Loss of Integrase Interactor 1 (INI1) Expression in a Subset of Differentiated Thyroid Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Immunohistochemistry
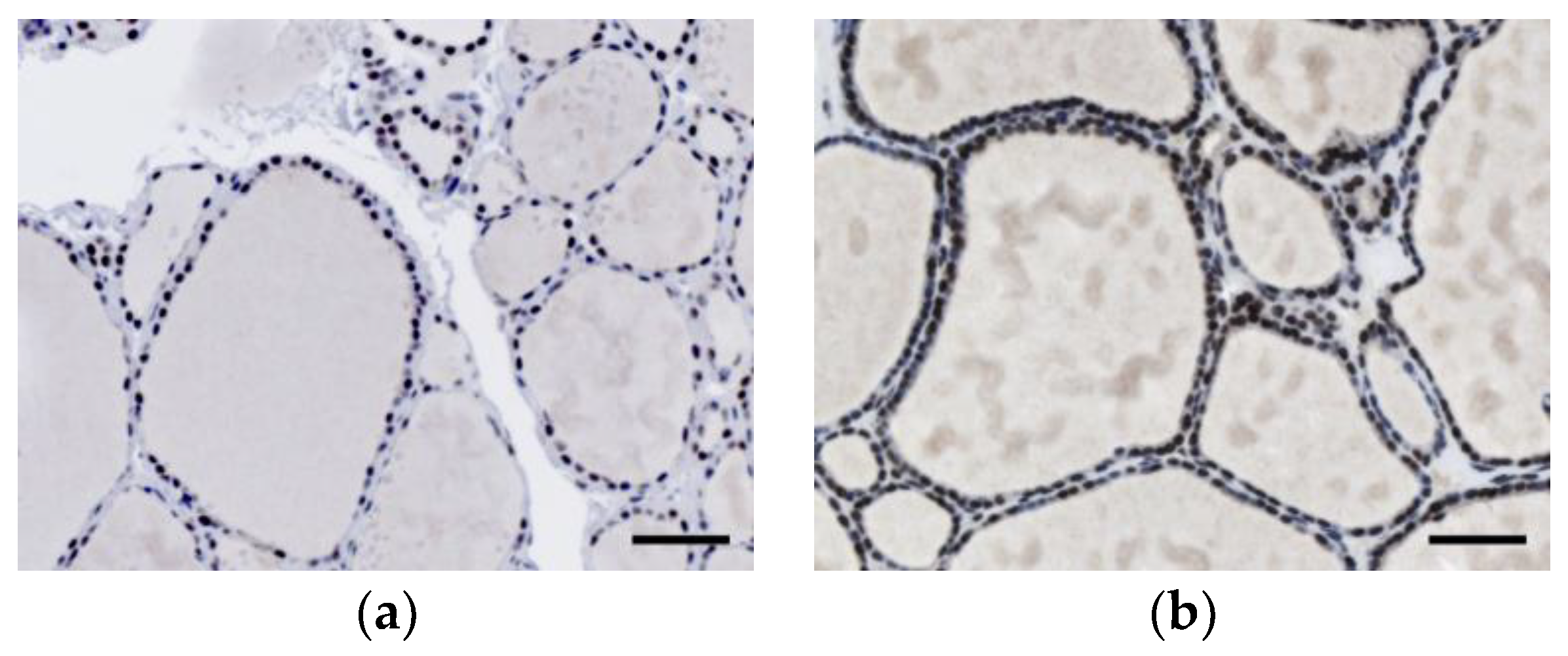
2.3. Interpretation of INI1 Staining
2.4. Analysis of Publicly Available Genomics Dataset
2.5. Analysis of The Cancer Genome Atlas (TCGA)
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Zaballos, M.A.; Santisteban, P. Key signaling pathways in thyroid cancer. J. Endocrinol. 2017, 235, R43–R61. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ghossein, R. Genomic Landscape of poorly Differentiated and Anaplastic Thyroid Carcinoma. Endocr. Pathol. 2016, 27, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Masliah-Planchon, J.; Bièche, I.; Guinebretière, J.-M.; Bourdeaut, F.; Delattre, O. SWI/SNF Chromatin Remodeling and Human Malignancies. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, G.; Marmon, S.; Wang, W.; Crabtree, G.; Goff, S. Binding and stimulation of HIV-1 integrase by a human homolog of yeast transcription factor SNF5. Science 1994, 266, 2002–2006. [Google Scholar] [CrossRef]
- Versteege, I.; Sevenet, N.; Lange, J.; Rousseau-Merck, M.F.; Ambros, P.; Handgretinger, R.; Aurias, A.; Delattre, O. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature 1998, 394, 203–206. [Google Scholar] [CrossRef]
- Kalimuthu, S.N.; Chetty, R. Gene of the month: SMARCB1. J. Clin. Pathol. 2016, 69, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.-P.; Liu, C.-L.; Chen, M.-J.; Chien, M.-N.; Leung, C.-H.; Lin, C.-H.; Hsu, Y.-C.; Lee, J.-J. CD74 expression and its therapeutic potential in thyroid carcinoma. Endocrine-Related Cancer 2015, 22, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-J.; Wang, T.-Y.; Liu, C.-L.; Chien, M.-N.; Chen, M.-J.; Hsu, Y.-C.; Leung, C.-H.; Cheng, S.-P. Dipeptidyl peptidase IV as a prognostic marker and therapeutic target in papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2017, 102, 2930–2940. [Google Scholar] [CrossRef]
- Cheng, S.-P.; Chen, M.-J.; Chien, M.-N.; Lin, C.-H.; Lee, J.-J.; Liu, C.-L. Overexpression of teneurin transmembrane protein 1 is a potential marker of disease progression in papillary thyroid carcinoma. Clin. Exp. Med. 2016, 17, 555–564. [Google Scholar] [CrossRef]
- Vasko, V.; Espinosa, A.V.; Scouten, W.; He, H.; Auer, H.; Liyanarachchi, S.; Larin, O.; Savchenko, V.; Francis, G.L.; De La Chapelle, A.; et al. Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc. Natl. Acad. Sci. USA 2007, 104, 2803–2808. [Google Scholar] [CrossRef] [Green Version]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, M.-N.; Yang, P.S.; Lee, J.-J.; Wang, T.-Y.; Hsu, Y.-C.; Cheng, S.-P. Recurrence-associated genes in papillary thyroid cancer: An analysis of data from The Cancer Genome Atlas. Surgery 2017, 161, 1642–1650. [Google Scholar] [CrossRef]
- Chien, M.-N.; Yang, P.S.; Hsu, Y.-C.; Liu, T.-P.; Lee, J.-J.; Cheng, S.-P. Transcriptome analysis of papillary thyroid cancer harboring telomerase reverse transcriptase promoter mutation. Head Neck 2018, 40, 2528–2537. [Google Scholar] [CrossRef]
- Hsu, Y.; Lee, J.; Chien, M.; Chen, M.; Leung, C.; Cheng, S.-P. Is papillary thyroid microcarcinoma a biologically different disease? A propensity score-matched analysis. J. Surg. Oncol. 2019, 120, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.-Y.; Yang, P.-S.; Chien, M.-N.; Cheng, S.-P. Preoperative Factors Associated with Extrathyroidal Extension in Papillary Thyroid Cancer. Eur. Thyroid. J. 2020, 1–7. [Google Scholar] [CrossRef]
- Kaneko, T.; Mitsui, T.; Kaneko, K.; Kadoya, M.; Initiative, A.D.N. New longitudinal Visual Rating Scale Identifies Structural Alterations in People with Mild Cognitive Impairment and Those who are Cognitively Normal. Int. J. Gerontol. 2018, 13, 69–75. [Google Scholar] [CrossRef]
- Taylor, A.M.; Zhang, X.; Wang, C.; Liu, J.; Caesar-Johnson, S.J.; Demchok, J.A.; Felau, I.; Kasapi, M.; Ferguson, M.L.; Hutter, C.M.; et al. Genomic and Functional Approaches to Understanding Cancer Aneuploidy. Cancer Cell 2018, 33, 676–689. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Ribeiro-Silva, C.; Vermeulen, W.; Lans, H. SWI/SNF: Complex complexes in genome stability and cancer. DNA Repair 2019, 77, 87–95. [Google Scholar] [CrossRef]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.; Shah, R.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahimpasic, T.; Xu, B.; Landa, I.; Dogan, S.; Middha, S.; Seshan, V.; Deraje, S.; Carlson, D.L.; Migliacci, J.; Knauf, J.A.; et al. Genomic Alterations in Fatal Forms of Non-Anaplastic Thyroid Cancer: Identification of MED12 and RBM10 as Novel Thyroid Cancer Genes Associated with Tumor Virulence. Clin. Cancer Res. 2017, 23, 5970–5980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozdeyev, N.; Gay, L.M.; Hartmaier, R.J.; Davis, S.N.; Borre, P.V.; Tan, A.-C.; Schweppe, R.; Fishbein, L.; Ross, J.S.; HaugenMD, B.R.; et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, E.; Song, D.E.; Ahn, J.; Kim, T.Y.; Kim, W.B.; Shong, Y.K.; Jeon, M.J.; Kim, W.G. Genetic profile of advanced thyroid cancers in relation to distant metastasis. Endocr. Relat. Cancer 2020, 27, 285–293. [Google Scholar] [CrossRef]
- Landa, I.; Pozdeyev, N.; Korch, C.; Marlow, L.A.; Smallridge, R.C.; Copland, J.A.; Henderson, Y.C.; Lai, S.Y.; Clayman, G.L.; Onoda, N.; et al. Comprehensive Genetic Characterization of Human Thyroid Cancer Cell Lines: A Validated Panel for Preclinical Studies. Clin. Cancer Res. 2019, 25, 3141–3151. [Google Scholar] [CrossRef]
- Agaimy, A.; Foulkes, W.D. Hereditary SWI/SNF complex deficiency syndromes. Semin. Diagn. Pathol. 2018, 35, 193–198. [Google Scholar] [CrossRef]
- Sondka, Z.; Bamford, S.; Cole, C.G.; Ward, S.A.; Dunham, I.; Forbes, S.A. The COSMIC Cancer Gene Census: Describing genetic dysfunction across all human cancers. Nat. Rev. Cancer 2018, 18, 696–705. [Google Scholar] [CrossRef]
- Borowczyk, M.; Szczepanek-Parulska, E.; Dębicki, S.; Budny, B.; A Verburg, F.; Filipowicz, D.; Więckowska, B.; Janicka-Jedyńska, M.; Gil, L.; Ziemnicka, K.; et al. Differences in Mutational Profile between Follicular Thyroid Carcinoma and Follicular Thyroid Adenoma Identified Using Next Generation Sequencing. Int. J. Mol. Sci. 2019, 20, 3126. [Google Scholar] [CrossRef] [Green Version]
- Wreesmann, V.; Ghossein, R.A.; Hezel, M.; Banerjee, D.; Shaha, A.R.; Tuttle, R.M.; Shah, J.P.; Rao, P.H.; Singh, B. Follicular variant of papillary thyroid carcinoma: Genome-wide appraisal of a controversial entity. Genes Chromosom. Cancer 2004, 40, 355–364. [Google Scholar] [CrossRef]
- Hemmer, S.; Wasenius, V.-M.; Knuutila, S.; Franssila, K.; Joensuu, H. DNA Copy Number Changes in Thyroid Carcinoma. Am. J. Pathol. 1999, 154, 1539–1547. [Google Scholar] [CrossRef] [Green Version]
- Agaimy, A. The expanding family of SMARCB1(INI1)-deficient neoplasia: Implications of phenotypic, biological, and molecular heterogeneity. Adv. Anat. Pathol. 2014, 21, 394–410. [Google Scholar] [CrossRef]
- Wang, J.; Andrici, J.; Sioson, L.; Clarkson, A.; Sheen, A.; Farzin, M.; Toon, C.; Turchini, J.; Gill, A.J. Loss of INI1 expression in colorectal carcinoma is associated with high tumor grade, poor survival, BRAFV600E mutation, and mismatch repair deficiency. Hum. Pathol. 2016, 55, 83–90. [Google Scholar] [CrossRef]
- Agarwal, S.; Kakkar, A.; Damle, N.A.; Kumar, C.; Sarangi, J.; Subudhi, K.; Jain, D.; Sharma, M.C. SMARCB1 (INI1)-deficient thyroid carcinoma: A novel entity expanding the spectrum of tumors with INI1 loss. Pathol. Res. Pr. 2020, 216, 152830. [Google Scholar] [CrossRef]
- Garcia-Rendueles, M.E.; Ricarte-Filho, J.C.; Untch, B.R.; Landa, I.; Knauf, J.; Voza, F.; Smith, V.; Ganly, I.; Taylor, B.S.; Persaud, Y.; et al. NF2 Loss Promotes Oncogenic RAS-Induced Thyroid Cancers via YAP-Dependent Transactivation of RAS Proteins and Sensitizes Them to MEK Inhibition. Cancer Discov. 2015, 5, 1178–1193. [Google Scholar] [CrossRef] [Green Version]
- Wilson, B.G.; Wang, X.; Shen, X.; McKenna, E.S.; Lemieux, M.E.; Cho, Y.J.; Koellhoffer, E.C.; Pomeroy, S.L.; Orkin, S.H.; Roberts, C.W. Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell 2010, 18, 316–328. [Google Scholar] [CrossRef] [Green Version]
- Knutson, S.K.; Warholic, N.M.; Wigle, T.J.; Klaus, C.R.; Allain, C.J.; Raimondi, A.; Scott, M.P.; Chesworth, R.; Moyer, M.P.; Copeland, R.A.; et al. Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc. Natl. Acad. Sci. USA 2013, 110, 7922–7927. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-C.; Chien, M.-N.; Chang, Y.-C.; Lee, J.-J.; Dai, S.-H.; Cheng, S.-P. Overexpression of Histone H3 Lysine 27 Trimethylation Is Associated with Aggressiveness and Dedifferentiation of Thyroid Cancer. Endocr. Pathol. 2019, 30, 305–311. [Google Scholar] [CrossRef]
- Kohashi, K.; Oda, Y. Oncogenic roles of SMARCB1/INI1 and its deficient tumors. Cancer Sci. 2017, 108, 547–552. [Google Scholar] [CrossRef] [Green Version]





| All | INI1 Intact | INI1 Loss | p-Value | ||
|---|---|---|---|---|---|
| (n = 63) | (n = 56) | (n = 7) | |||
| Age | 0.095 | ||||
| <55 years | 45 (71%) | 42 (75%) | 3 (43%) | ||
| ≥55 years | 18 (29%) | 14 (25%) | 4 (57%) | ||
| Sex | 0.646 | ||||
| Male | 14 (22%) | 12 (21%) | 2 (29%) | ||
| Female | 49 (78%) | 44 (79%) | 5 (71%) | ||
| Type | 0.073 | ||||
| Papillary | 53 (84%) | 49 (88%) | 4 (57%) | ||
| Follicular | 10 (16%) | 7 (13%) | 3 (43%) | ||
| Extrathyroidal extension | <0.001 | ||||
| No | 48 (76%) | 47 (84%) | 1 (14%) | ||
| Yes | 15 (24%) | 9 (16%) | 6 (86%) | ||
| Lymph node metastasis | 0.038 | ||||
| No | 35 (56%) | 34 (61%) | 1 (14%) | ||
| Yes | 28 (44%) | 22 (39%) | 6 (86%) | ||
| Under | Normal | Over | p-Value | ||
|---|---|---|---|---|---|
| (n = 47) | (n = 418) | (n = 20) | |||
| Expression (RSEM) | 1119 (982–1208) | 1879 (1682–2070) | 2775 (2653–2921) | <0.001 | |
| Age (years) | 51 (37–62) | 46 (34–58) | 51 (36–57) | 0.573 | |
| Sex | 0.647 | ||||
| Male | 12 (26%) | 117 (28%) | 3 (15%) | ||
| Female | 35 (74%) | 301 (72%) | 17 (85%) | ||
| Subtype | 0.012 | ||||
| Classic | 24 (51%) | 299 (72%) | 16 (80%) | ||
| Follicular variant | 20 (43%) | 79 (19%) | 2 (10%) | ||
| Other variants | 3 (6%) | 40 (10%) | 2 (10%) | ||
| Tumor size (cm) | 2.7 (2.0–4.3) | 2.5 (1.5–4.0) | 2.4 (2.0–3.3) | 0.266 | |
| Extrathyroidal extension | 0.068 | ||||
| None | 39 (83%) | 280 (67%) | 13 (65%) | ||
| Minimal | 5 (11%) | 123 (29%) | 6 (30%) | ||
| Advanced | 3 (6%) | 15 (4%) | 1 (5%) | ||
| Lymph node metastasis | 0.072 | ||||
| N0/NX | 29 (62%) | 229 (55%) | 7 (35%) | ||
| N1 | 18 (38%) | 189 (45%) | 13 (65%) | ||
| Average ploidy | 1.99 (1.96–1.99) | 2.00 (1.96–2.00) | 2.00 (2.00–2.00) | 0.002 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, K.-C.; Lee, J.-J.; Lin, C.-H.; Leung, C.-H.; Cheng, S.-P. Loss of Integrase Interactor 1 (INI1) Expression in a Subset of Differentiated Thyroid Cancer. Diagnostics 2020, 10, 280. https://doi.org/10.3390/diagnostics10050280
Ho K-C, Lee J-J, Lin C-H, Leung C-H, Cheng S-P. Loss of Integrase Interactor 1 (INI1) Expression in a Subset of Differentiated Thyroid Cancer. Diagnostics. 2020; 10(5):280. https://doi.org/10.3390/diagnostics10050280
Chicago/Turabian StyleHo, Kung-Chen, Jie-Jen Lee, Chi-Hsin Lin, Ching-Hsiang Leung, and Shih-Ping Cheng. 2020. "Loss of Integrase Interactor 1 (INI1) Expression in a Subset of Differentiated Thyroid Cancer" Diagnostics 10, no. 5: 280. https://doi.org/10.3390/diagnostics10050280
APA StyleHo, K. -C., Lee, J. -J., Lin, C. -H., Leung, C. -H., & Cheng, S. -P. (2020). Loss of Integrase Interactor 1 (INI1) Expression in a Subset of Differentiated Thyroid Cancer. Diagnostics, 10(5), 280. https://doi.org/10.3390/diagnostics10050280





