The Relationship between Coronary Artery Wall Shear Strain and Plaque Morphology: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Data Sources
2.2. Study Selection
2.3. Outcome Variables
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Search Results and Trial Flow
3.2. Characteristics of Included Studies
3.3. Characteristics of Coronary Plaques
3.3.1. Differences between Low WSS and High WSS
3.3.2. Differences between Intermediate WSS and High WSS
3.3.3. Differences between Low WSS and Intermediate WSS
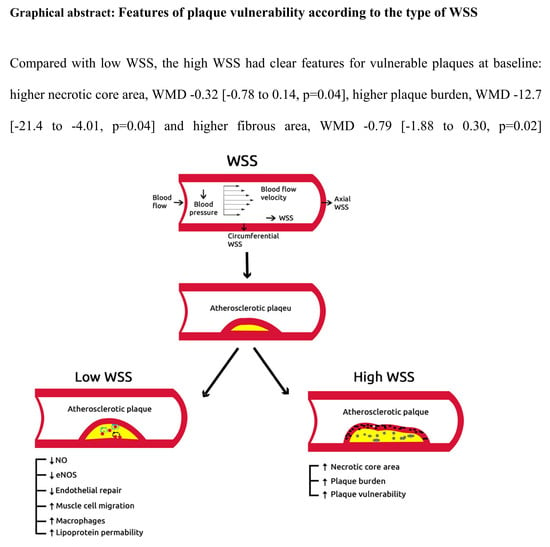
3.3.4. Features of Plaque Vulnerability according to the Type of WSS
3.3.5. Risk Assessment of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Murray, C.J.; Lopez, A.D. Measuring the Global Burden of Disease. New Engl. J. Med. 2013, 369, 448–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Feldman, C.L.; Chatzizisis, Y.S.; Coskun, A.U.; Jonas, M.; Maynard, C.; Baker, A.B.; Papafaklis, M.I.; Edelman, E.R.; Stone, P.H. Natural history of experimental coronary atherosclerosis and vascular remodeling in relation to endothelial shear stress: A serial, in vivo intravascular ultrasound study. Circulation 2010, 121, 2092–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slager, C.J.; Wentzel, J.J.; Gijsen, F.J.H.; Schuurbiers, J.C.H.; Van Der Wal, A.C.; Van Der Steen, A.F.W.; Serruys, P.W. The role of shear stress in the generation of rupture-prone vulnerable plaques. Nat. Clin. Pr. Neurol. 2005, 2, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.H.; Saito, S.; Takahashi, S.; Makita, Y.; Nakamura, S.; Kawasaki, T.; Takahashi, A.; Katsuki, T.; Nakamura, S.; Namiki, A. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: The PREDICTION Study. Circulation 2012, 126, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Samady, H.; Eshtehardi, P.; McDaniel, M.C.; Suo, J.; Dhawan, S.S.; Maynard, C.; Timmins, L.H.; Quyyumi, A.A.; Giddens, D.P.; Fiedler, J.; et al. Coronary Artery Wall Shear Stress Is Associated With Progression and Transformation of Atherosclerotic Plaque and Arterial Remodeling in Patients With Coronary Artery Disease. Circulation 2011, 124, 779–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatzizisis, Y.S.; Coskun, A.U.; Jonas, M.; Edelman, E.R.; Feldman, C.L.; Stone, P.H. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: Molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 2007, 49, 2379–2393. [Google Scholar] [CrossRef] [Green Version]
- Eshtehardi, P.; McDaniel, M.C.; Suo, J.; Dhawan, S.S.; Timmins, L.H.; Binongo, J.N.G.; Golub, L.J.; Corban, M.T.; Finn, A.V.; Oshinsk, J.N.; et al. Association of Coronary Wall Shear Stress With Atherosclerotic Plaque Burden, Composition, and Distribution in Patients With Coronary Artery Disease. J. Am. Hear. Assoc. 2012, 1, 002543. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Moher, D.; Cook, D.J.; Eastwood, S.; Olkin, I.; Rennie, D.; Stroup, D.F. Improving the quality of reports of meta-analyses of randomised controlled trials: The QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999, 354, 1896–1900. [Google Scholar] [CrossRef]
- Malek, A.M.; Alper, S.L.; Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999, 282, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Der Simonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Egger, M.; Smith, G.D. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001, 323, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Leeflang, M.M.; Sterne, J.A.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Bossuyt, P.M. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies FREE. Ann. Intern. Med. 2011, 155, 529. [Google Scholar] [CrossRef]
- Timmins, L.H.; Molony, D.S.; Eshtehardi, P.; McDaniel, M.C.; Oshinski, J.N.; Samady, H.; Giddens, D.P. Focal Association Between Wall Shear Stress and Clinical Coronary Artery Disease Progression. Ann. Biomed. Eng. 2014, 43, 94–106. [Google Scholar] [CrossRef]
- Timmins, L.H.; Molony, D.S.; Eshtehardi, P.; McDaniel, M.C.; Oshinski, J.N.; Giddens, D.P.; Samady, H. Oscillatory wall shear stress is a dominant flow characteristic affecting lesion progression patterns and plaque vulnerability in patients with coronary artery disease. J. R. Soc. Interface 2017, 14, 20160972. [Google Scholar] [CrossRef]
- Dhawan, S.S.; Nanjundappa, R.P.A.; Branch, J.R.; Taylor, W.R.; A Quyyumi, A.; Jo, H.; McDaniel, M.C.; Suo, J.; Giddens, D.; Samady, H. Shear stress and plaque development. Expert Rev. Cardiovasc. Ther. 2010, 8, 545–556. [Google Scholar] [CrossRef] [Green Version]
- Tuenter, A.; Selwaness, M.; Lorza, A.A.; Schuurbiers, J.; Speelman, L.; Cibis, M.; Van Der Lugt, A.; De Bruijne, M.; Van Der Steen, A.; Franco, O.; et al. High shear stress relates to intraplaque haemorrhage in asymptomatic carotid plaques. Atheroscler 2016, 251, 348–354. [Google Scholar] [CrossRef] [Green Version]
- Eshtehardi, P.; Teng, Z. Protective or destructive: High wall shear stress and atherosclerosis. Atheroscler 2016, 251, 501–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, U.; Ni, C.-W.; Rezvan, A.; Suo, J.; Budzyn, K.; Llanos, A.; Harrison, D.; Giddens, D.; Jo, H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am. J. Physiol. Circ. Physiol. 2009, 297, H1535–H1543. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone MAJr Topper, J.N.; Nagel, T.; Anderson, K.R.; Garcia-Cardena, G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann. N. Y. Acad. Sci. 2000, 902, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Chatzizisis, Y.S.; Jonas, M.; Coskun, A.U.; Beigel, R.; Stone, B.V.; Maynard, C.; Gerrity, R.G.; Daley, W.; Rogers, C.; Edelman, E.R.; et al. Prediction of the localization of high-risk coronary atherosclerotic plaques on the basis of low endothelial shear stress: An intravascular ultrasound and histopathology natural history study. Circulation 2008, 117, 993–1002. [Google Scholar] [CrossRef] [Green Version]
- Stone, P.H.; Coskun, A.U.; Kinlay, S.; Popma, J.J.; Sonka, M.; Wahle, A.; Yeghiazarians, Y.; Maynard, C.; Kuntz, R.E.; Feldman, C.L. Regions of low endothelial shear stress are the sites where coronary plaque progresses and vascular remodelling occurs in humans: An in vivo serial study. Eur. Hear. J. 2007, 28, 705–710. [Google Scholar] [CrossRef]
- Stone, P.H.; Coskun, A.U.; Kinlay, S.; Clark, M.E.; Sonka, M.; Wahle, A.; Ilegbusi, O.J.; Yeghiazarians, Y.; Popma, J.J.; Orav, J.; et al. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: In vivo 6-month follow-up study. Circulation 2003, 108, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Fukumoto, Y.; Hiro, T.; Fujii, T.; Hashimoto, G.; Fujimura, T.; Yamada, J.; Okamura, T.; Matsuzaki, M. Localized elevation of shear stress is related to coronary plaque rupture: A 3-dimensional intravascular ultrasound study with in-vivo color mapping of shear stress distribution. J. Am. Coll. Cardiol. 2008, 51, 645–650. [Google Scholar] [CrossRef] [Green Version]
- Gijsen, F.J.H.; Wentzel, J.J.; Thury, A.; Mastik, F.; Schaar, J.A.; Schuurbiers, J.C.H.; Slager, C.J.; Van Der Giessen, W.J.; De Feyter, P.J.; Van Der Steen, A.F.W.; et al. Strain distribution over plaques in human coronary arteries relates to shear stress. Am. J. Physiol. Circ. Physiol. 2008, 295, H1608–H1614. [Google Scholar] [CrossRef] [Green Version]
- Groen, H.; Gijsen, F.; van der Lugt, A.; Ferguson, M.; Hatsukami, T.; van der Steen, A.; Yuan, C.; Wentzel, J. Plaque rupture in the carotid artery is localized at the high shear stress region: A case report. Stroke 2007, 38, 2379–2381. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Teng, Z.; Canton, G.; Yang, C.; Ferguson, M.; Huang, X.; Zheng, J.; Woodard, P.K.; Yuan, C. Sites of rupture in human atherosclerotic carotid plaques are associated with high structural stresses: An in vivo MRI-based 3D fluid-structure interaction study. Stroke 2009, 40, 3258–3263. [Google Scholar] [CrossRef] [Green Version]
- Glagov, S.; Bassiouny, H.S.; Giddens, D.P.; Zarins, C.K. Pathobiology of Plaque Modeling and Complication. Surg. Clin. N. Am. 1995, 75, 545–556. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Burke, A.P.; Farb, A.; Gold, H.K.; Yuan, J.; Narula, J.; Finn, A.V.; Virmani, R. The thin-cap fibroatheroma: A type of vulnerable plaque: The major precursor lesion to acute coronary syndromes. Curr. Opin. Cardiol. 2001, 16, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of Vulnerable/Unstable Plaque. Arter. Thromb. Vasc. Boil. 2010, 30, 1282–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolodgie, F.D.; Virmani, R.; Burke, A.P.; Farb, A.; Weber, D.K.; Kutys, R.; Finn, A.V.; Gold, H.K. Pathologic assessment of the vulnerable human coronary plaque. Heart 2004, 90, 1385–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicoll, R.; Wiklund, U.; Zhao, Y.; Diederichsen, A.; Mickley, H.; Ovrehus, K.; Zamorano, J.; Gueret, P.; Schmermund, A.; Maffei, E.; et al. Gender and age effects on risk factor-based prediction of coronary artery calcium in symptomatic patients: A Euro-CCAD study. Atheroscler 2016, 252, 32–39. [Google Scholar] [CrossRef] [PubMed]




| Study (Trial) Year | Study Design | Types of WSS | No. of Segments | Inclusion Criteria | Exclusion Criteria | Primary Endpoints |
|---|---|---|---|---|---|---|
| Samady 2011 | Observational | Low WSS | 2249 | Abnormal | Myocardial infarction | Lumen area |
| prospective | Intermediate WSS | noninvasive stress test or | Cardiogenic shock | Plaque area | ||
| study | High WSS | stable angina | Hemodynamic instability | Necrotic core area | ||
| syndromes | CABG or PCI | Dense calcium area | ||||
| Myocardial infarction | Fibrofatty area | |||||
| Cardiogenic shock | Fibrous area | |||||
| Eshtehardi 2012 | Observational | Low WSS | 3581 | Abnormal | Myocardial infarction | Lumen area |
| prospective | Intermediate WSS | noninvasive stress test or | Cardiogenic shock | plaque area | ||
| study | High WSS | stable angina | Hemodynamic instability | plaque burden | ||
| syndromes | CABG or PCI | |||||
| Timmins 2015 | Prospective | Low WSS | 3871 | CAD | NR | Lumen area |
| observational | Intermediate WSS | |||||
| study | High WSS | |||||
| Timmins 2017 | Observational | Low WSS | 14,235 | Abnormal | NR | Plaque area |
| prospective | Intermediate WSS | noninvasive stress test or | Necrotic core area | |||
| study | High WSS | stable angina | Dense calcium area | |||
| syndromes | Fibrofatty area | |||||
| Fibrous area |
| Study (Trial) Year | Groups | No. of Patients | No. of Segments | Age Year | Male (%) | HTN (%) | DM (%) | TC (mg/dL) | Triglyceride (mg/dL) | Smoking (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Samady 2011 | L-WSS | 20 * | 205 | 54 ± 10 * | 65 | 70 | 35 | 186 ± 13 | 115.5+ | 25 |
| I-WSS | 1034 | NR | NR | NR | NR | NR | NR | NR | ||
| H = WSS | 27* | 1010 | NR | NR | NR | NR | NR | NR | NR | |
| Eshtehardi 2012 | L-WSS | 3851 * | 50 ± 10 * | 60 | 60 | 26 | 181.5 ± 34 | 114 ± 95 | 22 | |
| I-WSS | ||||||||||
| H = WSS | ||||||||||
| Timmins 2015 | L-WSS | 5 * | 3871 * | 62.1 ± 7.6 | 65.5 | 72.2 | 23.6 | NR | NR | NR |
| I-WSS | 61.7 ± 10.2 | 79.6 | 67.7 | 13.0 | NR | NR | NR | |||
| H = WSS | 62.9 ± 10.3 | 74.1 | 74.1 | 16.7 | NR | NR | NR | |||
| Timmins 2017 | L-WSS | 20 * | 1785 | 54 ±10 * | 65 * | 70 * | 35* | 186 ± 16 * | 107± 101 * | 25 * |
| I-WSS | 413 | |||||||||
| H = WSS | 929 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajraktari, A.; Bytyçi, I.; Henein, M.Y. The Relationship between Coronary Artery Wall Shear Strain and Plaque Morphology: A Systematic Review and Meta-Analysis. Diagnostics 2020, 10, 91. https://doi.org/10.3390/diagnostics10020091
Bajraktari A, Bytyçi I, Henein MY. The Relationship between Coronary Artery Wall Shear Strain and Plaque Morphology: A Systematic Review and Meta-Analysis. Diagnostics. 2020; 10(2):91. https://doi.org/10.3390/diagnostics10020091
Chicago/Turabian StyleBajraktari, Artan, Ibadete Bytyçi, and Michael Y. Henein. 2020. "The Relationship between Coronary Artery Wall Shear Strain and Plaque Morphology: A Systematic Review and Meta-Analysis" Diagnostics 10, no. 2: 91. https://doi.org/10.3390/diagnostics10020091
APA StyleBajraktari, A., Bytyçi, I., & Henein, M. Y. (2020). The Relationship between Coronary Artery Wall Shear Strain and Plaque Morphology: A Systematic Review and Meta-Analysis. Diagnostics, 10(2), 91. https://doi.org/10.3390/diagnostics10020091