Diagnosis of Parkinson’s Disease by A Metabolomics-Based Laboratory-Developed Test (LDT)
Abstract
1. Introduction
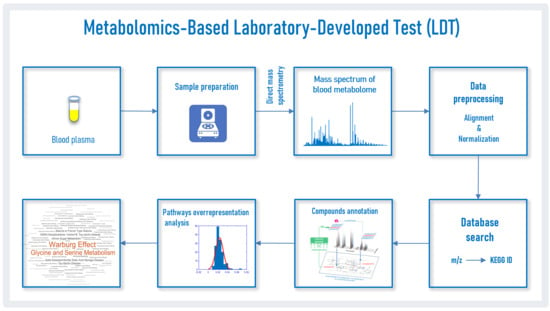
2. Materials and Methods
2.1. Mass Spectra of Blood Plasma
2.2. Mass Spectra Preprocessing
2.3. Search for Correspondence of Mass Peaks to Metabolite Identifiers
2.4. Compound Annotation Algorithm
2.5. Pathway Overrepresentation Analysis
2.6. Detection of the PD Pattern by the LDT
2.7. Analysis of Individual Samples by the LDT
3. Results
3.1. Mass Spectrometry Analysis of Compounds in Blood
3.2. Compound Annotations
3.3. Pathway Pattern of PD
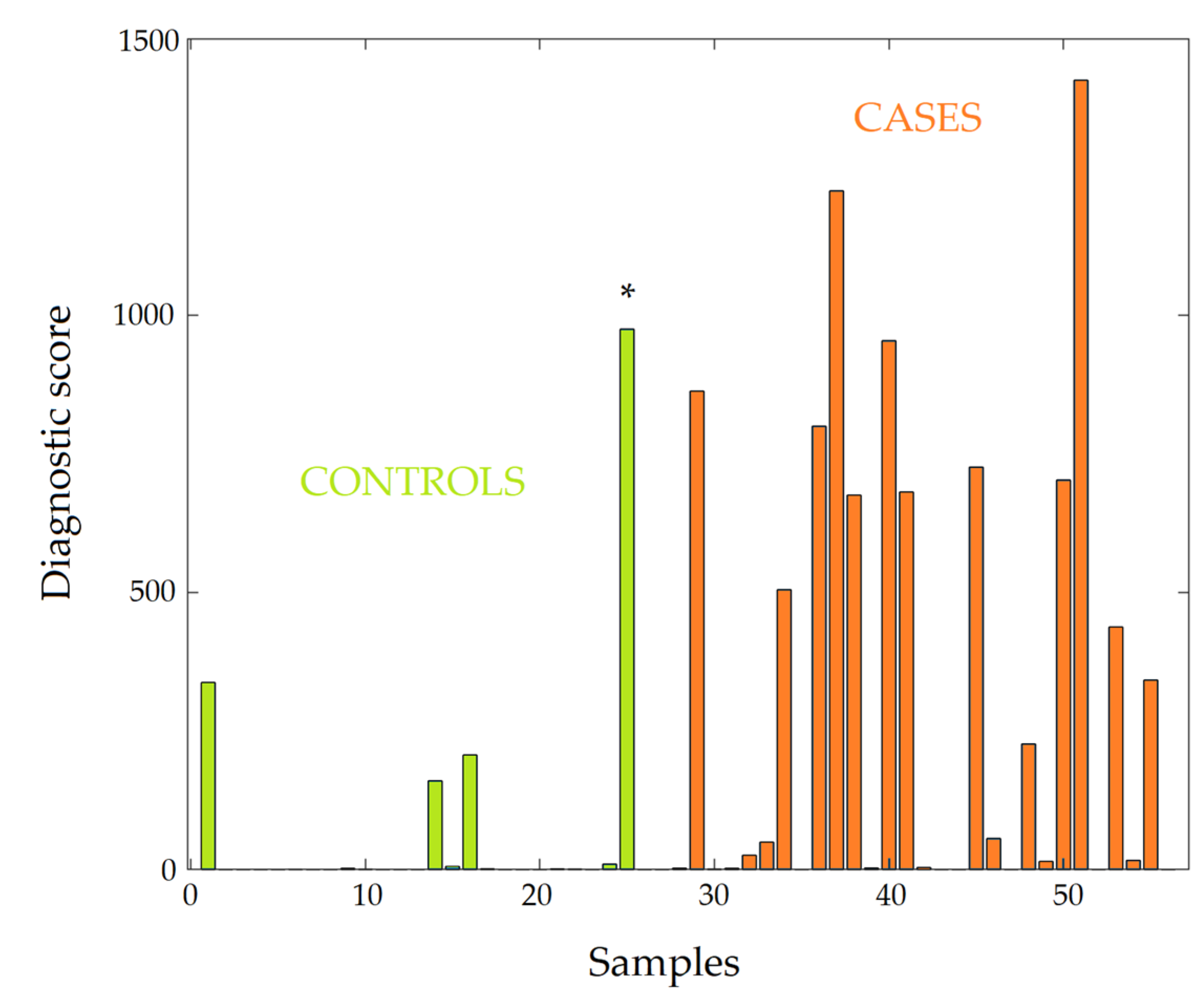
3.4. Diagnosis of PD by LDT
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sommer, A.; Winner, B.; Prots, I. The Trojan horse-Neuroinflammatory impact of T cells in neurodegenerative diseases. Mol. Neurodegener. 2017, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Powers, R. NMR Metabolomics Analysis of Parkinson’s Disease. Curr. Metab. 2013, 1, 191–209. [Google Scholar] [CrossRef]
- Chen-Plotkin, A.S.; Albin, R.; Alcalay, R.; Babcock, D.; Bajaj, V.; Bowman, D.; Buko, A.; Cedarbaum, J.; Chelsky, D.; Cookson, M.R.; et al. Finding useful biomarkers for Parkinson s disease. Sci. Transl. Med. 2018, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Trifonova, O.; Lokhov, P.; Archakov, A. Postgenomics diagnostics: Metabolomics approaches to human blood profiling. OMICS 2013, 17, 550–559. [Google Scholar] [CrossRef]
- Omenn, G.S.; DeAngelis, C.D.; DeMets, D.L.; Fleming, T.R.; Geller, G.; Gray, J.; Hayes, D.F.; Henderson, G.I.; Kessler, L.; Lapidus, S.; et al. Evolution of Translational Omics: Lessons Learned and the Path Forward-Institute of Medicine. Institute of Medicine; National Academies Press: Washington, DC, USA, 2012; ISBN 9780309224185. [Google Scholar]
- Nass, S.J.; Moses, H.L. Cancer Biomarkers: The Promises and Challenges of Improving Detection and Treatment; National Academies Press: Washington, DC, USA, 2007; ISBN 0309667119. [Google Scholar]
- FDA. Laboratory Developed Tests. Available online: https://www.fda.gov/medical-devices/vitro-diagnostics/laboratory-developed-tests (accessed on 5 October 2018).
- Genzen, J.R. Regulation of Laboratory-Developed Tests. Am. J. Clin. Pathol. 2019, 152, 122–131. [Google Scholar] [CrossRef]
- Sharfstein, J. FDA regulation of laboratory-developed diagnostic tests: Protect the public, advance the science. JAMA-J. Am. Med. Assoc. 2015, 313, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Schreier, J.; Feeney, R.; Keeling, P. Diagnostics Reform and Harmonization of Clinical Laboratory Testing. J. Mol. Diagn. 2019, 21, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Burchard, P.R.; Abou Tayoun, A.N.; Lefferts, J.A.; Lewis, L.D.; Tsongalis, G.J.; Cervinski, M.A. Development of a rapid clinical TPMT genotyping assay. Clin. Biochem. 2014, 47, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Munari, E.; Zamboni, G.; Lunardi, G.; Marconi, M.; Brunelli, M.; Martignoni, G.; Netto, G.J.; Quatrini, L.; Vacca, P.; Moretta, L.; et al. PD-L1 expression in non–small cell lung cancer: Evaluation of the diagnostic accuracy of a laboratory-developed test using clone E1L3N in comparison with 22C3 and SP263 assays. Hum. Pathol. 2019, 90, 54–59. [Google Scholar] [CrossRef]
- Fiset, P.O.; Labbé, C.; Young, K.; Craddock, K.J.; Smith, A.C.; Tanguay, J.; Pintilie, M.; Wang, R.; Torlakovic, E.; Cheung, C.; et al. Anaplastic lymphoma kinase 5A4 immunohistochemistry as a diagnostic assay in lung cancer: A Canadian reference testing center’s results in population-based reflex testing. Cancer 2019, 125, 4043–4051. [Google Scholar] [CrossRef]
- Tinawi-Aljundi, R.; King, L.; Knuth, S.T.; Gildea, M.; Ng, C.; Kahl, J.; Dion, J.; Young, C.; Schervish, E.W.; Frontera, J.R.; et al. One-year monitoring of an oligonucleotide fluorescence in situ hybridization probe panel laboratory-developed test for bladder cancer detection. Res. Rep. Urol. 2015, 7, 49–55. [Google Scholar] [PubMed]
- Brukner, I.; Eintracht, S.; Forgetta, V.; Papadakis, A.I.; Spatz, A.; Oughton, M. Laboratory-developed test for detection of acute Clostridium difficile infections with the capacity for quantitative sample normalization. Diagn. Microbiol. Infect. Dis. 2019, 95, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kulis-Horn, R.K.; Tiemann, C. Evaluation of a laboratory-developed test for simultaneous detection of norovirus and rotavirus by real-time RT-PCR on the Panther Fusion® system. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 103–112. [Google Scholar] [CrossRef]
- Calvert, J.; Saber, N.; Hoffman, J.; Das, R. Machine-learning-based laboratory developed test for the diagnosis of sepsis in high-risk patients. Diagnostics 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Lokhov, P.G.; Balashova, E.E.; Trifonova, O.P.; Maslov, D.L.; Ponomarenko, E.A.; Archakov, A.I. Mass Spectrometry-Based Metabolomics Analysis of Obese Patients’ Blood Plasma. Int. J. Mol. Sci. 2020, 21, 568. [Google Scholar] [CrossRef]
- Blochberger, A.; Jones, S. Parkinson’s disease clinical features and diagnosis. Clin. Pharm. 2011, 3, 361–366. [Google Scholar]
- Gelb, D.J.; Oliver, E.; Gilman, S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 1999, 56, 33–39. [Google Scholar] [CrossRef]
- Gibb, W.R.; Lees, A.J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 745–752. [Google Scholar] [CrossRef]
- Lokhov, P.G.; Dashtiev, M.I.; Moshkovskii, S.A.; Archakov, A.I. Metabolite profiling of blood plasma of patients with prostate cancer. Metabolomics 2009, 6, 156–163. [Google Scholar] [CrossRef]
- Lokhov, P.G.; Kharybin, O.N.; Archakov, A.I. Diagnosis of lung cancer based on direct-infusion electrospray mass spectrometry of blood plasma metabolites. Int. J. Mass Spectrom. 2011, 309, 200–205. [Google Scholar] [CrossRef]
- Lokhov, P.G.; Trifonova, O.P.; Maslov, D.L.; Archakov, A.I. Blood plasma metabolites and the risk of developing lung cancer in Russia. Eur. J. Cancer Prev. 2013, 22, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Lokhov, P.G.; Balashova, E.E.; Voskresenskaya, A.A.; Trifonova, O.P.; Maslov, D.L.; Archakov, A.I. Mass spectrometric signatures of blood plasma metabolome for disease diagnostics. Biomed. Rep. 2016, 4, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Lokhov, P.G.; Dashtiev, M.I.; Bondartsov, L.V.; Lisitsa, A.V.; Moshkovskiĭ, S.A.; Archakov, A.I. Metabolic fingerprinting of blood plasma for patients with prostate cancer. Biomeditsinskaia Khimiia 2009, 55, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Lokhov, P.G.; Trifonova, O.P.; Maslov, D.L.; Balashova, E.E.; Archakov, A.I.; Shestakova, E.A.; Shestakova, M.V.; Dedov, I.I. Diagnosing impaired glucose tolerance using direct infusion mass spectrometry of blood plasma. PLoS ONE 2014, 9, e105343. [Google Scholar] [CrossRef] [PubMed]
- Balashova, E.E.; Lokhov, P.G.; Maslov, D.L.; Trifonova, O.P.; Khasanova, D.M.; Zalyalova, Z.A.; Nigmatullina, R.R.; Archakov, A.I.; Ugrumov, M.V. Plasma Metabolome Signature in Patients with Early-stage Parkinson Disease. Curr. Metab. 2018, 6, 75–82. [Google Scholar] [CrossRef]
- Trifonova, O.P.; Lokhov, P.G.; Archakov, A.I. Metabolic profiling of human blood. Biochem. Suppl. Ser. B Biomed. Chem. 2013, 7, 179–186. [Google Scholar] [CrossRef]
- Lokhov, P.G.; Archakov, A.I. Mass spectrometry methods in metabolomics. Biochem. Suppl. Ser. B Biomed. Chem. 2009, 3, 1–9. [Google Scholar] [CrossRef]
- Lokhov, P.G.; Arckakov, A.I. Mass spectrometry methods in metabolomics. Biomeditsinskaya Khimiya 2008, 54, 497–511. [Google Scholar] [CrossRef]
- Lokhov, P.G.; Maslov, D.L.; Kharibin, O.N.; Balashova, E.E.; Archakov, A.I. Label-free data standardization for clinical metabolomics. BioData Min. 2017, 10, 10. [Google Scholar] [CrossRef]
- Viant, M.R.; Kurland, I.J.; Jones, M.R.; Dunn, W.B. How close are we to complete annotation of metabolomes? Curr. Opin. Chem. Biol. 2017, 36, 64–69. [Google Scholar] [CrossRef]
- De Jong, F.; Beecher, C.; Raskind, A.; Chamberlain, C.; Guingab, J.; Garrett, T. MetaboNews. Available online: http://www.metabonews.ca/Aug2017/MetaboNews_Aug2017.htm (accessed on 10 August 2017).
- Rogers, S.; Scheltema, R.A.; Girolami, M.; Breitling, R. Probabilistic assignment of formulas to mass peaks in metabolomics experiments. Bioinformatics 2009, 25, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.R.; Jourdan, F.; Salvanha, D.M.; Letisse, F.; Jamin, E.L.; Guidetti-Gonzalez, S.; Labate, C.A.; Vêncio, R.Z.N. ProbMetab: An R package for Bayesian probabilistic annotation of LC-MS-based metabolomics. Bioinformatics 2014, 30, 1336–1337. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Dorval, V.; Hébert, S.S. LRRK2 in transcription and translation regulation: Relevance for Parkinson’s disease. Front. Neurol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Parlato, R.; Liss, B. How Parkinson’s disease meets nucleolar stress. Biochim. Biophys. Acta-Mol. Basis Dis. 2014, 1842, 791–797. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Eckert, G.P.; Lipka, U.; Muller, W.E. Omega-3 fatty acids in neurodegenerative diseases: Focus on mitochondria. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 105–114. [Google Scholar] [CrossRef]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef]
- Steuer, R.; Kurths, J.; Fiehn, O.; Weckwerth, W. Observing and interpreting correlations in metabolomic networks. Bioinformatics 2003, 19, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Trifonova, O.P.; Maslov, D.L.; Balashova, E.E.; Lokhov, P.G. Evaluation of dried blood spot sampling for clinical metabolomics: Effects of different papers and sample storage stability. Metabolites 2019, 9, 277. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Values | |
|---|---|---|
| Control Subjects | Subjects with PD 1 | |
| Number | 28 | 28 |
| Age (years; mean ± s.d. (range) | 62.8 ± 8.7 (45–77) | 62.6 ± 8.6 (37–77) |
| Gender (male/female) | 14/14 | 14/14 |
| PD stages (1/1.5/2/2.5) | – | 6/6/12/4 |
| Parameter | Value |
|---|---|
| Detection mass range of compounds (m/z) | 45–900 |
| Number of detected compound mass peaks | 9664 ± 620 1 |
| Number of masses submitted to search engine block | 14,857 |
| Number of mass peaks/compound candidate submitted to the annotation algorithm | 31,724 |
| Number of mass peaks with putatively annotated compound(s) by the annotation algorithm | 2741 |
| Number of unique compound names retrieved by the annotation algorithm | 709 |
| Pathway | Pathway Representation Score 1 | Pathway Overrepresentation (Fold) | Wilcoxon Rank-Sum Test | |
|---|---|---|---|---|
| Case Samples | Control Samples | |||
| Transcription/translation | 27.8 | 8.6 | 3.2 | 0.003 |
| Dopa-responsive dystonia | 21.4 | 2.7 | 7.9 | 0.3 |
| Fatty acid elongation in mitochondria | 21.4 | 2.7 | 7.9 | 0.3 |
| Long-chain-3-hydroxyacyl-coa dehydrogenase deficiency (LCHAD) | 21.4 | 2.7 | 7.9 | 0.3 |
| Hyperphenylalaninemia due to guanosine triphosphate cyclohydrolase deficiency | 21.4 | 2.7 | 7.9 | 0.3 |
| Hyperphenylalaninemia due to 6-pyruvoyltetrahydropterin synthase (PTPS) deficiency | 21.4 | 2.7 | 7.9 | 0.3 |
| Hyperphenylalaninemia due to DHPR deficiency | 21.4 | 2.7 | 7.9 | 0.3 |
| Pterine biosynthesis | 21.4 | 2.7 | 7.9 | 0.3 |
| Segawa syndrome | 21.4 | 2.7 | 7.9 | 0.3 |
| Sepiapterin reductase deficiency | 21.4 | 2.7 | 7.9 | 0.3 |
| Warburg effect | 20.3 | 4.6 | 4.4 | 0.0004 |
| Glutaminolysis and cancer | 14.4 | 2.9 | 5.0 | 0.029 |
| Mercaptopurine action pathway | 11.7 | 4.6 | 2.5 | 0.24 |
| Thioguanine action pathway | 11.7 | 4.6 | 2.5 | 0.014 |
| Glycine and serine metabolism | 9.4 | 5.3 | 1.8 | 0.014 |
| AICA-ribosiduria | 9.4 | 4.7 | 2.0 | 0.014 |
| Adenine phosphoribosyltransferase deficiency (APRT) | 9.4 | 4.7 | 2.0 | 0.36 |
| Adenosine deaminase deficiency | 9.4 | 4.7 | 2.0 | 0.36 |
| Lesch–Nyhan Syndrome (LNS) | 9.4 | 4.7 | 2.0 | 0.004 |
| Mitochondrial DNA depletion syndrome | 9.4 | 4.7 | 2.0 | 0.29 |
| Criteria | Value | |
|---|---|---|
| Score Threshold #1 | Score Threshold #2 | |
| Score threshold | 12 | 340 |
| True positive | 18 | 12 |
| False positive | 4 | 1 1 |
| True negative | 24 | 27 |
| False negative | 10 | 16 |
| Sensitivity | 64% | 43% |
| Specificity | 86% | 96% |
| Accuracy | 75% | 70% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lokhov, P.G.; Trifonova, O.P.; Maslov, D.L.; Lichtenberg, S.; Balashova, E.E. Diagnosis of Parkinson’s Disease by A Metabolomics-Based Laboratory-Developed Test (LDT). Diagnostics 2020, 10, 332. https://doi.org/10.3390/diagnostics10050332
Lokhov PG, Trifonova OP, Maslov DL, Lichtenberg S, Balashova EE. Diagnosis of Parkinson’s Disease by A Metabolomics-Based Laboratory-Developed Test (LDT). Diagnostics. 2020; 10(5):332. https://doi.org/10.3390/diagnostics10050332
Chicago/Turabian StyleLokhov, Petr G., Oxana P. Trifonova, Dmitry L. Maslov, Steven Lichtenberg, and Elena E. Balashova. 2020. "Diagnosis of Parkinson’s Disease by A Metabolomics-Based Laboratory-Developed Test (LDT)" Diagnostics 10, no. 5: 332. https://doi.org/10.3390/diagnostics10050332
APA StyleLokhov, P. G., Trifonova, O. P., Maslov, D. L., Lichtenberg, S., & Balashova, E. E. (2020). Diagnosis of Parkinson’s Disease by A Metabolomics-Based Laboratory-Developed Test (LDT). Diagnostics, 10(5), 332. https://doi.org/10.3390/diagnostics10050332