Elevated LysoGb3 Concentration in the Neuronopathic Forms of Mucopolysaccharidoses
Abstract
1. Introduction
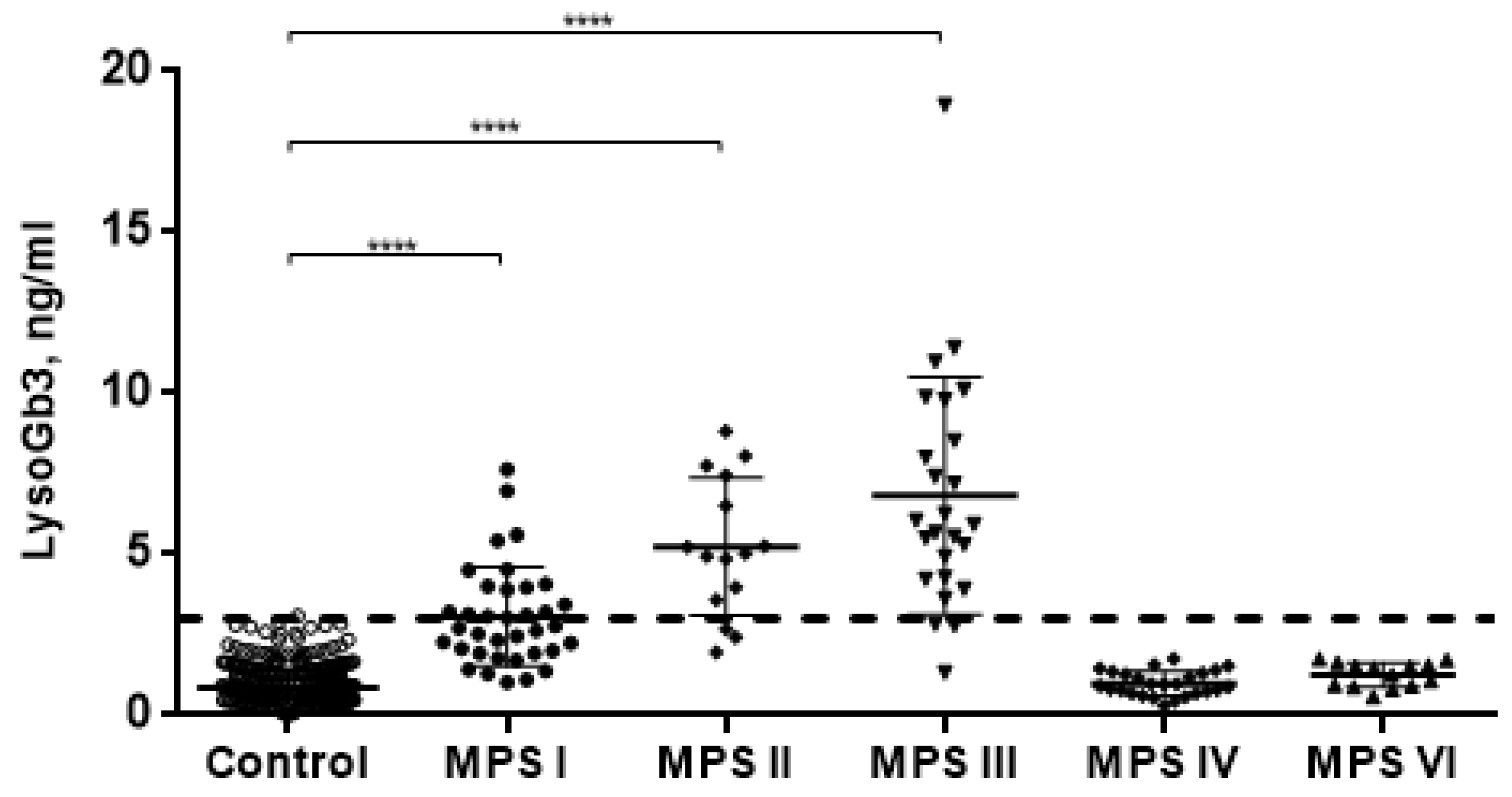
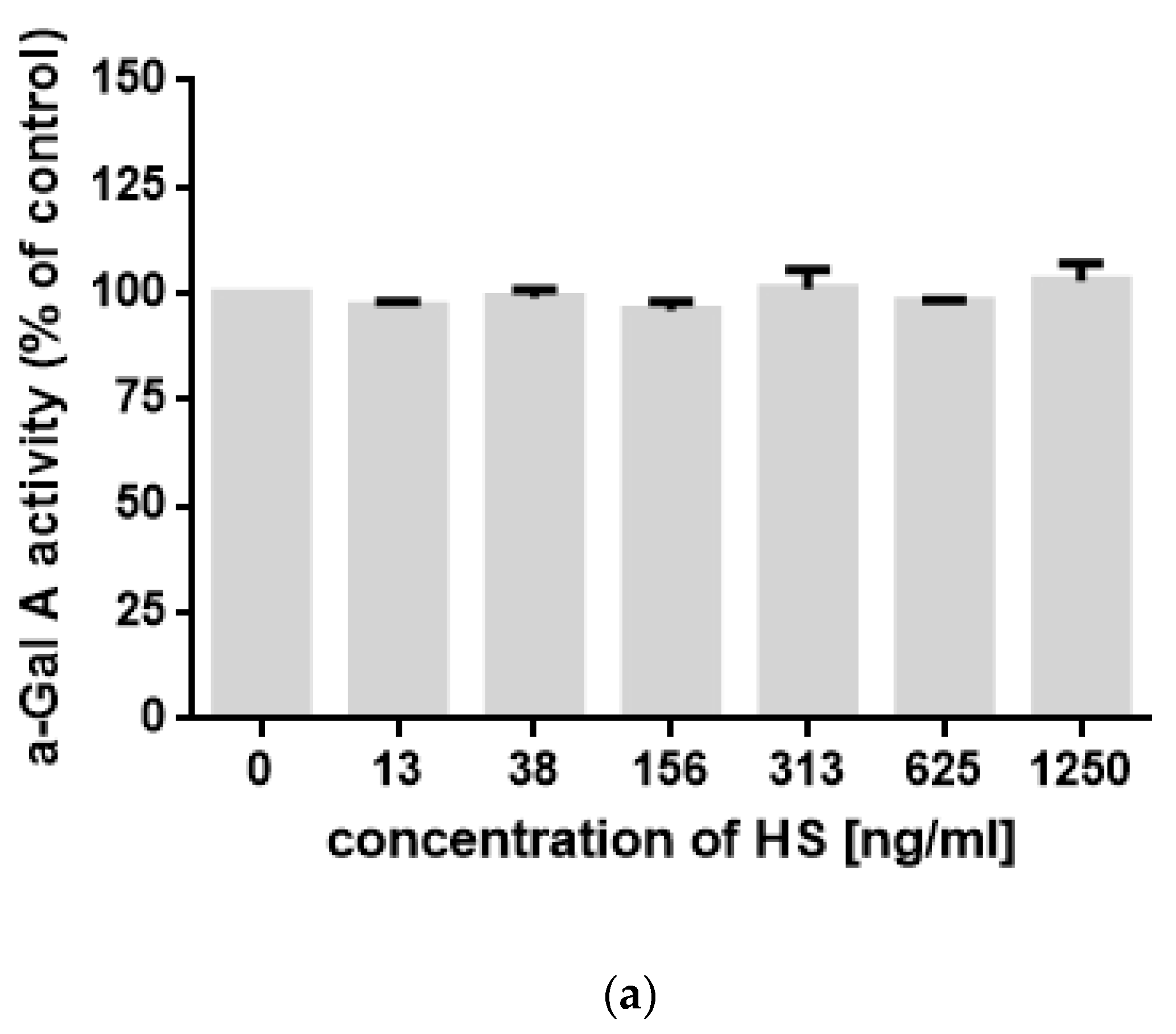
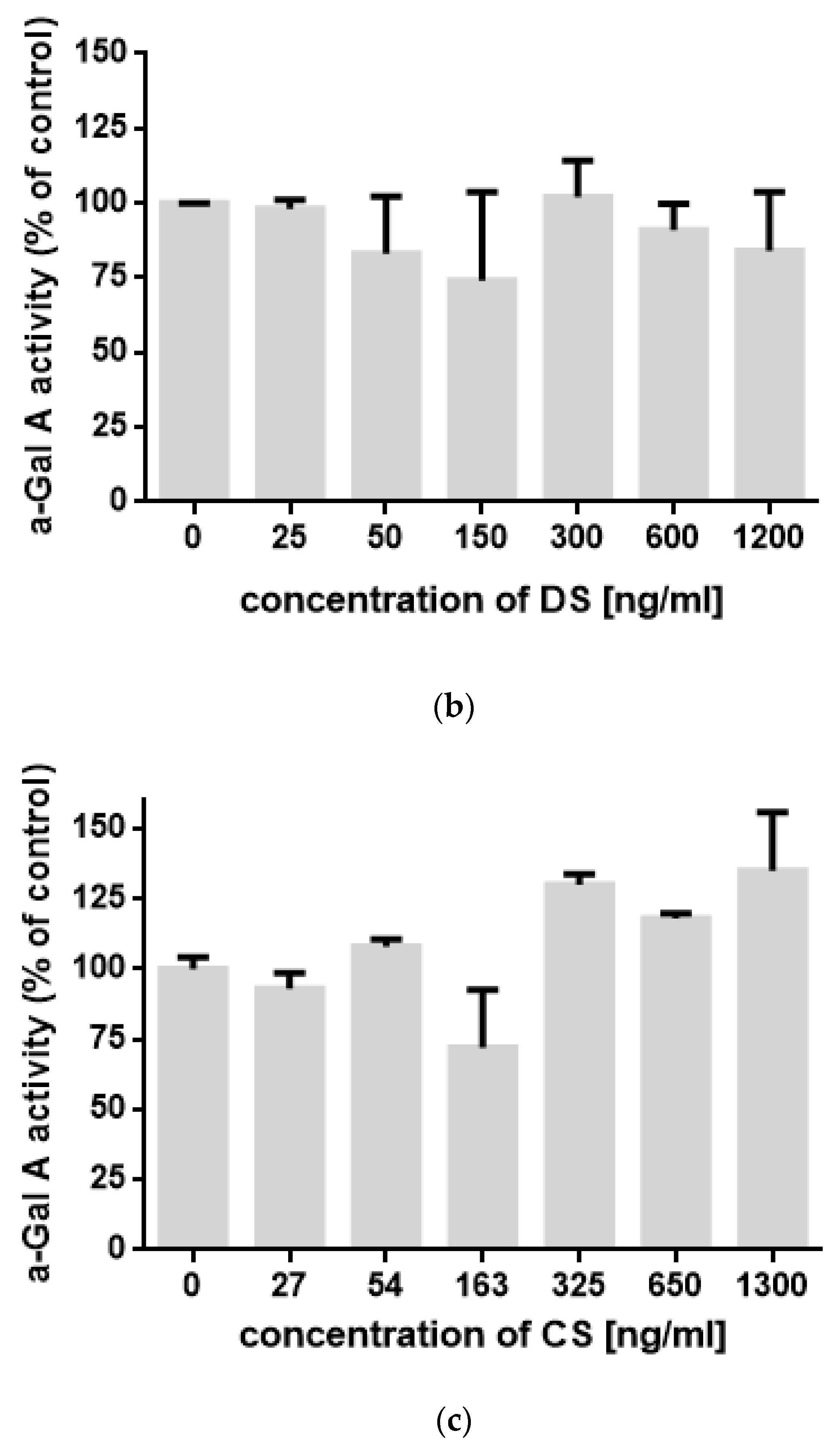
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Sample Preparation
4.3. Measurement of LysoGb3 Concentration
4.4. Measurement of α-Gal A Activity In Vitro
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MPSs | Mucopolysaccharidoses |
| GAGs | Glycosaminoglycans |
| CNS | Central nervous system |
| HS | Heparan sulfate |
| LacCer | Lactosylceramide |
| Gb3 | Globotriaosylceramide |
| LysoGb3 | Globotriaosylsphingosine |
| DBSs | Dried blood spots |
| HPLC-MS/MS | High pressure liquid chromatography–tandem mass spectrometry |
| LSDs | Lysosomal storage disorders |
| LysoSLs | Lysosphingolipids |
| α-Gal A | α-galactosidase A |
References
- Jurecka, A.; Ługowska, A.; Golda, A.; Czartoryska, B.; Tylki-Szymańska, A. Prevalence rates of mucopolysaccharidoses in Poland. J. Appl. Genet. 2015, 56, 205–210. [Google Scholar] [CrossRef]
- Baehner, F.; Schmiedeskamp, C.; Krummenauer, F.; Miebach, E.; Bajbouj, M.; Whybra, C.; Kohlschütter, A.; Kampmann, C.; Beck, M. Cumulative incidence rates of the mucopolysaccharidoses in Germany. JIMD 2005, 28, 1011–1017. [Google Scholar] [CrossRef]
- Turkia, B.; Tebib, N.; Azzouz, H.; Abdelmoula, M.S.; Ben Chehida, A.; Chemli, J.; Monastiri, K.; Chaabouni, M.; Sanhagi, H.; Zouari, B.; et al. Incidence of mucopolysaccharidoses in Tunisia. Tunis Med. 2009, 87, 782–785. [Google Scholar] [PubMed]
- Barone, R.; Pellico, A.; Pittalà, A.; Gasperini, S. Neurobehavioral phenotypes of neuronopathic mucopolysaccharidoses. Ital. J. Pediatr. 2018, 44, 121. [Google Scholar] [CrossRef] [PubMed]
- Zafeiriou, D.I.; Batzios, S.P. Brain and spinal MR imaging findings in mucopolysaccharidoses: A review. Am. J. Neuroradiol. 2013, 34, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Seto, T.; Kono, K.; Morimoto, K.; Inoue, Y.; Shintaku, H.; Hattori, H.; Matsuoka, O.; Yamano, T.; Tanaka, A. Brain magnetic resonance imaging in 23 patients with mucopolysaccharidoses and the effect of bone marrow transplantation. Ann. Neurol. 2001, 50, 79–92. [Google Scholar] [CrossRef]
- Villani, G.; Gargiulo, N.; Faraonio, R.; Castaldo, S.; Gonzalez Y Reyero, E.; Di Natale, P. Cytokines, neurotrophins, and oxidative stress in brain disease from mucopolysaccharidosis IIIB. J. Neurosci. Res. 2007, 85, 612–622. [Google Scholar] [CrossRef]
- Langereis, E.J.; Wagemans, T.; Kulik, W.; Lefeber, D.J.; van Lenthe, H.; Oussoren, E.; van der Ploeg, A.T.; Ruijter, G.J.; Wevers, R.A.; Wijburg, F.A.; et al. A Multiplex assay for the diagnosis of mucopolysaccharidoses and mucolipidoses. PLoS ONE 2015, 10, e0138622. [Google Scholar]
- Stapleton, M.; Kubaski, F.; Mason, R.W.; Shintaku, H.; Kobayashi, H.; Yamaguchi, S.; Taketani, T.; Suzuki, Y.; Orii, K.; Orii, T.; et al. Newborn screening for mucopolysaccharidoses: Measurement of glycosaminoglycans by LC-MS/MS. Mol. Genet. Metab. Rep. 2020, 22, 100563. [Google Scholar] [CrossRef]
- Kubaski, F.; Suzuki, Y.; Orii, K.; Giugliani, R.; Church, H.J.; Mason, R.W.; Dũng, V.C.; Ngoc, C.T.; Yamaguchi, S.; Kobayashi, H.; et al. Glycosaminoglycan levels in dried blood spots of patients with mucopolysaccharidoses and mucolipidoses. Mol. Genet. Metab. 2017, 120, 247–254. [Google Scholar] [CrossRef]
- Muenzer, J. Early initiation of enzyme replacement therapy for the mucopolysaccharidoses. Mol. Genet. Metab. 2014, 111, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Breen, C.; Heap, F.; Rust, S.; de Ruijter, J.; Tump, E.; Marchal, J.P.; Pan, L.; Qiu, Y.; Chung, J.K.; et al. A phase 1/2 study of intrathecal heparan-N-sulfatase in patients with mucopolysaccharidosis IIIA. Mol. Genet. Metab. 2016, 118, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.; Convit, J. Inhibition of leucocytic lysosomal enzymes by glycosaminoglycans in vitro. Biochem. J. 1975, 152, 57–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kint, J.; Dacremont, G.; Carton, D.; Orye, E.; Hooft, C. Mucopolysaccharidosis: Secondarily induced abnormal distribution of lysosomal isoenzymes. Science 1973, 181, 352–354. [Google Scholar] [CrossRef]
- Walkley, S.; Vanier, M.T. Pathomechanisms in Lysosomal Storage Disorders. Biochim. Biophys. Acta 2009, 1793, 726–736. [Google Scholar] [CrossRef]
- Hulkova, H.; Ledvinova, J.; Asfaw, B.; Koubek, K.; Kopriva, K.; Elleder, M. Lactosylceramide in lysosomal storage disorders: A comparative immunohistochemical and biochemical study. J. Pathol. 2005, 447, 31–44. [Google Scholar]
- Hara, A.; Kitazawa, N.; Taketomi, T. Abnormalities of glycosphingolipids in mucopolysaccharidosis type III B. J. Lipid. Res. 1984, 25, 175–184. [Google Scholar]
- Moskot, M.; Bocheńska, K.; Jakóbkiewicz-Banecka, J.; Banecki, B.; Gabig-Cimińska, M. Abnormal Sphingolipid World in Inflammation Specific for Lysosomal Storage Diseases and Skin Disorders. Int. J. Mol. Sci. 2018, 19, 247. [Google Scholar] [CrossRef]
- Viana, G.; Priestman, A.; Platt, F.; Khan, S.; Tomatsu, S.; Pshezhetsky, A. Brain Pathology in Mucopolysaccharidoses (MPS) Patients with Neurological Forms. J. Clin. Med. 2020, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.J.; Aoki, K.; Moehring, F.; Murphy, C.A.; O’Hara, C.L.; Tiemeyer, M.; Stucky, C.L.; Dahms, N.M. Neuropathic pain in a Fabry disease rat model. JCI Insight. 2018, 3, e99171. [Google Scholar] [CrossRef] [PubMed]
- Heywood, W.E.; Doykov, I.; Spiewak, J.; Hallqvist, J.; Mills, K.; Nowak, A. Global glycosphingolipid analysis in urine and plasma of female Fabry disease patients. BBA 2019, 1865, 2726–2735. [Google Scholar] [CrossRef] [PubMed]
- Vitner, E.B.; Platt, F.M.; Futerman, A.H. Common and uncommon pathogenic cascades in lysosomal storage diseases. J. Biol. Chem. 2010, 285, 20423–20427. [Google Scholar] [CrossRef] [PubMed]
- Pchelina, S.; Baydakova, G.; Nikolaev, M.; Senkevich, K.; Emelyanov, A.; Kopytova, A.; Miliukhina, I.; Yakimovskii, A.; Timofeeva, A.; Berkovich, O.; et al. Blood lysosphingolipids accumulation in patients with parkinson’s disease with glucocerebrosidase 1 mutations. Mov. Disord. 2018, 33, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Kotanko, P.; Kramar, R.; Devrnja, D.; Paschke, E.; Voigtländer, T.; Auinger, M.; Pagliardini, S.; Spada, M.; Demmelbauer, K.; Lorenz, M.; et al. Results of a nationwide screening for Anderson-Fabry disease among dialysis patients. J. Am. Soc. Nephrol. 2004, 15, 1323–1329. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baydakova, G.; Ilyushkina, A.; Gaffke, L.; Pierzynowska, K.; Bychkov, I.; Ługowska, A.; Wegrzyn, G.; Tylki-Szymanska, A.; Zakharova, E. Elevated LysoGb3 Concentration in the Neuronopathic Forms of Mucopolysaccharidoses. Diagnostics 2020, 10, 155. https://doi.org/10.3390/diagnostics10030155
Baydakova G, Ilyushkina A, Gaffke L, Pierzynowska K, Bychkov I, Ługowska A, Wegrzyn G, Tylki-Szymanska A, Zakharova E. Elevated LysoGb3 Concentration in the Neuronopathic Forms of Mucopolysaccharidoses. Diagnostics. 2020; 10(3):155. https://doi.org/10.3390/diagnostics10030155
Chicago/Turabian StyleBaydakova, Galina, Alex Ilyushkina, Lidia Gaffke, Karolina Pierzynowska, Igor Bychkov, Agnieszka Ługowska, Grzegorz Wegrzyn, Anna Tylki-Szymanska, and Ekaterina Zakharova. 2020. "Elevated LysoGb3 Concentration in the Neuronopathic Forms of Mucopolysaccharidoses" Diagnostics 10, no. 3: 155. https://doi.org/10.3390/diagnostics10030155
APA StyleBaydakova, G., Ilyushkina, A., Gaffke, L., Pierzynowska, K., Bychkov, I., Ługowska, A., Wegrzyn, G., Tylki-Szymanska, A., & Zakharova, E. (2020). Elevated LysoGb3 Concentration in the Neuronopathic Forms of Mucopolysaccharidoses. Diagnostics, 10(3), 155. https://doi.org/10.3390/diagnostics10030155









