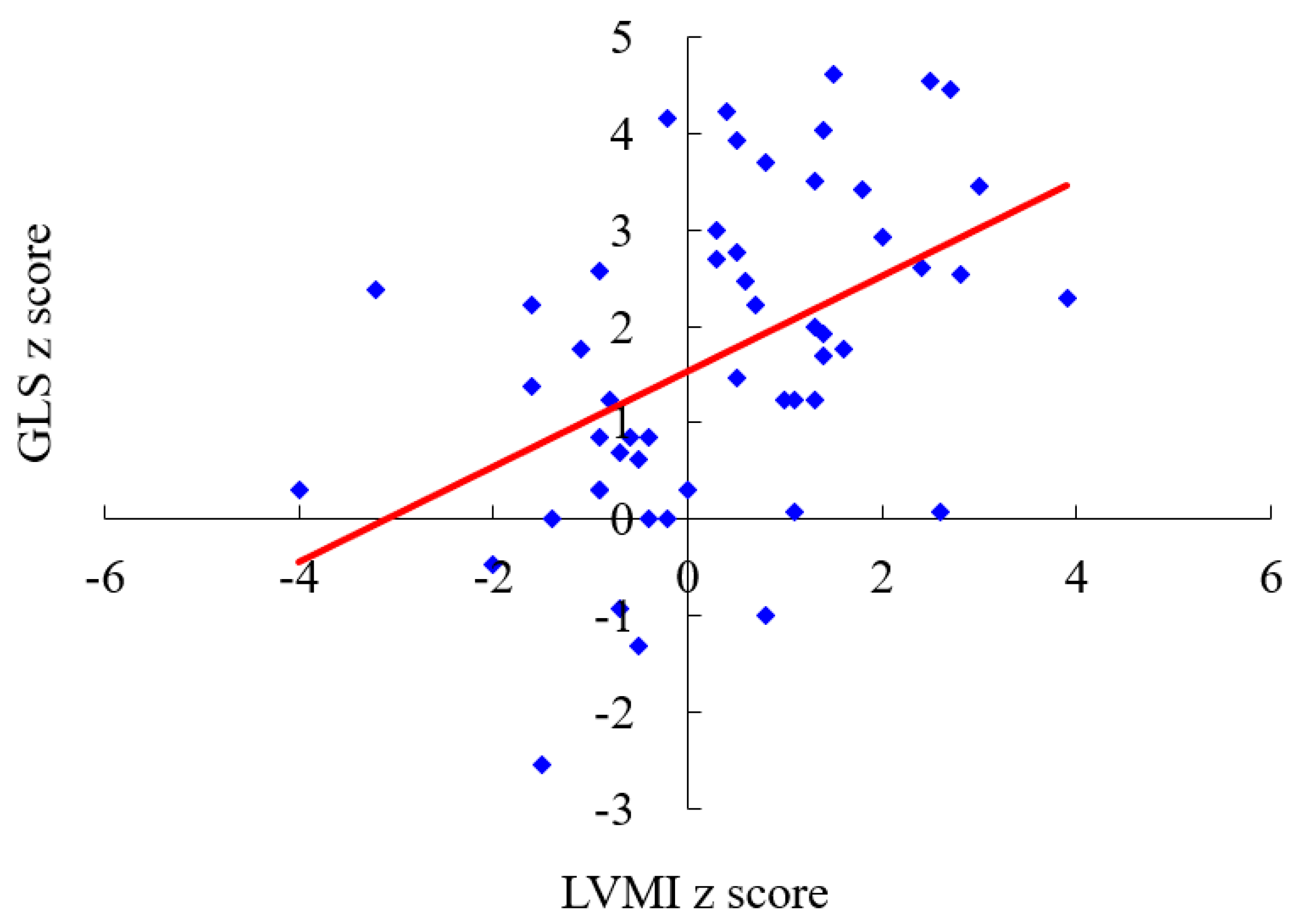
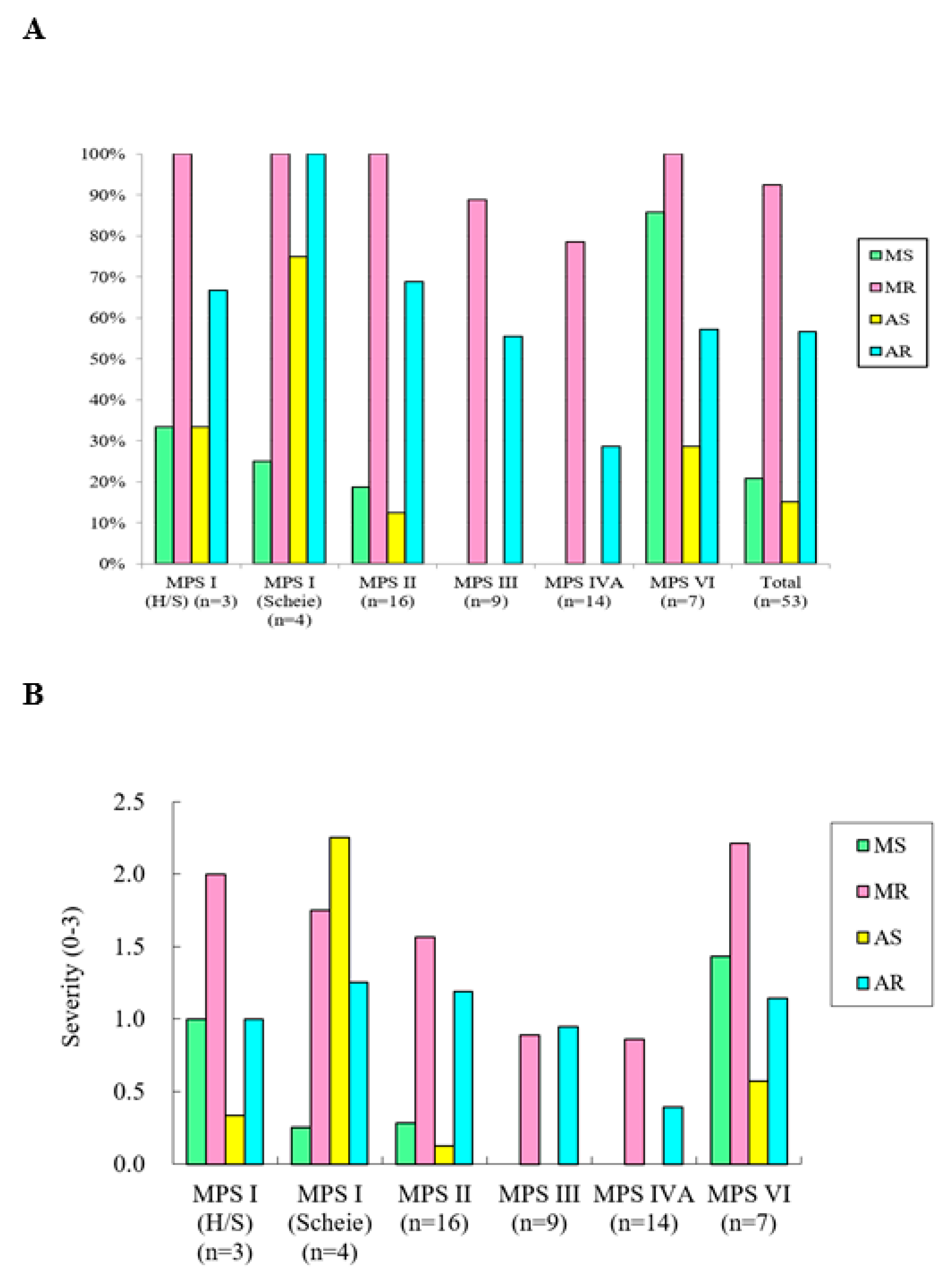
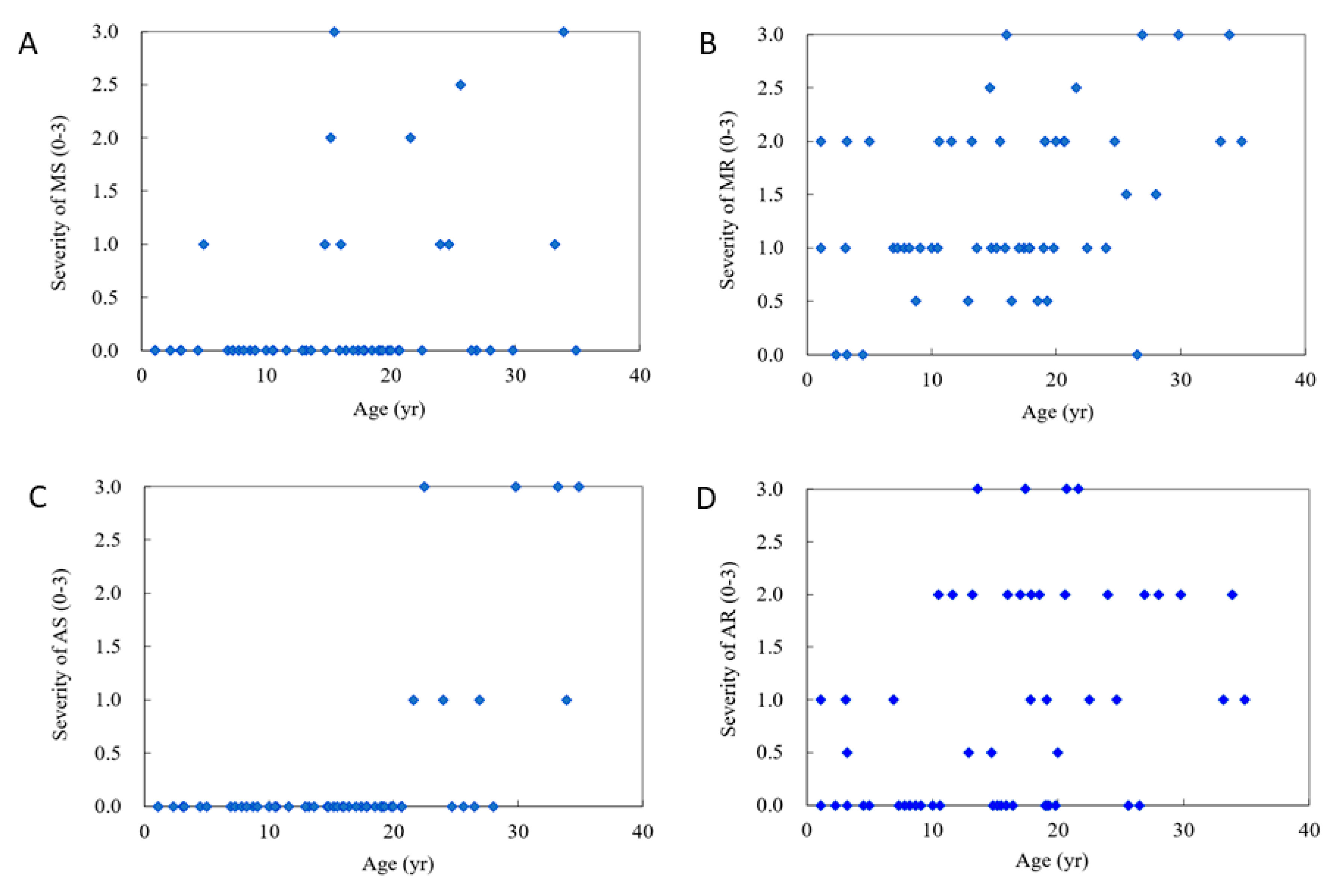
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MPS | Mucopolysaccharidosis |
| GAGs | Glycosaminoglycans |
| H/S | Hurler-Scheie |
| 2D STE | Two-dimensional speckle-tracking echocardiography |
| LV | Left ventricular |
| ERT | Enzyme replacement therapy |
| GLS | Global longitudinal strain |
| E/A | Ratio between early and late (atrial) ventricular filling velocity |
| AS | Aortic stenosis |
| MS | Mitral stenosis |
| LVMI | Left ventricular mass index |
| IVSd | Interventricular septal end-diastolic dimension |
| LVPWd | Left ventricular posterior wall end-diastolic dimension |
| LVIDd | Left ventricular end-diastolic dimension |
| RWT | Relative wall thickness |
References
- Neufeld, E.F.; Muenzer, J. The mucoplysaccharidoses. In The Metabolic and Molecular Bases of Inherited Disease, 8th ed.; Scriver, C.R., Beaudet, A.L., Sly, W.S., Valle, D., Childs, B., Kinzler, K.W., Vogelstein, B., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 3421–3452. [Google Scholar]
- Muenzer, J. Overview of the mucopolysaccharidoses. Rheumatology (Oxford) 2011, 50 (Suppl. 5), v4–v12. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Lin, S.P.; Chuang, C.K.; Niu, D.M.; Chen, M.R.; Tsai, F.J.; Chao, M.C.; Chiu, P.C.; Lin, S.J.; Tsai, L.P.; et al. Incidence of the mucopolysaccharidoses in Taiwan, 1984–2004. Am. J. Med. Genet. A 2009, 149, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Dangel, J.H. Cardiovascular changes in children with mucopolysaccharide storage diseases and related disorders--clinical and echocardiographic findings in 64 patients. Eur. J. Pediatr. 1998, 157, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Fesslová, V.; Corti, P.; Sersale, G.; Rovelli, A.; Russo, P.; Mannarino, S.; Butera, G.; Parini, R. The natural course and the impact of therapies of cardiac involvement in the mucopolysaccharidoses. Cardiol. Young 2009, 19, 170–178. [Google Scholar] [CrossRef]
- Wippermann, C.F.; Beck, M.; Schranz, D.; Huth, R.; Michel-Behnke, I.; Jüngst, B.K. Mitral and aortic regurgitation in 84 patients with mucopolysaccharidoses. Eur. J. Pediatr. 1995, 154, 98–101. [Google Scholar] [CrossRef]
- Braunlin, E.A.; Harmatz, P.R.; Scarpa, M.; Furlanetto, B.; Kampmann, C.; Loehr, J.P.; Ponder, K.P.; Roberts, W.C.; Rosenfeld, H.M.; Giugliani, R. Cardiac disease in patients with mucopolysaccharidosis: Presentation, diagnosis and management. J. Inherit. Metab. Dis. 2011, 34, 1183–1197. [Google Scholar] [CrossRef]
- Leal, G.N.; de Paula, A.C.; Leone, C.; Kim, C.A. Echocardiographic study of paediatric patients with mucopolysaccharidosis. Cardiol Young 2010, 20, 254–261. [Google Scholar] [CrossRef]
- Mohan UR, Hay AA, Cleary MA, Wraith JE, Patel RG: Cardiovascular changes in children with mucopolysaccharide disorders. Acta Paediatr. 2002, 91, 799–804. [CrossRef]
- Brands, M.M.; Frohn-Mulder, I.M.; Hagemans, M.L.; Hop, W.C.; Oussoren, E.; Helbing, W.A.; van der Ploeg, A.T. Mucopolysaccharidosis: Cardiologic features and effects of enzyme-replacement therapy in 24 children with MPS I, II and VI. J. Inherit. Metab. Dis. 2013, 36, 227–234. [Google Scholar] [CrossRef]
- Chen, M.R.; Lin, S.P.; Hwang, H.K.; Yu, C.H. Cardiovascular changes in mucopolysaccharidoses in Taiwan. Acta Cardiol. 2005, 60, 51–53. [Google Scholar] [CrossRef]
- Lin, S.M.; Lin, H.Y.; Chuang, C.K.; Lin, S.P.; Chen, M.R. Cardiovascular abnormalities in Taiwanese patients with mucopolysaccharidosis. Mol. Genet. Metab. 2014, 111, 493–498. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chuang, C.K.; Chen, M.R.; Lin, S.M.; Hung, C.L.; Chang, C.Y.; Chiu, P.C.; Tsai, W.H.; Niu, D.M.; Tsai, F.J.; et al. Cardiac structure and function and effects of enzyme replacement therapy in patients with mucopolysaccharidoses I, II, IVA and VI. Mol. Genet. Metab. 2016, 117, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Chen, M.R.; Lin, S.M.; Hung, C.L.; Niu, D.M.; Chuang, C.K.; Lin, S.P. Cardiac Features and Effects of Enzyme Replacement Therapy in Taiwanese Patients with Mucopolysaccharidosis IVA. Orphanet. J. Rare Dis. 2018, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Chen, M.R.; Lin, S.M.; Hung, C.L.; Niu, D.M.; Chang, T.M.; Chuang, C.K.; Lin, S.P. Cardiac Characteristics and Natural Progression in Taiwanese Patients with Mucopolysaccharidosis III. Orphanet. J. Rare Dis. 2019, 14, 140. [Google Scholar] [CrossRef] [PubMed]
- Braunlin, E.A.; Berry, J.M.; Whitley, C.B. Cardiac findings after enzyme replacement therapy for mucopolysaccharidosis type I. Am. J. Cardiol. 2006, 98, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Almássy, Z.; Beck, M.; Burt, K.; Clarke, J.T.; Giugliani, R.; Hendriksz, C.; Kroepfl, T.; Lavery, L.; Lin, S.P.; et al. Mortality and cause of death in mucopolysaccharidosis type II-a historical review based on data from the Hunter Outcome Survey (HOS). J. Inherit. Metab. Dis. 2009, 32, 534–543. [Google Scholar] [CrossRef]
- Lavery, C.; Hendriksz, C. Mortality in patients with Morquio syndrome A. JIMD Rep. 2015, 15, 59–66. [Google Scholar]
- Lin, H.Y.; Chuang, C.K.; Huang, Y.H.; Tu, R.Y.; Lin, F.J.; Lin, S.J.; Chiu, P.C.; Niu, D.M.; Tsai, F.J.; Hwu, W.L.; et al. Causes of death and clinical characteristics of 34 patients with Mucopolysaccharidosis II in Taiwan from 1995–2012. Orphanet. J. Rare Dis. 2016, 11, 85. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lin, S.P.; Chuang, C.K.; Chen, M.R.; Chen, B.F.; Wraith, J.E. Mucopolysaccharidosis I under enzyme replacement therapy with laronidase-a mortality case with autopsy report. J. Inherit. Metab. Dis. 2005, 28, 1146–1148. [Google Scholar] [CrossRef]
- Andrade, M.F.A.; Guimarães, I.C.B.; Acosta, A.X.; Leão, E.K.E.A.; Moreira, M.I.G.; Mendes, C.M.C. Left ventricular assessment in patients with mucopolysaccharidosis using conventional echocardiography and myocardial deformation by two-dimensional speckle-tracking method. J. Pediatr. (Rio J.) 2019, 95, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Mor-Avi, V.; Lang, R.M.; Badano, L.P.; Belohlavek, M.; Cardim, N.M.; Derumeaux, G. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur. J. Echocardiogr. 2011, 12, 167–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Khoury, D.S.; Yue, Y.; Torre-Amione, G.; Nagueh, S.F. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur. Heart. J. 2008, 29, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Kouzu, H.; Yuda, S.; Muranaka, A.; Doi, T.; Yamamoto, H.; Shimoshige, S.; Hase, M.; Hashimoto, A.; Saitoh, S.; Tsuchihashi, K.; et al. Left ventricular hypertrophy causes different changes in longitudinal, radial, and circumferential mechanics in patients with hypertension: A two-dimensional speckle tracking study. J. Am. Soc. Echocardiogr. 2011, 24, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Vinereanu, D.; Nicolaides, E.; Tweddel, A.C.; Mädler, C.F.; Holst, B.; Boden, L.E.; Cinteza, M.; Rees, A.E.; Fraser, A.G. Subclinical left ventricular dysfunction in asymptomatic patients with Type II diabetes mellitus, related to serum lipids and glycated haemoglobin. Clin. Sci. (Lond) 2003, 105, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.R.; Ko, H.S.; Chao, T.F.; Liu, H.C.; Kuo, J.Y.; Bulwer, B.E.; Yeh, H.I.; Hung, C.L. Relation of myocardial systolic mechanics to serum ferritin level as a prognosticator in thalassemia patients undergoing repeated transfusion. Echocardiography 2015, 32, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Nijmeijer, S.C.M.; de Bruin-Bon, R.H.A.C.M.; Wijburg, F.A.; Kuipers, I.M. Cardiac disease in mucopolysaccharidosis type III. J. Inherit. Metab. Dis. 2019, 42, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Pezzullo, E.; Schiano Lomoriello, V.; Sorrentino, R.; Lo Iudice, F.; Cocozza, S.; Della Casa, R.; Parenti, G.; Strisciuglio, P.; Trimarco, B.; et al. Myocardial deformation in pediatric patients with mucopolysaccharidoses: A two-dimensional speckle tracking echocardiography study. Echocardiography 2017, 34, 240–249. [Google Scholar] [CrossRef]
- Chuang, C.K.; Lin, S.P.; Lin, S.J.; Wang, T.J. MPS screening methods, the Berry spot and acid turbidity tests, cause a high incidence of false-negative results in sanfilippo and morquio syndromes. J. Clin. Lab. Anal. 2002, 16, 253–258. [Google Scholar]
- Chuang, C.K.; Lin, H.Y.; Wang, T.J.; Tsai, C.C.; Liu, H.L.; Lin, S.P. A modified liquid chromatography/tandem mass spectrometry method for predominant disaccharide units of urinary glycosaminoglycans in patients with mucopolysaccharidoses. Orphanet. J. Rare Dis. 2014, 9, 135. [Google Scholar] [CrossRef][Green Version]
- Hung, C.L.; Goncalves, A.; Lai, Y.J.; Lai, Y.H.; Sung, K.T.; Lo, C.I.; Liu, C.C.; Kuo, J.Y.; Hou, C.J.Y.; Chao, T.F.; et al. Light to Moderate Habitual Alcohol Consumption Is Associated with Subclinical Ventricular and Left Atrial Mechanical Dysfunction in an Asymptomatic Population: Dose-Response and Propensity Analysis. J. Am. Soc. Echocardiogr. 2016, 29, 1043–1051. [Google Scholar] [CrossRef]
- Marcus, K.A.; Mavinkurve-Groothuis, A.M.; Barends, M.; van Dijk, A.; Feuth, T.; de Korte, C.; Kapusta, L. Reference values for myocardial two-dimensional strain echocardiography in a healthy pediatric and young adult cohort. J. Am. Soc. Echocardiogr. 2011, 24, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart. J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Eidem, B.W.; McMahon, C.J.; Cohen, R.R.; Wu, J.; Finkelshteyn, I.; Kovalchin, J.P.; Ayres, N.A.; Bezold, L.I.; Smith, E.O.B.; Pignatelli, R.H. Impact of cardiac growth on Doppler tissue imaging velocities: A study in healthy children. J. Am. Soc. Echocardiogr. 2004, 17, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quiñones, M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J. Am. Soc. Echocardiogr. 2009, 22, 1–23. [Google Scholar] [CrossRef]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar]
- Foster, B.J.; Mackie, A.S.; Mitsnefes, M.; Ali, H.; Mamber, S.; Colan, S.D. A novel method of expressing left ventricular mass relative to body size in children. Circulation 2008, 117, 2769–2775. [Google Scholar] [CrossRef]
- Kampmann, C.; Wiethoff, C.M.; Wenzel, A.; Stolz, G.; Betancor, M.; Wippermann, C.F.; Huth, R.G.; Habermehl, P.; Knuf, M.; Emschermann, T. Normal values of M mode echocardiographic measurements of more than 2000 healthy infants and children in central Europe. Heart 2000, 83, 667–672. [Google Scholar] [CrossRef]
- Singh, A.; Voss, W.B.; Lentz, R.W.; Thomas, J.D.; Akhter, N. The Diagnostic and Prognostic Value of Echocardiographic Strain. JAMA Cardiol. 2019, 4, 580–588. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lee, C.L.; Lo, Y.T.; Wang, T.J.; Huang, S.F.; Chen, T.L.; Wang, Y.S.; Niu, D.M.; Chuang, C.K.; Lin, S.P. The Relationships between Urinary Glycosaminoglycan Levels and Phenotypes of Mucopolysaccharidoses. Mol. Genet. Genomic. Med. 2018, 6, 982–992. [Google Scholar] [CrossRef]
- Collins, J.A.; Munoz, J.V.; Patel, T.R.; Loukas, M.; Tubbs, R.S. The anatomy of the aging aorta. Clin. Anat. 2014, 27, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Bolourchi, M.; Renella, P.; Wang, R.Y. Aortic Root Dilatation in Mucopolysaccharidosis I-VII. Int. J. Mol. Sci. 2016, 17, 2004. [Google Scholar] [CrossRef] [PubMed]
- Konstam, M.A.; Kramer, D.G.; Patel, A.R.; Maron, M.S.; Udelson, J.E. Left ventricular remodeling in heart failure: Current concepts in clinical significance and assessment. JACC Cardiovasc. Imaging 2011, 4, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.P.; Lin, H.Y.; Wang, T.J.; Chang, C.Y.; Lin, C.H.; Huang, S.F.; Tsai, C.C.; Liu, H.L.; Keutzer, J.; Chuang, C.K. A Pilot Newborn Screening Program for Mucopolysaccharidosis Type I in Taiwan. Orphanet. J. Rare Dis. 2013, 8, 147. [Google Scholar] [CrossRef]
- Chuang, C.K.; Lin, H.Y.; Wang, T.J.; Huang, Y.H.; Chan, M.J.; Liao, H.C.; Lo, Y.T.; Wang, L.Y.; Tu, R.Y.; Fang, Y.Y.; et al. Status of newborn screening and follow up investigations for Mucopolysaccharidoses I and II in Taiwan. Orphanet. J. Rare Dis. 2018, 13, 84. [Google Scholar] [CrossRef]
- Chan, M.J.; Liao, H.C.; Gelb, M.H.; Chuang, C.K.; Liu, M.Y.; Chen, H.J.; Kao, S.M.; Lin, H.Y.; Huang, Y.H.; Kumar, A.B. Taiwan National Newborn Screening Program by Tandem Mass Spectrometry for Mucopolysaccharidoses Types, I, II, and VI. J. Pediatr. 2019, 205, 176–182. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lee, C.L.; Lo, Y.T.; Tu, R.Y.; Chang, Y.H.; Chang, C.Y.; Chiu, P.C.; Chang, T.M.; Tsai, W.H.; Niu, D.M.; et al. An At-Risk Population Screening Program for Mucopolysaccharidoses by Measuring Urinary Glycosaminoglycans in Taiwan. Diagnostics 2019, 9, 140. [Google Scholar] [CrossRef]
- Levy, P.T.; Machefsky, A.; Sanchez, A.A.; Patel, M.D.; Rogal, S.; Fowler, S.; Yaeger, L.; Hardi, A.; Holland, M.R.; Hamvas, A.; et al. Reference Ranges of Left Ventricular Strain Measures by Two-Dimensional Speckle-Tracking Echocardiography in Children: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2016, 29, 209–225. [Google Scholar] [CrossRef]
- Cantinotti, M.; Scalese, M.; Giordano, R.; Franchi, E.; Assanta, N.; Marotta, M.; Viacava, C.; Molinaro, S.; Iervasi, G.; Santoro, G.; et al. Normative Data for Left and Right Ventricular Systolic Strain in Healthy Caucasian Italian Children by Two-Dimensional Speckle-Tracking Echocardiography. J. Am. Soc. Echocardiogr. 2018, 31, 712–720. [Google Scholar] [CrossRef]


| No. | Gender | MPS Type | Age (Years) | ERT Duration (Years) | GLS z Score | LVMI z Score | IVSd z Score | LVPWd z Score | AoD z Score | EF (%) | SF (%) | E/A Ratio | RWT | Left Ventricular Remodeling Pattern | MS | MR | AS | AR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | I (H/S) | 1.1 | 1.9 | 1.4 | 2.9 | 2.1 | −0.1 | 54 | 27 | 3.22 | 0.413 | Normal geometry | 0 | 2 | 0 | 1 | |
| 2 | F | I (H/S) | 1.1 | 0.8 | −0.4 | 2.3 | 1.7 | 3.6 | 71 | 35 | 1.44 | 0.449 | Concentric remodeling | 0 | 1 | 0 | 0 | |
| 3 | F | I (H/S) | 33.9 | 2.7 | 0.3 | 1.0 | 1.1 | 4.4 | 62 | 33 | 0.94 | 0.337 | Normal geometry | 3 | 3 | 1 | 2 | |
| 4 | M | I (Scheie) | 13.2 | 2.8 | 0.5 | 1.1 | 0.7 | 0.4 | 71 | 40 | 2.56 | 0.346 | Normal geometry | 0 | 2 | 0 | 2 | |
| 5 | F | I (Scheie) | 22.5 | 0.3 | −0.9 | 1.7 | 0.2 | −0.4 | 71 | 40 | 1.71 | 0.423 | Concentric remodeling | 0 | 1 | 3 | 1 | |
| 6 | M | I (Scheie) | 33.2 | 4.0 | 1.4 | 1.7 | 0.7 | 1.4 | 66 | 37 | 1.16 | 0.376 | Normal geometry | 1 | 2 | 3 | 1 | |
| 7 | M | I (Scheie) | 34.9 | 0.0 | −0.2 | 0.7 | 0.4 | 0.9 | 60 | 32 | 0.91 | 0.380 | Normal geometry | 0 | 2 | 3 | 1 | |
| 8 | M | II (mild) | 6.9 | 1.2 | 1.1 | 3.2 | 1.5 | 3.3 | 74 | 42 | 1.05 | 0.434 | Concentric remodeling | 0 | 1 | 0 | 1 | |
| 9 | M | II (mild) | 7.3 | 2.2 | −1.6 | 0.8 | −0.2 | 7.0 | 68 | 37 | 1.44 | 0.348 | Normal geometry | 0 | 1 | 0 | 0 | |
| 10 | M | II (mild) | 10.0 | 5.0 | 0.0 | −1.4 | −0.9 | −0.1 | 2.9 | 71 | 39 | 1.73 | 0.335 | Normal geometry | 0 | 1 | 0 | 0 |
| 11 | M | II (mild) | 10.6 | 6.5 | 3.0 | 0.3 | 2.4 | 1.4 | 2.0 | 62 | 32 | 1.13 | 0.455 | Concentric remodeling | 0 | 2 | 0 | 0 |
| 12 | M | II (mild) | 11.6 | 5.7 | 2.5 | 2.8 | 1.6 | 1.7 | 4.2 | 41 | 20 | 0.89 | 0.351 | Eccentric hypertrophy | 0 | 2 | 0 | 2 |
| 13 | M | II (mild) | 17.4 | 2.4 | 2.9 | 2 | 2.3 | 1.2 | 5.3 | 73 | 42 | 2.61 | 0.349 | Normal geometry | 0 | 1 | 0 | 3 |
| 14 | M | II (mild) | 19.8 | 3.5 | 3 | 3.7 | 2.4 | 4.2 | 74 | 43 | 2.92 | 0.421 | Concentric hypertrophy | 0 | 1 | 0 | 0 | |
| 15 | M | II (mild) | 20.6 | 7.4 | 2.0 | 1.3 | 0.5 | 0.3 | 0.7 | 50 | 26 | 1.36 | 0.309 | Normal geometry | 0 | 2 | 0 | 2 |
| 16 | M | II (mild) | 20.7 | 2.4 | 4.5 | 2.7 | 2.6 | 1.9 | 4.7 | 51 | 26 | 1.45 | 0.381 | Eccentric hypertrophy | 0 | 2 | 0 | 3 |
| 17 | M | II (mild) | 24.0 | 6.5 | 1.2 | 1 | 1.9 | 1.4 | 3.7 | 62 | 33 | 0.85 | 0.391 | Normal geometry | 1 | 1 | 1 | 2 |
| 18 | M | II (mild) | 25.6 | 3.5 | 3.4 | 1.8 | 2.7 | 1.7 | 1.3 | 73 | 41 | 0.67 | 0.451 | Concentric remodeling | 2.5 | 1.5 | 0 | 0 |
| 19 | M | II (severe) | 3.1 | −1.3 | −0.5 | 0.7 | 0.9 | 3.8 | 63 | 33 | 1.17 | 0.359 | Normal geometry | 0 | 1 | 0 | 1 | |
| 20 | M | II (severe) | 3.2 | 2.6 | 2.4 | 4.3 | 2.5 | 3.8 | 63 | 33 | 1.48 | 0.421 | Concentric hypertrophy | 0 | 2 | 0 | 0.5 | |
| 21 | M | II (severe) | 14.7 | 3.7 | 0.8 | 4.1 | 2.1 | 2.4 | 76 | 44 | 0.95 | 0.4204 | Concentric remodeling | 1 | 2.5 | 0 | 0.5 | |
| 22 | M | II (severe) | 17.9 | 1.7 | 1.4 | 1.4 | 1.5 | 2.6 | 76 | 44 | 1.42 | 0.382 | Normal geometry | 0 | 1 | 0 | 2 | |
| 23 | M | II (severe) | 26.9 | 3.5 | 1.3 | 3.9 | 2.1 | 6.0 | 65 | 35 | 1.14 | 0.444 | Concentric remodeling | 0 | 3 | 1 | 2 | |
| 24 | F | IIIA | 8.7 | 2.4 | −3.2 | 0.0 | −0.6 | 0.9 | 52 | 26 | 1.31 | 0.301 | Normal geometry | 0 | 0.5 | 0 | 0 | |
| 25 | F | IIIB | 10.5 | 0.0 | −0.4 | 1.4 | 0.7 | 1.8 | 71 | 40 | 1.46 | 0.366 | Normal geometry | 0 | 1 | 0 | 2 | |
| 26 | F | IIIB | 12.9 | 1.4 | −1.6 | 0.1 | 0.4 | 1.3 | 75 | 43 | 1.06 | 0.391 | Normal geometry | 0 | 0.5 | 0 | 0.5 | |
| 27 | M | IIIA | 13.6 | 1.5 | 0.5 | 0.9 | 0.4 | 1.7 | 73 | 42 | 1.26 | 0.338 | Normal geometry | 0 | 1 | 0 | 3 | |
| 28 | M | IIIA | 16.4 | 4.2 | −0.2 | 2.0 | 0.9 | 5.2 | 68 | 38 | 1.37 | 0.382 | Normal geometry | 0 | 0.5 | 0 | 0 | |
| 29 | F | IIIB | 18.5 | 2.5 | 0.6 | 2.4 | 1.5 | 2.4 | 60 | 32 | 0.88 | 0.345 | Normal geometry | 0 | 0.5 | 0 | 2 | |
| 30 | F | IIIB | 19.1 | 0.7 | −0.7 | 1.0 | 0.1 | 2.6 | 69 | 38 | 0.89 | 0.349 | Normal geometry | 0 | 2 | 0 | 0 | |
| 31 | F | IIIB | 19.1 | 0.8 | −0.6 | 0.2 | 0.8 | 3.8 | 72 | 41 | 0.93 | 0.382 | Normal geometry | 0 | 2 | 0 | 1 | |
| 32 | M | IIIC | 26.5 | 2.6 | −0.9 | 0.5 | −0.1 | 2.7 | 56 | 29 | 1.08 | 0.333 | Normal geometry | 0 | 0 | 0 | 0 | |
| 33 | M | IVA | 2.3 | 0.3 | − 4 | 0.4 | 0.5 | 2.5 | 62 | 31 | 1.11 | 0.476 | Concentric remodeling | 0 | 0 | 0 | 0 | |
| 34 | M | IVA | 3.2 | 1.7 | −0.9 | −0.7 | 0.4 | 0.3 | 2.5 | 72 | 40 | 1.73 | 0.312 | Normal geometry | 0 | 0 | 0 | 0 |
| 35 | M | IVA | 4.5 | 1.7 | −2.5 | −1.5 | 0.1 | 0.5 | 2.5 | 81 | 49 | 1.24 | 0.347 | Normal geometry | 0 | 0 | 0 | 0 |
| 36 | F | IVA | 7.8 | −1.0 | 0.8 | 2.3 | 1.9 | 1.0 | 56 | 28 | 1.10 | 0.394 | Normal geometry | 0 | 1 | 0 | 0 | |
| 37 | F | IVA | 8.2 | 0.3 | 0 | 3.5 | 1.6 | 3.7 | 71 | 39 | 1.16 | 0.429 | Concentric remodeling | 0 | 1 | 0 | 0 | |
| 38 | M | IVA | 9.1 | 1.8 | 0.8 | −0.9 | 0.0 | −0.2 | 3.9 | 61 | 31 | 1.40 | 0.297 | Normal geometry | 0 | 1 | 0 | 0 |
| 39 | F | IVA | 14.8 | 1.8 | −1.1 | 1.3 | 0.6 | 2.6 | 72 | 40 | 1.82 | 0.373 | Normal geometry | 0 | 1 | 0 | 0 | |
| 40 | M | IVA | 15.9 | 2.0 | 0.3 | −0.9 | 1.2 | 0.8 | 8.4 | 70 | 38 | 1.42 | 0.375 | Normal geometry | 0 | 1 | 0 | 0 |
| 41 | F | IVA | 17.0 | 2.2 | 1.8 | 1.6 | 0.7 | 0.1 | 5.3 | 76 | 45 | 1.42 | 0.255 | Normal geometry | 0 | 1 | 0 | 2 |
| 42 | F | IVA | 17.8 | 1.2 | 1.3 | 2.9 | 2.7 | 4.9 | 68 | 37 | 1.19 | 0.448 | Concentric remodeling | 0 | 1 | 0 | 1 | |
| 43 | F | IVA | 19.0 | 2.2 | 0.1 | 1.1 | 1.3 | 0.5 | 5.3 | 70 | 39 | 1.06 | 0.349 | Normal geometry | 0 | 1 | 0 | 0 |
| 44 | F | IVA | 19.3 | 4.6 | 1.5 | 1.2 | 0.5 | 4.5 | 67 | 36 | 1.29 | 0.312 | Normal geometry | 0 | 0.5 | 0 | 0 | |
| 45 | F | IVA | 20.0 | 1.2 | −0.8 | 0.5 | 0.1 | 6.8 | 78 | 45 | 1.06 | 0.323 | Normal geometry | 0 | 2 | 0 | 0.5 | |
| 46 | M | IVA | 28.0 | 2.3 | 0.1 | 2.6 | 2.1 | 1.0 | 6.3 | 53 | 26 | 1.06 | 0.324 | Eccentric hypertrophy | 0 | 1.5 | 0 | 2 |
| 47 | M | VI | 5.0 | −0.5 | −2 | 0.1 | 0.0 | 1.0 | 65 | 34 | 0.91 | 0.321 | Normal geometry | 1 | 2 | 0 | 0 | |
| 48 | M | VI | 15.2 | 7.7 | 3.9 | 0.5 | 3.3 | 3.2 | 3.8 | 72 | 39 | 9.40 | 0.510 | Concentric remodeling | 2 | 1 | 0 | 0 |
| 49 | F | VI | 15.5 | 1.5 | 4.2 | 0.4 | 2.8 | 1.2 | 1.2 | 75 | 43 | 0.81 | 0.424 | Concentric remodeling | 3 | 2 | 0 | 0 |
| 50 | M | VI | 16.0 | 7.7 | 4.5 | 2.5 | 2.3 | 1.5 | 4.6 | 76 | 44 | 1.11 | 0.371 | Eccentric hypertrophy | 1 | 3 | 0 | 2 |
| 51 | F | VI | 21.6 | 9.9 | 2.3 | 3.9 | 2.2 | 1.5 | 2.6 | 50 | 25 | 0.83 | 0.323 | Eccentric hypertrophy | 2 | 2.5 | 1 | 3 |
| 52 | F | VI | 24.7 | 8.0 | 2.2 | 0.7 | 2.1 | 1.5 | 3.0 | 72 | 40 | 1.35 | 0.463 | Concentric remodeling | 1 | 2 | 0 | 1 |
| 53 | F | VI | 29.8 | 3.3 | 0.6 | −0.5 | 2.0 | 1.1 | 0.2 | 76 | 44 | 1.22 | 0.379 | Normal geometry | 0 | 3 | 3 | 2 |
| MPS Type | n | Gender (M/F) | Age (Years) | Age Range (Years) | GLS z Score | LVMI z Score | IVSd z Score | LVPWd z Score | AoD z Score |
|---|---|---|---|---|---|---|---|---|---|
| MPS I (H/S) | 3 | 0/3 | 12.0 (18.9) | 1.1–33.9 | 1.82 (0.93) | 0.43 (0.91) | 2.04 (0.95) | 1.67 (0.50) | 2.62 (2.38) |
| MPS I (Scheie) | 4 | 3/1 | 26.0 (10.1) | 13.2–34.9 | 1.78 (1.95) | 0.20 (0.98) | 1.31 (0.47) | 0.51 (0.25) | 1.69 (1.66) |
| MPS II | 16 | 16/0 | 15.0 (7.7) | 6.9–26.9 | 2.29 (1.48) | 1.15 (1.39) | 2.20 (1.47) | 1.39 (0.81) | 3.61 (1.66) |
| MPS III | 9 | 3/6 | 16.1 (5.4) | 8.7–26.5 | 1.77 (1.25) | −0.72 (1.15) | 0.95 (0.86) | 0.45 (0.64) | 2.49 (1.33) |
| MPS IVA | 14 | 6/8 | 13.3 (7.6) | 2.3–28.0 | 0.58 (1.65) | −0.07 (1.69) | 1.29 (1.06) | 0.79 (0.78) | 4.30 (2.02) |
| MPS VI | 7 | 3/4 | 18.3 (8.0) | 5.0–29.8 | 2.48 (1.90) | 0.79 (1.93) | 2.13 (0.99) | 1.45 (0.95) | 2.35 (1.59) |
| Total | 53 | 31/22 | 15.3 (8.2) | 1.1–34.9 | 1.71 (1.66) | 0.35 (1.57) | 1.66 (1.20) | 1.03 (0.84) | 3.15 (1.93) |
| MPS Type | n | Gender (M/F) | Age (Years) | MS | MR | AS | AR |
|---|---|---|---|---|---|---|---|
| MPS I (H/S) | 3 | 0/3 | 12.0 (18.9) | 1.00 | 2.00 | 0.33 | 1.00 |
| MPS I (Scheie) | 4 | 3/1 | 26.0 (10.1) | 0.25 | 1.75 | 2.25 | 1.25 |
| MPS II | 16 | 16/0 | 15.0 (7.7) | 0.28 | 1.56 | 0.13 | 1.19 |
| MPS III | 9 | 3/6 | 16.1 (5.4) | 0.00 | 0.89 | 0.00 | 0.94 |
| MPS IVA | 14 | 6/8 | 13.3 (7.6) | 0.00 | 0.86 | 0.00 | 0.39 |
| MPS VI | 7 | 3/4 | 18.3 (8.0) | 1.43 | 2.21 | 0.57 | 1.14 |
| Total | 53 | 31/22 | 15.3 (8.2) | 0.35 | 1.39 | 0.30 | 0.92 |
| Left Ventricular Remodeling Pattern | Normal | Concentric Remodeling | Eccentric Hypertrophy | Concentric Hypertrophy |
|---|---|---|---|---|
| MPS I (n = 7) | 5 (71%) | 2 (29%) | 0 | 0 |
| MPS II (n =16) | 7 (44%) | 5 (31%) | 2 (13%) | 2 (13%) |
| MPS III (n = 9) | 9 (100%) | 0 | 0 | 0 |
| MPS IVA (n = 14) | 10 (71%) | 3 (21%) | 1 (7%) | 0 |
| MPS VI (n = 7) | 2 (29%) | 3 (43%) | 2 (29%) | 0 |
| Total (n = 53) | 33 (62%) | 13 (25%) | 5 (9%) | 2 (4%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-Y.; Chuang, C.-K.; Lee, C.-L.; Chen, M.-R.; Sung, K.-T.; Lin, S.-M.; Hou, C.J.-Y.; Niu, D.-M.; Chang, T.-M.; Hung, C.-L.; et al. Cardiac Evaluation Using Two-Dimensional Speckle-Tracking Echocardiography and Conventional Echocardiography in Taiwanese Patients with Mucopolysaccharidoses. Diagnostics 2020, 10, 62. https://doi.org/10.3390/diagnostics10020062
Lin H-Y, Chuang C-K, Lee C-L, Chen M-R, Sung K-T, Lin S-M, Hou CJ-Y, Niu D-M, Chang T-M, Hung C-L, et al. Cardiac Evaluation Using Two-Dimensional Speckle-Tracking Echocardiography and Conventional Echocardiography in Taiwanese Patients with Mucopolysaccharidoses. Diagnostics. 2020; 10(2):62. https://doi.org/10.3390/diagnostics10020062
Chicago/Turabian StyleLin, Hsiang-Yu, Chih-Kuang Chuang, Chung-Lin Lee, Ming-Ren Chen, Kuo-Tzu Sung, Shan-Miao Lin, Charles Jia-Yin Hou, Dau-Ming Niu, Tung-Ming Chang, Chung-Lieh Hung, and et al. 2020. "Cardiac Evaluation Using Two-Dimensional Speckle-Tracking Echocardiography and Conventional Echocardiography in Taiwanese Patients with Mucopolysaccharidoses" Diagnostics 10, no. 2: 62. https://doi.org/10.3390/diagnostics10020062
APA StyleLin, H.-Y., Chuang, C.-K., Lee, C.-L., Chen, M.-R., Sung, K.-T., Lin, S.-M., Hou, C. J.-Y., Niu, D.-M., Chang, T.-M., Hung, C.-L., & Lin, S.-P. (2020). Cardiac Evaluation Using Two-Dimensional Speckle-Tracking Echocardiography and Conventional Echocardiography in Taiwanese Patients with Mucopolysaccharidoses. Diagnostics, 10(2), 62. https://doi.org/10.3390/diagnostics10020062









