Cardiac Magnetic Resonance versus Single-Photon Emission Computed Tomography for Detecting Coronary Artery Disease and Myocardial Ischemia: Comparison with Coronary Angiography
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. SPECT MPI
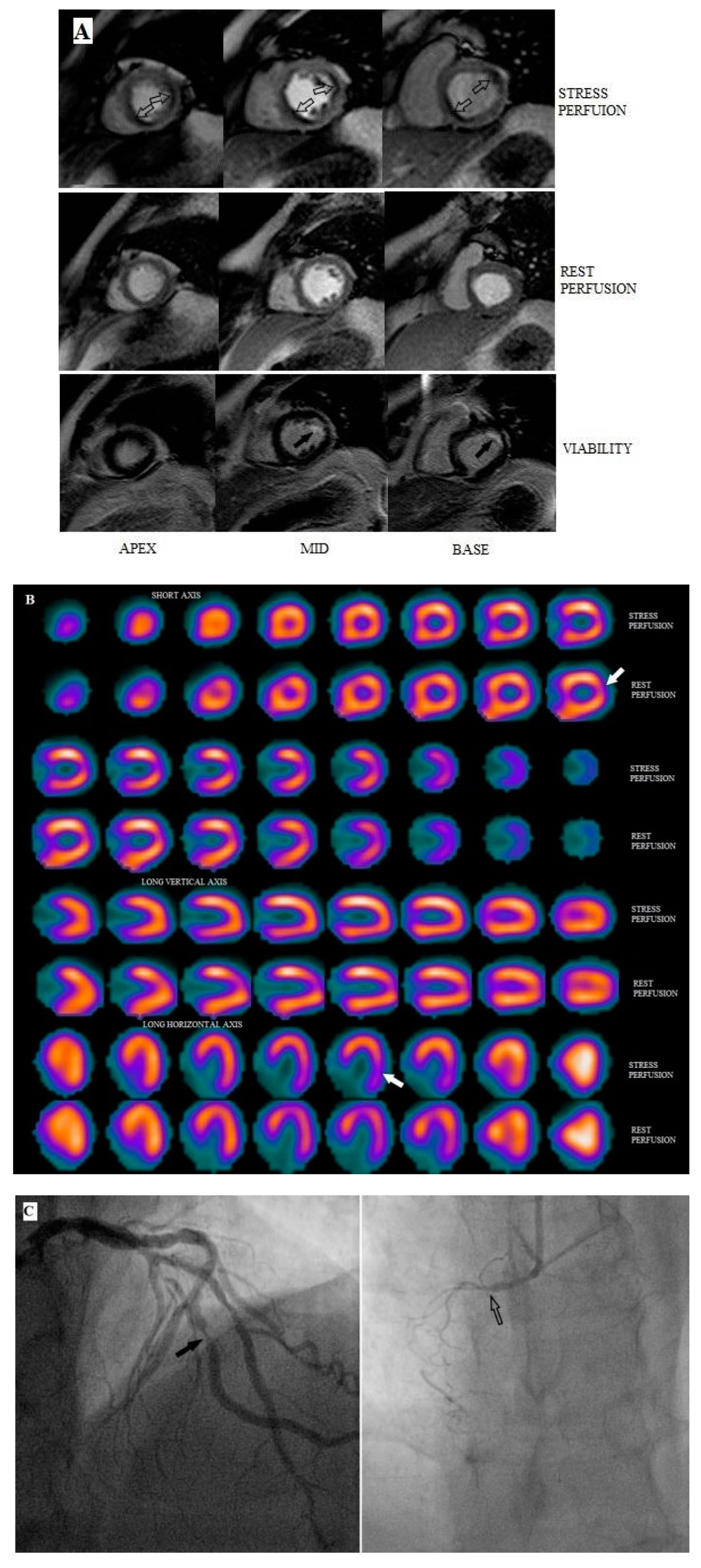
2.3. CMR MPI
2.4. Coronary Angiography
2.5. Comparison of the Procedures
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart Disease and Stroke Statistics--2015 Update: A Report From the American Heart Association. Circulation 2015, 131, 29–322. [Google Scholar] [CrossRef]
- Schuijf, J.D.; Shaw, L.J.; Wijns, W.; Lamb, H.J.; Poldermans, D.; de Roos, A.; van der Wall, E.E.; Bax, J.J. Cardiac imaging in coronary artery disease: Differing modalities. Heart 2005, 91, 1110–1117. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Neglia, D.; Rovai, D.; Caselli, C.; Pietila, M.; Teresinska, A.; Aguadé-Bruix, S.; Pizzi, M.N.; Todiere, G.; Gimelli, A.; Schroeder, S.; et al. Detection of Significant Coronary Artery Disease by Noninvasive Anatomical and Functional Imaging. Circ. Cardiovasc. Imaging 2015, 8, e002179. [Google Scholar] [CrossRef]
- Greenwood, J.P.; Maredia, N.; Younger, J.F.; Brown, J.M.; Nixon, J.; Everett, C.C.; Bijsterveld, P.; Ridgway, J.P.; Radjenovic, A.; Dickinson, C.J.; et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): A prospective trial. Lancet 2012, 379, 453–460. [Google Scholar] [CrossRef]
- Sparrow, P.; Plein, S.; Jones, T.R.; Thorley, P.J.; Hale, C.; Sivananthan, M.U. Tolerance of MRI vs. SPECT myocardial perfusion studies--a patient survey. J. Magn. Reson. Imaging 2004, 19, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Sakuma, H.; Motoyasu, M.; Okinaka, T.; Isaka, N.; Nakano, T.; Takeda, K. Noninfarcted myocardium: Correlation between dynamic first-pass contrast-enhanced myocardial MR imaging and quantitative coronary angiography. Radiology 2003, 229, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Pilz, G.; Eierle, S.; Heer, T.; Klos, M.; Ali, E.; Scheck, R.; Wild, M.; Bernhardt, P.; Hoefling, B. Negative predictive value of normal adenosine-stress cardiac MRI in the assessment of coronary artery disease and correlation with semiquantitative perfusion analysis. J. Magn. Reson. Imaging 2010, 32, 615–621. [Google Scholar] [CrossRef]
- Ebersberger, U.; Makowski, M.R.; Schoepf, U.J.; Platz, U.; Schmidtler, F.; Rose, J.; Kessel, A.; Roth, P.; Antoni, D.; Schnackenburg, B.; et al. Magnetic resonance myocardial perfusion imaging at 3.0 Tesla for the identification of myocardial ischaemia: Comparison with coronary catheter angiography and fractional flow reserve measurements. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 1174–1180. [Google Scholar] [CrossRef]
- Schwitter, J.; Wacker, C.M.; Van Rossum, A.C.; Lombardi, M.; Al-Saadi, N.; Ahlstrom, H.; Dill, T.; Larsson, H.B.; Flamm, S.D.; Marquardt, M.; et al. MR-IMPACT: Comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur. Heart J. 2008, 29, 480–489. [Google Scholar] [CrossRef]
- Schwitter, J.; Wacker, C.M.; Wilke, N.; Al-Saadi, N.; Sauer, E.; Huettle, K.; Schönberg, S.O.; Luchner, A.; Strohm, O.; Ahlstrom, H.; et al. MR-IMPACT II: Magnetic resonance imaging for myocardial perfusion assessment in coronary artery disease trial: Perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: A comparative. Eur. Heart J. 2013, 34, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Plein, S.; Herzog, B.A.; Maredia, N.; Kidambi, A.; Motwani, M.; Uddin, A.; Ripley, D.P.; Dickinson, C.J.; Brown, J.; Nixon, J.; et al. The ischaemic and scar burden measured by cardiac magnetic resonance imaging in patients with ischaemic coronary heart disease from the CE-MARC study. J. Cardiovasc. Magn. Reson. 2013, 15, 15–16. [Google Scholar] [CrossRef]
- Jogiya, R.; Morton, G.; De Silva, K.; Reyes, E.; Hachamovitch, R.; Kozerke, S.; Nagel, E.; Underwood, S.R.; Plein, S. Ischemic burden by 3-dimensional myocardial perfusion cardiovascular magnetic resonance: Comparison with myocardial perfusion scintigraphy. Circ. Cardiovasc. Imaging 2014, 7, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Alfakih, K.; Greenwood, J.P.; Plein, S. The 2016 update to NICE CG95 guideline for the investigation of new onset stable chest pain: More innovation, but at a cost? Clin. Med. J. R. Coll. Phys. Lond. 2017, 17, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Montalescot, G.; Sechtem, U.; Achenbach, S.; Andreotti, F.; Arden, C.; Budaj, A.; Bugiardini, R.; Crea, F.; Cuisset, T.; Di Mario, C.; et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 2013, 34, 2949–3003. [Google Scholar] [PubMed]
- Dvorak, R.A.; Brown, R.K.; Corbett, J.R. Interpretation of SPECT/CT Myocardial Perfusion Images: Common Artifacts and Quality Control Techniques. Radiographics 2011, 31, 2041–2057. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. J. Nucl. Cardiol. 2002, 9, 240–245. [Google Scholar] [CrossRef]
- De Jong, M.C.; Genders, T.S.; van Geuns, R.J.; Moelker, A.; Hunink, M.G. Diagnostic performance of stress myocardial perfusion imaging for coronary artery disease: A systematic review and meta-analysis. Eur. Radiol. 2012, 22, 1881–1895. [Google Scholar] [CrossRef]
- Jaarsma, C.; Leiner, T.; Bekkers, S.C.; Crijns, H.J.; Wildberger, J.E.; Nagel, E.; Nelemans, P.J.; Schalla, S. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: A meta-anal. J. Am. Coll. Cardiol. 2012, 59, 1719–1728. [Google Scholar] [CrossRef]
- Buckert, D.; Dewes, P.; Walcher, T.; Rottbauer, W.; Bernhardt, P. Intermediate-term prognostic value of reversible perfusion deficit diagnosed by adenosine CMR: A prospective follow-up study in a consecutive patient population. JACC Cardiovasc. Imaging 2013, 6, 56–63. [Google Scholar] [CrossRef]
- Steel, K.; Broderick, R.; Gandla, V.; Larose, E.; Resnic, F.; Jerosch-Herold, M.; Brown, K.A.; Kwong, R.Y. Complementary prognostic values of stress myocardial perfusion and late gadolinium enhancement imaging by cardiac magnetic resonance in patients with known or suspected coronary artery disease. Circulation 2009, 120, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Takx, R.A.; Blomberg, B.A.; El Aidi, H.E.; Habets, J.; de Jong, P.A.; Nagel, E.; Hoffmann, U.; Leiner, T. Diagnostic Accuracy of Stress Myocardial Perfusion Imaging Compared to Invasive Coronary Angiography With Fractional Flow Reserve Meta-Analysis. Circ. Cardiovasc. Imaging 2015, 8, e002666. [Google Scholar] [CrossRef] [PubMed]
- Ohba, T.; Takano, H.; Kunimi, T.; Fujita, N.; Kodani, E.; Mizuno, K. Direct comparison between pharmacological stress with adenosine triphosphate disodium and exercise stress myocardial perfusion imagings. J. Cardiol. 2008, 52, 30–38. [Google Scholar] [CrossRef] [PubMed]


| Patient Characteristics | N (%) |
|---|---|
| Patients enrolled | 30 |
| Male gender | 24 (80%) |
| Mean age (Age range) (years) | 65.3 (46–75) |
| Mean Body Mass Index (kg/m2) | 28.4 |
| Previous stent placement | 5 (17%) |
| Previous coronary artery bypass graft surgery (CABG) | 2 (7%) |
| Family history of coronary artery disease (CAD) | 14 (47%) |
| Current Smokers | 5 (17%) |
| Hypertension | 17 (57%) |
| Hypercholesterolemia | 16 (53%) |
| Diabetes mellitus | 5 (17%) |
| ≥70% stenosis by selective coronary angiography (SCA) | 9 (30%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laspas, F.; Pipikos, T.; Karatzis, E.; Georgakopoulos, N.; Prassopoulos, V.; Andreou, J.; Moulopoulos, L.A.; Chatziioannou, A.; Danias, P.G. Cardiac Magnetic Resonance versus Single-Photon Emission Computed Tomography for Detecting Coronary Artery Disease and Myocardial Ischemia: Comparison with Coronary Angiography. Diagnostics 2020, 10, 190. https://doi.org/10.3390/diagnostics10040190
Laspas F, Pipikos T, Karatzis E, Georgakopoulos N, Prassopoulos V, Andreou J, Moulopoulos LA, Chatziioannou A, Danias PG. Cardiac Magnetic Resonance versus Single-Photon Emission Computed Tomography for Detecting Coronary Artery Disease and Myocardial Ischemia: Comparison with Coronary Angiography. Diagnostics. 2020; 10(4):190. https://doi.org/10.3390/diagnostics10040190
Chicago/Turabian StyleLaspas, Fotios, Theodoros Pipikos, Emmanouil Karatzis, Nikolaos Georgakopoulos, Vasileios Prassopoulos, John Andreou, Lia A. Moulopoulos, Achilleas Chatziioannou, and Peter G. Danias. 2020. "Cardiac Magnetic Resonance versus Single-Photon Emission Computed Tomography for Detecting Coronary Artery Disease and Myocardial Ischemia: Comparison with Coronary Angiography" Diagnostics 10, no. 4: 190. https://doi.org/10.3390/diagnostics10040190
APA StyleLaspas, F., Pipikos, T., Karatzis, E., Georgakopoulos, N., Prassopoulos, V., Andreou, J., Moulopoulos, L. A., Chatziioannou, A., & Danias, P. G. (2020). Cardiac Magnetic Resonance versus Single-Photon Emission Computed Tomography for Detecting Coronary Artery Disease and Myocardial Ischemia: Comparison with Coronary Angiography. Diagnostics, 10(4), 190. https://doi.org/10.3390/diagnostics10040190




