Left Atrial Structural Remodelling in Non-Valvular Atrial Fibrillation: What Have We Learnt from CMR?
Abstract
:1. Introduction
2. Atrial Cardiomyopathy and Left Atrial Remodelling
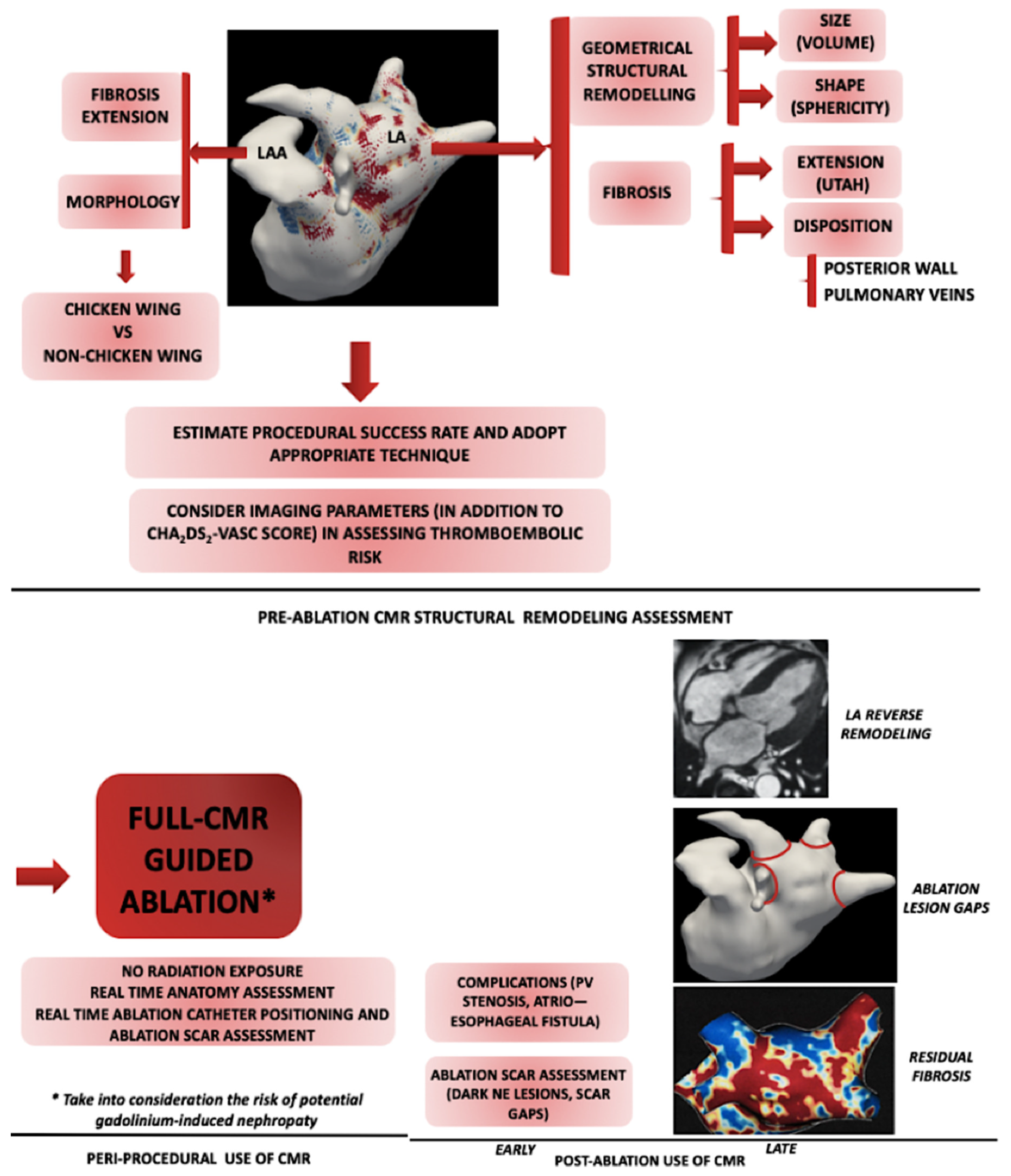
3. Left Atrial Structural Remodeling
3.1. Left Atrial Size
3.2. Left Atrial Shape
3.3. Left Atrial Fibrosis
3.3.1. Left Atrial Fibrosis as a Predictor of Post-Ablation Recurrences
3.3.2. Left Atrial Fibrosis and Thromboembolic Risk
3.3.3. Left Atrial Fibrosis and LA Dysfunction
3.3.4. Left Atrial Fibrosis and Heart Failure
3.3.5. Left Atrial Fibrosis and Electroanatomic Mapping
4. Left atrial Appendage Structural Remodelling
5. Therapeutic Implications of CMR
5.1. Pre-ablation Fibrosis Assessment
5.2. Peri-Procedural CMR
5.3. Post-Procedural CMR
5.3.1. Assessing the Ablation Scar
5.3.2. Left Atrial Reverse Remodeling
5.3.3. Post-Ablation Fibrosis Assessment
5.3.4. Post-Ablation Complications
6. CMR Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.; Abhayaratna, W.P. Left Atrial Reverse Remodeling: Mechanisms, Evaluation, and Clinical Significance. JACC Cardiovasc. Imaging 2017, 10, 65–77. [Google Scholar] [CrossRef]
- Habibi, M.; Lima, J.A.; Khurram, I.M.; Zimmerman, S.L.; Zipunnikov, V.; Fukumoto, K.; Spragg, D.; Ashikaga, H.; Rickard, J.; Marine, J.E.; et al. Association of Left Atrial Function and Left Atrial Enhancement in Patients With Atrial Fibrillation: Cardiac Magnetic Resonance Study. Circ. Cardiovasc. Imaging 2015, 8, e002769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandon, K.; Tirschwell, D.; Longstreth, W.T., Jr.; Smith, B.; Akoum, N. Embolic stroke of undetermined source correlates to atrial fibrosis without atrial fibrillation. Neurology 2019, 93, e381–e387. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on Atrial cardiomyopathies: Definition, characterisation, and clinical implication. J. Arrhythm. 2016, 32, 247–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benussi, S.; de Maat, G.E. Atrial remodelling and function: Implications for atrial fibrillation surgery. Eur. J. Cardiothorac Surg. 2018, 53, i2–i8. [Google Scholar] [CrossRef]
- Habibi, M.; Samiei, S.; Ambale Venkatesh, B.; Opdahl, A.; Helle-Valle, T.M.; Zareian, M.; Almeida, A.L.; Choi, E.Y.; Wu, C.; Alonso, A.; et al. Cardiac Magnetic Resonance-Measured Left Atrial Volume and Function and Incident Atrial Fibrillation: Results From MESA (Multi-Ethnic Study of Atherosclerosis). Circ. Cardiovasc. Imaging 2016, 9, e004299. [Google Scholar] [CrossRef] [Green Version]
- Zghaib, T.; Nazarian, S. New Insights into the Use of Cardiac Magnetic Resonance Imaging to Guide Decision-Making in AF Management. Can. J. Cardiol. 2018, 34, 1461–1470. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: The DECAAF study. JAMA 2014, 311, 498–506. [Google Scholar] [CrossRef]
- Bisbal, F.; Baranchuk, A.; Braunwald, E.; Bayés de Luna, A.; Bayés-Genís, A. Atrial Failure as a Clinical Entity: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 222–232. [Google Scholar] [CrossRef]
- Guichard, J.B.; Nattel, S. Atrial Cardiomyopathy: A Useful Notion in Cardiac Disease Management or a Passing Fad? J. Am. Coll. Cardiol. 2017, 70, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B.; American Heart Association; et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, M.J.; Arora, R.; Jalife, J. Atrial Myopathy. JACC Basic Transl. Sci. 2019, 4, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, B.J.; Copeland-Halperin, R.S.; Halperin, J.L. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism: Mechanistic links and clinical inferences. J. Am. Coll. Cardiol. 2015, 65, 2239–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiog. 2015, 28, 1–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamori, S.; Ngo, L.H.; Tugal, D.; Manning, W.J.; Nezafat, R. Incremental Value of Left Atrial Geometric Remodeling in Predicting Late Atrial Fibrillation Recurrence After Pulmonary Vein Isolation: A Cardiovascular Magnetic Resonance Study. J. Am. Heart Assoc. 2018, 7, e009793. [Google Scholar] [CrossRef] [Green Version]
- Bisbal, F.; Guiu, E.; Calvo, N.; Marin, D.; Berruezo, A.; Arbelo, E.; Ortiz-Pérez, J.; de Caralt, T.M.; Tolosana, J.M.; Borràs, R.; et al. Left atrial sphericity: A new method to assess atrial remodeling. Impact on the outcome of atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 2013, 24, 752–759. [Google Scholar] [CrossRef]
- Bisbal, F.; Guiu, E.; Cabanas, P.; Calvo, N.; Berruezo, A.; Tolosana, J.M.; Arbelo, E.; Vidal, B.; de Caralt, T.M.; Sitges, M.; et al. Reversal of spherical remodelling of the left atrium after pulmonary vein isolation: Incidence and predictors. Europace 2014, 16, 840–847. [Google Scholar] [CrossRef]
- Moon, J.; Lee, H.J.; Yu, J.; Pak, H.N.; Ha, J.W.; Lee, M.H.; Kim, Y.J.; Joung, B. Prognostic implication of left atrial sphericity in atrial fibrillation patients undergoing radiofrequency catheter ablation. Pacing Clin. Electrophysiol. 2017, 40, 713–720. [Google Scholar] [CrossRef]
- Njoku, A.; Kannabhiran, M.; Arora, R.; Reddy, P.; Gopinathannair, R.; Lakkireddy, D. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: A meta-analysis. Europace 2018, 20, 33–42. [Google Scholar] [CrossRef]
- Oakes, R.S.; Badger, T.J.; Kholmovski, E.G.; Akoum, N.; Burgon, N.S.; Fish, E.N.; Blauer, J.J.; Rao, S.N.; DiBella, E.V.; Segerson, N.M.; et al. Detection and Quantification of Left Atrial Structural Remodeling With Delayed-Enhancement Magnetic Resonance Imaging in Patients With Atrial Fibrillation. Circulation 2009, 119, 1758–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisbal, F.; Fernández-Armenta, J.; Berruezo, A.; Mont, L.; Brugada, J. Use of MRI to guide electrophysiology procedures. Heart 2014, 100, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Sohns, C.; Marrouche, N.F. Atrial fibrillation and cardiac fibrosis. Eur. Heart J. 2019. (Epub ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Bisbal, F.; Alarcón, F.; Ferrero-de-Loma-Osorio, A.; González-Ferrer, J.J.; Alonso, C.; Pachón, M.; Tizón, H.; Cabanas-Grandío, P.; Sanchez, M.; Benito, E.; et al. Left atrial geometry and outcome of atrial fibrillation ablation: Results from the multicentre LAGO-AF study. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Siebermair, J.; Kholmovski, E.G.; Marrouche, N. Assessment of Left Atrial Fibrosis by Late Gadolinium Enhancement Magnetic Resonance Imaging: Methodology and Clinical Implications. JACC Clin. Electrophysiol. 2017, 3, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Navaravong, L.; Marrouche, N. CMR Guidance of RFA to Atrial Arrhythmias. In Cardiovascular Magnetic Resonance Imaging, 2nd ed.; Kwong, R.Y., Jerosch-Herold, M., Heydari, B., Eds.; Humana Press: New York, NY, USA, 2019; pp. 407–418. [Google Scholar]
- Gal, P.; Marrouche, N.F. Magnetic resonance imaging of atrial fibrosis: Redefining atrial fibrillation to a syndrome. Eur. Heart J. 2017, 38, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Khurram, I.M.; Habibi, M.; Gucuk Ipek, E.; Chrispin, J.; Yang, E.; Fukumoto, K.; Dewire, J.; Spragg, D.D.; Marine, J.E.; Berger, R.D.; et al. Left Atrial LGE and Arrhythmia Recurrence Following Pulmonary Vein Isolation for Paroxysmal and Persistent AF. JACC Cardiovasc. Imaging. 2016, 9, 142–148. [Google Scholar] [CrossRef]
- Chubb, H.; Karim, R.; Mukherjee, R.; Williams, S.E.; Whitaker, J.; Harrison, J.; Niederer, S.A.; Staab, W.; Gill, J.; Schaeffter, T.; et al. A comprehensive multi-index cardiac magnetic resonance-guided assessment of atrial fibrillation substrate prior to ablation: Prediction of long-term outcomes. J. Cardiovasc. Electrophysiol. 2019, 30, 1894–1903. [Google Scholar] [CrossRef]
- Chrispin, J.; Ipek, E.G.; Habibi, M.; Yang, E.; Spragg, D.; Marine, J.E.; Ashikaga, H.; Rickard, J.; Berger, R.D.; Zimmerman, S.L.; et al. Clinical predictors of cardiac magnetic resonance late gadolinium enhancement in patients with atrial fibrillation. Europace 2017, 19, 371–377. [Google Scholar] [CrossRef]
- Gal, P.; Pacchia, C.; Morris, A.; Cates, J.; Kaur, G.; Elvan, A.; Marrouche, N. P1304: Architecture of fibrosis predicts atrial fibrillation recurrences after ablation. Europace 2015, 7, iii176. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, K.; Cates, J.; Gardner, G.; Morris, A.; Burgon, N.S.; Akoum, N.; Marrouche, N.F. The Spatial Distribution of Late Gadolinium Enhancement of Left Atrial Magnetic Resonance Imaging in Patients With Atrial Fibrillation. JACC Clin. Electrophysiol. 2018, 4, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Benito, E.M.; Cabanelas, N.; Nuñez-Garcia, M.; Alarcón, F.; Figueras, I.; Ventura, R.M.; Soto-Iglesias, D.; Guasch, E.; Prat-Gonzalez, S.; Perea, R.J.; et al. Preferential regional distribution of atrial fibrosis in posterior wall around left inferior pulmonary vein as identified by late gadolinium enhancement cardiac magnetic resonance in patients with atrial fibrillation. Europace 2018, 20, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Spronk, H.M.; De Jong, A.M.; Verheule, S.; De Boer, H.C.; Maass, A.H.; Lau, D.H.; Rienstra, M.; van Hunnik, A.; Kuiper, M.; Lumeij, S.; et al. Hypercoagulability causes atrial fibrosis and promotes atrial fibrillation. Eur. Heart J. 2017, 38, 38–50. [Google Scholar] [CrossRef] [PubMed]
- King, J.B.; Azadani, P.N.; Suksaranjit, P.; Bress, A.P.; Witt, D.M.; Han, F.T.; Chelu, M.G.; Silver, M.A.; Biskupiak, J.; Wilson, B.D.; et al. Left Atrial Fibrosis and Risk of Cerebrovascular and Cardiovascular Events in Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 70, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Daccarett, M.; Badger, T.J.; Akoum, N.; Burgon, N.S.; Mahnkopf, C.; Vergara, G.; Kholmovski, E.; McGann, C.J.; Parker, D.; Brachmann, J.; et al. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2011, 57, 831–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akoum, N.; Fernandez, G.; Wilson, B.; Mcgann, C.; Kholmovski, E.; Marrouche, N. Association of atrial fibrosis quantified using LGE-MRI with atrial appendage thrombus and spontaneous contrast on transesophageal echocardiography in patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 2013, 24, 1104–1109. [Google Scholar] [CrossRef] [Green Version]
- Inoue, Y.Y.; Alissa, A.; Khurram, I.M.; Fukumoto, K.; Habibi, M.; Venkatesh, B.A.; Zimmerman, S.L.; Nazarian, S.; Berger, R.D.; Calkins, H.; et al. Quantitative tissue-tracking cardiac magnetic resonance (CMR) of left atrial deformation and the risk of stroke in patients with atrial fibrillation. J. Am. Heart Assoc. 2015, 4, e001844. [Google Scholar] [CrossRef] [Green Version]
- Habibi, M.; Lima, J.A.C.; Gucuk Ipek, E.; Zimmerman, S.L.; Zipunnikov, V.; Spragg, D.; Ashikaga, H.; Rickard, J.; Marine, J.E.; Berger, R.D.; et al. The association of baseline left atrial structure and function measured with cardiac magnetic resonance and pulmonary vein isolation outcome in patients with drug-refractory atrial fibrillation. Heart Rhythm. 2016, 13, 1037–1044. [Google Scholar] [CrossRef]
- Ciuffo, L.; Inoue, Y.Y.; Tao, S.; Gucuk Ipek, E.; Balouch, M.; Lima, J.A.C.; Nazarian, S.; Spragg, D.D.; Marine, J.E.; Berger, R.D.; et al. Mechanical dyssynchrony of the left atrium during sinus rhythm is associated with history of stroke in patients with atrial fibrillation. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Akkaya, M.; Higuchi, K.; Koopmann, M.; Damal, K.; Burgon, N.S.; Kholmovski, E.; McGann, C.; Marrouche, N. Higher degree of left atrial structural remodeling in patients with atrial fibrillation and left ventricular systolic dysfunction. J. Cardiovasc. Electrophysiol. 2013, 24, 485–491. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; Ma, X.; Bai, R.; Liu, N.; Li, N.; Schoenhagen, P.; Ma, C. Prognostic Significance of Left Ventricular Fibrosis Assessed by T1 Mapping in Patients with Atrial Fibrillation and Heart Failure. Sci. Rep. 2019, 9, 13374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. CASTLE-AF Investigators. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Fukui, A.; Node, K. Bipolar Voltage Mapping for the Evaluation of Atrial Substrate: Can We Overcome the Challenge of Directionality? J. Atr Fibrillation. 2019, 11, 2116. [Google Scholar] [CrossRef] [PubMed]
- Parmar, B.R.; Jarrett, T.R.; Burgon, N.S.; Kholmovski, E.G.; Akoum, N.W.; Hu, N.; Macleod, R.S.; Marrouche, N.F.; Ranjan, R. Comparison of left atrial area marked ablated in electroanatomical maps with scar in MRI. J. Cardiovasc. Electrophysiol. 2014, 25, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Tsuchiya, T.; Fukui, A.; Kawano, Y.; Otsubo, T.; Takahashi, Y.; Hirota, K.; Murotani, K.; Eshima, K.; Takahash, N. Impact of the extent of low-voltage zone on outcomes after voltage-based catheter ablation for persistent atrial fibrillation. J. Cardiol. 2018, 72, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.S.; Yamashita, S.; Cochet, H.; Haïssaguerre, M. Delineating atrial scar by electroanatomic voltage mapping versus cardiac magnetic resonance imaging: Where to draw the line? J. Cardiovasc. Electrophysiol. 2014, 25, 1053–1056. [Google Scholar] [CrossRef]
- Platonov, P.G.; Mitrofanova, L.B.; Orshanskaya, V.; Ho, S.Y. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J. Am. Coll. Cardiol. 2011, 58, 2225–2232. [Google Scholar] [CrossRef] [Green Version]
- Chrispin, J.; Gucuk Ipek, E.; Zahid, S.; Prakosa, A.; Habibi, M.; Spragg, D.; Marine, J.E.; Ashikaga, H.; Rickard, J.; Trayanova, N.A.; et al. Lack of regional association between atrial late gadolinium enhancement on cardiac magnetic resonance and atrial fibrillation rotors. Heart Rhythm. 2016, 13, 654–660. [Google Scholar] [CrossRef]
- Zghaib, T.; Keramati, A.; Chrispin, J.; Huang, D.; Balouch, M.A.; Ciuffo, L.; Berger, R.D.; Marine, J.E.; Ashikaga, H.; Calkins, H.; et al. Multimodal Examination of Atrial Fibrillation Substrate: Correlation of Left Atrial Bipolar Voltage Using Multi-Electrode Fast Automated Mapping, Point-by-Point Mapping, and Magnetic Resonance Image Intensity Ratio. JACC Clin. Electrophysiol. 2018, 4, 59–68. [Google Scholar] [CrossRef]
- Siebermair, J.; Suksaranjit, P.; McGann, C.J.; Peterson, K.A.; Kheirkhahan, M.; Baher, A.A.; Damal, K.; Wakili, R.; Marrouche, N.F.; Wilson, B.D. Atrial fibrosis in non-atrial fibrillation individuals and prediction of atrial fibrillation by use of late gadolinium enhancement magnetic resonance imaging. J. Cardiovasc. Electrophysiol. 2019, 30, 550–556. [Google Scholar] [CrossRef]
- Tsai, L.M.; Chen, J.H.; Lin, L.J.; Yang, Y.J. Role of transesophageal echocardiography in detecting left atrial thrombus and spontaneous echo contrast in patients with mitral valve disease or non-rheumatic atrial fibrillation. J. Formos Med. Assoc. 1990, 89, 270–274. [Google Scholar] [PubMed]
- Manning, W.J.; Silverman, D.I.; Keighley, C.S.; Oettgen, P.; Douglas, P.S. Transesophageal echocardiographically facilitated early cardioversion from atrial fibrillation using short-term anticoagulation: Final results of a prospective 4.5-year study. J. Am. Coll. Cardiol. 1995, 25, 1354–1361. [Google Scholar] [CrossRef] [Green Version]
- Khurram, I.M.; Dewire, J.; Mager, M.; Maqbool, F.; Zimmerman, S.L.; Zipunnikov, V.; Beinart, R.; Marine, J.E.; Spragg, D.D.; Berger, R.D.; et al. Relationship between left atrial appendage morphology and stroke in patients with atrial fibrillation. Heart Rhythm. 2013, 10, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Suksaranjit, P.; Marrouche, N.F.; Han, F.T.; Morris, A.; Kaur, G.; Oswald, T.; Wilson, B.D. Relation of Left Atrial Appendage Remodeling by Magnetic Resonance Imaging and Outcome of Ablation for Atrial Fibrillation. Am. J. Cardiol. 2018, 122, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Lu, R.; Zhao, D.; Jiang, Z.; Tang, M.; Bao, C.; Mei, J. Left Atrial Appendage Fibrosis and 3-Year Clinical Outcomes in Atrial Fibrillation After Endoscopic Ablation: A Histologic Analysis. Ann. Thorac. Surg. 2020, 109, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Biase, L.; Burkhardt, J.D.; Mohanty, P.; Sanchez, J.; Mohanty, S.; Horton, R.; Gallinghouse, G.J.; Bailey, S.M.; Zagrodzky, J.D.; Santangeli, P.; et al. Left atrial appendage: An underrecognized trigger site of atrial fibrillation. Circulation 2010, 122, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Kottkamp, H. Human atrial fibrillation substrate: Towards a specific fibrotic atrial cardiomyopathy. Eur. Heart J. 2013, 34, 2731–2738. [Google Scholar] [CrossRef] [Green Version]
- Marrouche, N.F. Efficacy of Delayed Enhancement MRI-Guided Ablation vs Conventional Catheter Ablation of Atrial Fibrillation. Available online: https://clinicaltrials.gov/ct2/show/NCT02529319 (accessed on 8 September 2019).
- Zahid, S.; Whyte, K.N.; Schwarz, E.L.; Blake, R.C., 3rd; Boyle, P.M.; Chrispin, J.; Prakosa, A.; Ipek, E.G.; Pashakhanloo, F.; Halperin, H.R.; et al. Feasibility of using patient-specific models and the “minimum cut” algorithm to predict optimal ablation targets for left atrial flutter. Heart Rhythm. 2016, 13, 1687–1698. [Google Scholar] [CrossRef]
- Zghaib, T.; Malayeri, A.A.; Ipek, E.G.; Habibi, M.; Huang, D.; Balouch, M.A.; Bluemke, D.A.; Calkins, H.; Nazarian, S.; Zimmerman, S.L. Visualization of acute edema in the left atrial myocardium after radiofrequency ablation: Application of a novel high-resolution 3-dimensional magnetic resonance imaging sequence. Heart Rhythm. 2018, 15, 1189–1197. [Google Scholar] [CrossRef]
- Elbes, D.; Magat, J.; Govari, A.; Ephrath, Y.; Vieillot, D.; Beeckler, C.; Weerasooriya, R.; Jais, P.; Quesson, B. Magnetic resonance imaging-compatible circular mapping catheter: An in vivo feasibility and safety study. Europace 2017, 19, 458–464. [Google Scholar] [CrossRef] [Green Version]
- Paetsch, I.; Sommer, P.; Jahnke, C.; Hilbert, S.; Loebe, S.; Schoene, K.; Oebel, S.; Krueger, S.; Weiss, S.; Smink, J.; et al. Clinical workflow and applicability of electrophysiological cardiovascular magnetic resonance-guided radiofrequency ablation of isthmus-dependent atrial flutter. Eur. Heart J. Cardiovasc. Imaging. 2019, 20, 147–156. [Google Scholar] [CrossRef] [PubMed]
- McGann, C.; Kholmovski, E.; Blauer, J.; Vijayakumar, S.; Haslam, T.; Cates, J.; DiBella, E.; Burgon, N.; Wilson, B.; Alexander, A.; et al. Dark regions of no-reflow on late gadolinium enhancement magnetic resonance imaging result in scar formation after atrial fibrillation ablation. J. Am. Coll. Cardiol. 2011, 58, 177–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGann, C.; Akoum, N.; Patel, A.; Kholmovski, E.; Revelo, P.; Damal, K.; Wilson, B.; Cates, J.; Harrison, A.; Ranjan, R.; et al. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ. Arrhythm. Electrophysiol. 2014, 7, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriatselis, C.; Unruh, T.; Kaufmann, J.; Gerds-Li, J.H.; Kelle, S.; Gebker, R.; Jahnke, C.; Paetsch, I.; Pieske, B. Long-term left atrial remodeling after ablation of persistent atrial fibrillation: 7-year follow-up by cardiovascular magnetic resonance imaging. J. Interv. Card Electrophysiol. 2019. (Epub ahead of print). [Google Scholar] [CrossRef]
- Markman, T.M.; Nazarian, S. Cardiac Magnetic Resonance for Lesion Assessment in the Electrophysiology Laboratory. Circ. Arrhythm. Electrophysiol. 2017, 10, e005839. [Google Scholar] [CrossRef]
- Goo, H.W.; Al-Otay, A.; Grosse-Wortmann, L.; Wu, S.; Macgowan, C.K.; Yoo, S.J. Phase-contrast magnetic resonance quantification of normal pulmonary venous return. J. Magn Reson Imaging. 2009, 29, 588–594. [Google Scholar] [CrossRef]
- Chang, L.; Verma, D.R.; Kholmovski, E.; Vijayakumar, S.; Burgon, N.S.; Anderson, P.A.; Marrouche, N.F.; McGann, C. Pulmonary vein stenosis detection by early cardiac magnetic resonance imaging post-atrial fibrillation ablation. J. Cardiovasc. Magn. Reson. 2012, 14, 208. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Peters, D.C.; Hsing, J.M.; Chuang, M.L.; Chan, J.; Fish, A.; Josephson, M.E.; Manning, W.J. Late gadolinium enhancement of the esophagus is common on cardiac MR several months after pulmonary vein isolation: Preliminary observations. Pacing Clin. Electrophysiol. 2010, 33, 661–666. [Google Scholar] [CrossRef]
- Baher, A.; Kheirkhahan, M.; Rechenmacher, S.J.; Marashly, Q.; Kholmovski, E.G.; Siebermair, J.; Acharya, M.; Aljuaid, M.; Morris, A.K.; Kaur, G.; et al. High-Power Radiofrequency Catheter Ablation of Atrial Fibrillation: Using Late Gadolinium Enhancement Magnetic Resonance Imaging as a Novel Index of Esophageal Injury. JACC Clin. Electrophysiol. 2018, 4, 1583–1594. [Google Scholar] [CrossRef]
- Arnold, J.R.; McCann, G.P. Cardiovascular magnetic resonance: Applications and practical considerations for the general cardiologist. Heart 2019. [Google Scholar] [CrossRef]
- Floria, M.; Tanase, D.M. Atrial fibrillation type and renal dysfunction: New challenges in thromboembolic risk assessment. Heart 2019, 105, 1295–1297. [Google Scholar] [CrossRef] [PubMed]



| Level | Change | Effects | Additional Remarks |
|---|---|---|---|
| Metabolic | Switch to fetal glycolysis (fatty acid beta-oxidation) | Reduced energy levels | − |
| Neuro- hormonal | Increased NPs, Ang II, aldosterone, TGF- β1 levels | Increased fibrosis | Ang II * + TGF−β1 ≥ fibroblasts ≥ increased collagen production; |
| Cellular | Fibroblast activation Fibroblast-to-myofibroblast differentiation | Increased fibrosis | Fibroblasts can conduct electrical impulses via connexins ≥ anisotropy and spontaneous phase 4 cardiomyocyte depolarization; Myofibroblasts are typical of a structurally abnormal myocardium |
| Electrical | ↓ L-type Ca2+ current; ↑ K+ inward rectifier current IK,Ach activation Abnormal gap junctions distribution | Reentry, AP shortening Atrial refractoriness shortening | Calcium overload promotes reentry through action potential shortening and membrane hyperpolarization |
| Authors, Year | Number of Patients | Type of Remodeling | Imaging Parameters * | Conclusion | Reference |
|---|---|---|---|---|---|
| Habibi et al. 2016 | 509 | Size | LAV/LAVI | LAV predicts incident AF | [7] |
| Kriatselis et al. 2019 | 42 | Size | LAV | Greater LA reverse remodeling in normoponderal patients | [66] |
| Bisbal et al. 2013 | 106 | Size, shape | LAV, SI | Baseline sphericity predicts recurrences | [17] |
| Bisbal et al. 2014 | 102 | Size, shape | LAV, SI | Baseline sphericity better than LAV in predicting recurrences | [18] |
| Nakamori et al. 2018 | 227 | Size, shape | LAV/LAVI, SI | Baseline sphericity predicts recurrences | [16] |
| Oakes et al. 2009 | 81 | Fibrosis | LGE | Fibrosis predicts recurrences Fibrosis correlates with low-voltage areas | [21] |
| Marrouche et al. 2014 | 272 | Fibrosis | Utah | Fibrosis predicts recurrences | [9] |
| McGann et al. 2014 | 386 | Fibrosis | % of LA wall LGE | Fibrosis predicts recurrences LGE- correlates with histological fibrosis | [65] |
| Habibi et al. 2015 | 90 | Size, fibrosis | LAV, LGE | Fibrosis=dysfunction | [3] |
| Khurram et al.2016 | 165 | Fibrosis | LGE | Fibrosis predicts recurrences, especially in persistent AF patients | [28] |
| Higuchi et al. 2018 | 160 | Fibrosis | LGE extension in 6 LA segments | Fibrosis = inhomogeneous distribution; ↑ posterior wall and inferior PV antrum | [32] |
| Chrispin et al. 2017 | 179 | Fibrosis, size | LGE, LAV | Weak fibrosis-LAV correlation; | [30] |
| Siebermair J et al. 2019 | 182 | Fibrosis, size | Utah, LAV | LAV and obesity predicted fibrosis in non-AF patients | [51] |
| Chubb et al. 2019 | 89 | Fibrosis, shape, size | LGE, LAV, SI, LAEF | Fibrosis and dysfunction predict recurrences | [29] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floria, M.; Radu, S.; Gosav, E.M.; Cozma, D.; Mitu, O.; Ouatu, A.; Tanase, D.M.; Scripcariu, V.; Serban, L.I. Left Atrial Structural Remodelling in Non-Valvular Atrial Fibrillation: What Have We Learnt from CMR? Diagnostics 2020, 10, 137. https://doi.org/10.3390/diagnostics10030137
Floria M, Radu S, Gosav EM, Cozma D, Mitu O, Ouatu A, Tanase DM, Scripcariu V, Serban LI. Left Atrial Structural Remodelling in Non-Valvular Atrial Fibrillation: What Have We Learnt from CMR? Diagnostics. 2020; 10(3):137. https://doi.org/10.3390/diagnostics10030137
Chicago/Turabian StyleFloria, Mariana, Smaranda Radu, Evelina Maria Gosav, Dragos Cozma, Ovidiu Mitu, Anca Ouatu, Daniela Maria Tanase, Viorel Scripcariu, and Lacramioara Ionela Serban. 2020. "Left Atrial Structural Remodelling in Non-Valvular Atrial Fibrillation: What Have We Learnt from CMR?" Diagnostics 10, no. 3: 137. https://doi.org/10.3390/diagnostics10030137
APA StyleFloria, M., Radu, S., Gosav, E. M., Cozma, D., Mitu, O., Ouatu, A., Tanase, D. M., Scripcariu, V., & Serban, L. I. (2020). Left Atrial Structural Remodelling in Non-Valvular Atrial Fibrillation: What Have We Learnt from CMR? Diagnostics, 10(3), 137. https://doi.org/10.3390/diagnostics10030137









