Craniofacial Morphology in Children with Growth Hormone Deficiency and Turner Syndrome
Abstract
:1. Introduction
2. Literature Review
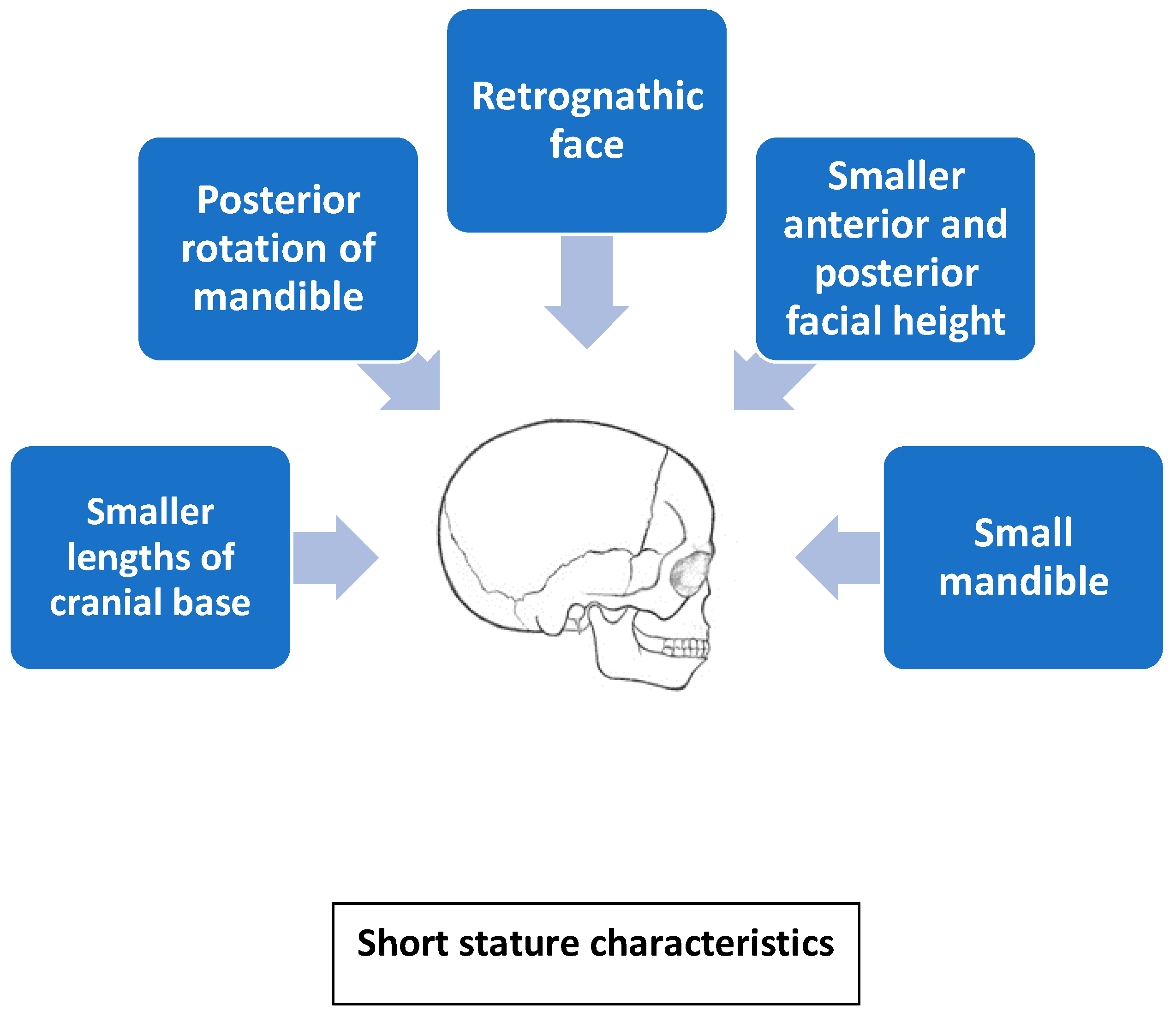
2.1. Craniofacial Morphology in Children with GHD and IGHD
2.2. Craniofacial Morphology in Children with IGHD
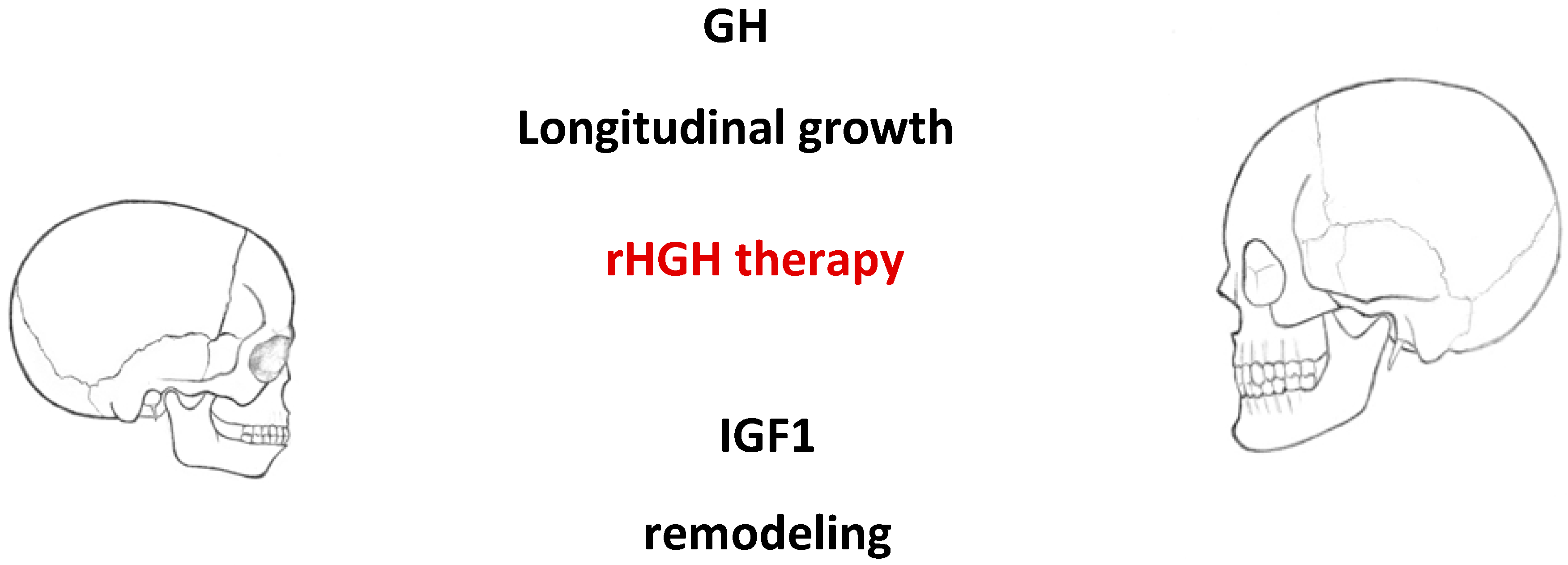
2.3. Effects of GH in Children with IGHD
2.4. Mean SD for N-ANS, A-Ptm, and Cd-Go Following Administration of GH
2.5. Turner Syndrome
2.6. Orthodontic Treatment in Children Affected by Disorders Associated with GH Deficiency in the Aspect of Craniofacial Growth—Review of Case Reports
3. Future Perspectives—Growth Hormone Receptor Polymorphism and Craniofacial Morphology
4. Conclusions
- Children subjected to long-term GH therapy (i.e., more than two years) showed augmented growth of the craniofacial skeleton, especially the maxilla and mandibular rami.
- According to these findings, GH accelerates craniofacial development, improving occlusion and facial profile.
- Treatment with human recombinant growth hormone positively influences longitudinal growth and bone remodeling. The period of treatment seems to be a good time for orthodontic treatment in children with some orthodontic anomalies.
- Long-term GH therapy in patients with Turner syndrome has a positive effect on craniofacial development; GH exerts the strongest effect on posterior facial height and mandibular ramus. However, GH is not able to compensate for the absence of the X chromosome and correct craniofacial features.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GHD | growth hormone deficiency |
| rHGH | recombinant human growth hormone |
| GH | growth hormone |
| IGF-I | insulin-like growth factor-I |
| MSCs | mesenchymal stem cells |
| TGFβ | transforming growth factorβ |
| BMPs | bone morphogenetic protein |
| FGFs | fibroblast growth factor |
| RANK-L | receptor activator of nuclear factor κB |
| IGHD | Isolated GHD |
| N-ANS | upper face height—linear measurement from nasion to anterior nasal spine |
| Gn-Cd | total height of the face—gnathion–condylion |
| Cd-Go | height of the mandibular ramus—condylion–gonion |
| N-S | anterior cranial base—nasion–sella |
| ANS-Me | lower facial height—anterior nasal spine–menton |
| A-Ptm | mandibular length—subspinale–pterygomaxillary fissure |
| Pog-Go | mandibular body length—pogonion–gonion |
| SNA | angle between sella, nasion, and subspinale point A |
| N-Me | total facial height dimensions—nasion–menton |
| N-ANS | upper anterior facial height—nasion–anterior nasal spine |
| TS | Turner syndrome |
| IGF | insulin-like growth factor |
| IGFBP | IGF binding protein |
| ALS | acid-labile subunit |
| HRT | hormone replacement therapy |
| SHOX | short stature homeobox-containing gene |
| P561T | P561T heterozygous missense mutation in the growth hormone receptor |
| d3-GHR | d3-growth hormone receptor, an isoform of GHR that lacks exon 3 |
| LD | linkage disequilibrium |
References
- Pirinen, S. Endocrine regulation of craniofacial growth. Acta Odontol. Scand. 1995, 53, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.-H. Fixed-functional appliance treatment combined with growth hormone therapy. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Almengló, C.; Devesa, P. Multiple Effects of Growth Hormone in the Body: Is it Really the—Hormone for Growth? Clin. Med. Insights: Endocrinol. Diabetes 2016, 9, 47–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, T.L.; Sondergaard, T.E.; Skorzynska, K.E.; Dagnaes-Hansen, F.; Plesner, T.L.; Hauge, E.M.; Plesner, T.; Delaisse, J.-M. A Physical Mechanism for Coupling Bone Resorption and Formation in Adult Human Bone. Am. J. Pathol. 2009, 174, 239–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohlsson, C.; Bengtsson, B.; Isaksson, O.G.P.; Andreassen, T.T.; Slootweg, M.C. Growth hormone and bone. Endocr. Rev. 1998, 19, 55–79. [Google Scholar] [PubMed] [Green Version]
- Krishnan, V. Regulation of bone mass by Wnt signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [CrossRef]
- Hou, P.; Sato, T.; Hofstetter, W.; Foged, N.T. Identification and Characterization of the Insulin-like Growth Factor I Receptor in Mature Rabbit Osteoclasts. J. Bone Min. Res 1997, 12, 534–540. [Google Scholar] [CrossRef]
- Niu, T.; Rosen, C.J. The insulin-like growth factor-I gene and osteoporosis: A critical appraisal. Gene 2005, 361, 38–56. [Google Scholar] [CrossRef]
- Mazziotti, G.; Bianchi, A.; Bonadonna, S.; Cimino, V.; Patelli, I.; Fusco, A.; Pontecorvi, A.; De Marinis, L.; Giustina, A. Prevalence of Vertebral Fractures in Men with Acromegaly. J. Clin. Endocrinol. Metab. 2008, 93, 4649–4655. [Google Scholar] [CrossRef] [Green Version]
- Mrak, E.; Villa, I.; Lanzi, R.T.; Losa, M.; Guidobono, F.; Rubinacci, A. Growth hormone stimulates osteoprotegerin expression and secretion in human osteoblast-like cells. J. Endocrinol. 2007, 192, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Menagh, P.J.; Turner, R.; Jump, D.B.; Wong, C.P.; Lowry, M.B.; Yakar, S.; Rosen, C.J.; Iwaniec, U.T. Growth Hormone Regulates the Balance Between Bone Formation and Bone Marrow Adiposity. J. Bone Miner. Res. 2009, 25, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Olney, R.C. Regulation of bone mass by growth hormone. Med. Pediatr. Oncol. 2003, 41, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.; Anawalt, B.; Boyce, A.; Chrousos, G.; Dungan, K.; Grossman, A.; Hershman, J.; Kaltsas, G.; Koch, C.; Kopp, P.; et al. Disorders of Growth Hormone in Childhood—Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Sinona, B.; Franceca, G.; Alessandro, R.; Isabella, V. The Role of Growth Hormone in Mesenchymal Stem Cell Commitment. Int. J. Mol. Sci. 2019, 20, 5264. [Google Scholar]
- Hwang, C.-J.; Cha, J.-Y. Orthodontic treatment with growth hormone therapy in a girl of short stature. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Fan, D.; Hwang, M.-S.; Lee, H.-K.; Hwang, C.-J. Effect of growth hormone treatment on craniofacial growth in children: Idiopathic short stature versus growth hormone deficiency. J. Formos. Med. Assoc. 2017, 116, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Juloski, J.; Dumančić, J.; Šćepan, I.; Lauc, T.; Milašin, J.; Kaić, Z.; Dumić, M.; Babić, M. Growth hormone positive effects on craniofacial complex in Turner syndrome. Arch. Oral Biol. 2016, 71, 10–15. [Google Scholar] [CrossRef]
- Funatsu, M.; Sato, K.; Mitani, H. Effects of Growth Hormone on Craniofacial Growth: Duration of Replacement Therapy. Angle Orthod. 2006, 76, 970–977. [Google Scholar] [CrossRef] [Green Version]
- Kjellberg, H.; Beiring, M.; Wikland, K.A. Craniofacial morphology, dental occlusion, tooth eruption, and dental maturity. Oral Sci. 2000, 108, 359–367. [Google Scholar] [CrossRef]
- Davidopoulou, S.; Chatzigianni, A. Craniofacial morphology and dental maturity in children with reduced somatic growth of different aetiology and the effect of growth hormone treatment. Prog. Orthod. 2017, 18, 10. [Google Scholar] [CrossRef] [Green Version]
- Wit, J.M.; Oostdijk, W.; Losekoot, M.; van Duyvenvoorde, H.A.; Ruivenkamp, C.A.L.; Kant, S.G. Mechanism in endocrinology: Novel genetic causes of short stature. Eur. J. Endocrinol. 2016, 174, R145–R173. [Google Scholar] [CrossRef] [Green Version]
- MacGillivray, M.H. Disorders of Growth and Development. Endocrinology and Metabolism, 2nd ed.; McGraw-Hill Book Co.: New York, NY, USA, 1987; pp. 1581–1628. [Google Scholar]
- Poole, A.; Greene, I.; Buschang, P. The effect of growth hormone therapy on longitudinal growth of the oral facial structures in children. Prog. Clin. Biol. Res. 1982, 101, 499–516. [Google Scholar] [PubMed]
- Takano, K.; Ogiuchi, H.; Hizuka, N.; Sangu, Y.; Shizume, K. Oro-maxillofacial development in patients with GH deficiency and in normal short children. Endocrinol. Jpn. 1986, 33, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantu, G.; Buschang, P.H.; Gonzalez, J.L. Differential growth and maturation in idiopathic growth-hormone-deficient children. Eur. J. Orthod. 1997, 19, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevis, R.R.; Hayles, A.B.; Isaacson, R.J.; Sather, A.H. Facial growth response to human growth hormone in hypopituitary dwarfs. Angle Orthod. 1977, 47, 193–205. [Google Scholar]
- Isaksson, O.G.; Lindahl, A.; Nilsson, A.; Isgaard, J. Mechanism of the stimulatory effect of growth hormone on longitudinal bone growth. Endocr. Rev. 1987, 8, 426–438. [Google Scholar] [CrossRef]
- Rongen-Westerlaken, C.; Born, E.V.; Prahl-Andersen, B.; Teunenbroek, A.V.; Manesse, P.; Otten, B.; Tweel, I.V.; Kuijpers-Jagtman, A.; Delemarre vd Waal, H.; Drayer, N.; et al. Effect of growth hormone treatment on craniofacial growth in Turner’s syndrome. Acta Paediatr. 1993, 82, 364–368. [Google Scholar] [CrossRef]
- Forsberg, C.-M. The effect of growth hormone therapy on mandibular and cranial base development in children treated with total body irradiation. Eur. J. Orthod. 2002, 24, 285–292. [Google Scholar] [CrossRef]
- Gravholt, C.H.; Frystyk, J.; Flyvbjerg, A.; Ørskov, H.; Christiansen, J.S. Reduced free IGF-I and increased IGFBP-3 proteolysis in Turner syndrome: Modulation by female sex steroids. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E308–E314. [Google Scholar] [CrossRef] [Green Version]
- Gravholt, C.H.; Chen, J.-W.; Oxvig, C.; Overgaard, M.T.; Christiansen, J.S.; Frystyk, J.; Flyvbjerg, A. The GH–IGF–IGFBP axis is changed in Turner syndrome: Partial normalization by HRT. Growth Horm. Igf Res. 2006, 16, 332–339. [Google Scholar] [CrossRef]
- Rao, E.; Weiss, B.; Fukami, M.; Rump, A.; Niesler, B.; Mertz, A.; Muroya, K.; Binder, G.; Kirsch, S.; Winkelmann, M.; et al. Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nat. Genet. 1997, 16, 54–63. [Google Scholar] [CrossRef]
- Rao, E. The Leri-Weill and Turner syndrome homeobox gene SHOX encodes a cell-type specific transcriptional activator. Hum. Mol. Genet. 2001, 10, 3083–3091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchini, A.; Marttila, T.; Winter, A.; Caldeira, S.; Malanchi, I.; Blaschke, R.J.; Häcker, B.; Rao, E.; Karperien, M.; Wit, J.M.; et al. The Short Stature Homeodomain Protein SHOX Induces Cellular Growth Arrest and Apoptosis and Is Expressed in Human Growth Plate Chondrocytes. J. Biol. Chem. 2004, 279, 37103–37114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaschke, R.J.; Rappold, G. The pseudoautosomal regions, SHOX and disease. Curr. Opin. Genet. Dev. 2006, 16, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Binder, G.; Baur, F.; Schweizer, R.; Ranke, M.B. The d3-Growth Hormone (GH) Receptor Polymorphism Is Associated with Increased Responsiveness to GH in Turner Syndrome and Short Small-for-Gestational-Age Children. J. Clin. Endocrinol. Metab. 2006, 91, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Filipsson, R.; Lindsten, J.; Almqvist, S. Time of eruption of the permanent teeth, cephalometric and tooth measurement and sulphation factor activity in 45 patients with Turner’s syndrome with different tyoes of X chromosome aberrations. Acta Endocrinol. 1965, 48, 91–113. [Google Scholar] [CrossRef]
- Babic, M.; Glisic, B.; Scepan, I. Mandibular growth pattern in Turner’s syndrome. Eur. J. Orthod. 1997, 19, 161–164. [Google Scholar] [CrossRef] [Green Version]
- Dumancic, J.; Kaic, Z.; Varga, M.L.; Lauc, T.; Dumic, M.; Milosevic, S.A.; Brkic, H. Characteristics of the craniofacial complex in Turner syndrome. Arch. Oral Biol. 2010, 55, 81–88. [Google Scholar] [CrossRef]
- Babić, M.; Šćepan, I.; Mićić, M. Comparative cephalometric analysis in patients with X-chromosome aneuploidy. Arch. Oral Biol. 1993, 38, 179–183. [Google Scholar] [CrossRef]
- Grön, M.; Pietilä, K.; Alvesalo, L. The craniofacial complex in 45, X/46, XX females. Arch. Oral Biol. 1999, 44, 1077–1084. [Google Scholar] [CrossRef]
- Peltomäki, T.; Alvesalo, L.; Isotupa, K. Shape of the craniofacial complex in 45, X females: Cephalometric study. J. Craniofac. Genet. Dev. Biol. 1989, 9, 331–338. [Google Scholar]
- Perkiomaki, M.R. The relationship of distinct craniofacial features between Turner syndrome females and their parents. Eur. J. Orthod. 2005, 27, 48–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, K.E. Growth Hormone and Craniofacial Changes: Preliminary Data From Studies in Turner’s Syndrome. Pediatrics 1999, 104, 1021–1024. [Google Scholar] [PubMed]
- Juloski, J.; Glisic, B.; Scepan, I.; Milasin, J.; Mitrovic, K.; Babic, M. Ontogenetic changes of craniofacial complex in Turner syndrome patients treated with growth hormone. Clin. Oral Investig. 2013, 17, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.A. Orthodontic treatment for patients with Turner syndrome. Am. J. Orthod. Dentofac. Orthop. 2001, 120, 314–322. [Google Scholar] [CrossRef]
- Cazzolla, A.P.; Lo Muzio, L.; Di Fede, O.; Lacarbonara, V.; Colaprico, A.; Testa, N.F.; Giuseppe, T.; Zhurakivska, K.; Marzo, G.; Lacaita, M.G. Orthopedic-orthodontic treatment of the patient with Turner’s syndrome: Review of the literature and case report. Spec. Care Dent. 2018, 38, 239–248. [Google Scholar] [CrossRef]
- Kang, E.H.; Yamaguchi, T.; Tajima, A.; Nakajima, T.; Tomoyasu, Y.; Watanabe, M.; Yamaguchi, M.; Park, S.B.; Maki, K.; Inoue, I. Association of the growth hormone receptor gene polymorphisms with mandibular height in a Korean population. Arch. Oral Biol. 2009, 54, 556–562. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, Y.; Gao, X.; Chen, Y.; Lu, J.; Bai, Y.; Shen, Y.; Wang, B. The growth hormone receptor gene is associated with mandibular height in a Chinese population. J. Dent. Res. 2005, 84, 1052–1056. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Maki, K.; Shibasaki, Y. Growth hormone receptor gene variant and mandibular height in the normal Japanese population. Am. J. Orthod. Dentofac. Orthop. 2001, 119, 650–653. [Google Scholar] [CrossRef]
- Dos Santos, C.; Essioux, L.; Teinturier, C.; Tauber, M.; Goffin, V.; Bougnères, P. A common polymorphism of the growth hormone receptor is associated with increased responsiveness to growth hormone. Nat. Genet. 2004, 36, 720–724. [Google Scholar] [CrossRef]
- Jorge, A.A.L.; Marchisotti, F.G.; Montenegro, L.R.; Carvalho, L.R.; Mendonca, B.B.; Arnhold, I.J.P. Growth Hormone (GH) Pharmacogenetics: Influence of GH Receptor Exon 3 Retention or Deletion on First-Year Growth Response and Final Height in Patients with Severe GH Deficiency. J. Clin. Endocrinol. Metab. 2006, 91, 1076–1080. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, D.; Beń-Skowronek, I. Craniofacial Morphology in Children with Growth Hormone Deficiency and Turner Syndrome. Diagnostics 2020, 10, 88. https://doi.org/10.3390/diagnostics10020088
Wójcik D, Beń-Skowronek I. Craniofacial Morphology in Children with Growth Hormone Deficiency and Turner Syndrome. Diagnostics. 2020; 10(2):88. https://doi.org/10.3390/diagnostics10020088
Chicago/Turabian StyleWójcik, Dorota, and Iwona Beń-Skowronek. 2020. "Craniofacial Morphology in Children with Growth Hormone Deficiency and Turner Syndrome" Diagnostics 10, no. 2: 88. https://doi.org/10.3390/diagnostics10020088




