Novel Diagnostic Options without Contrast Media or Radiation: Triggered Angiography Non-Contrast-Enhanced Sequence Magnetic Resonance Imaging in Treating Different Leg Venous Diseases
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. MRI Acquisition
2.3. Statistical Analysis
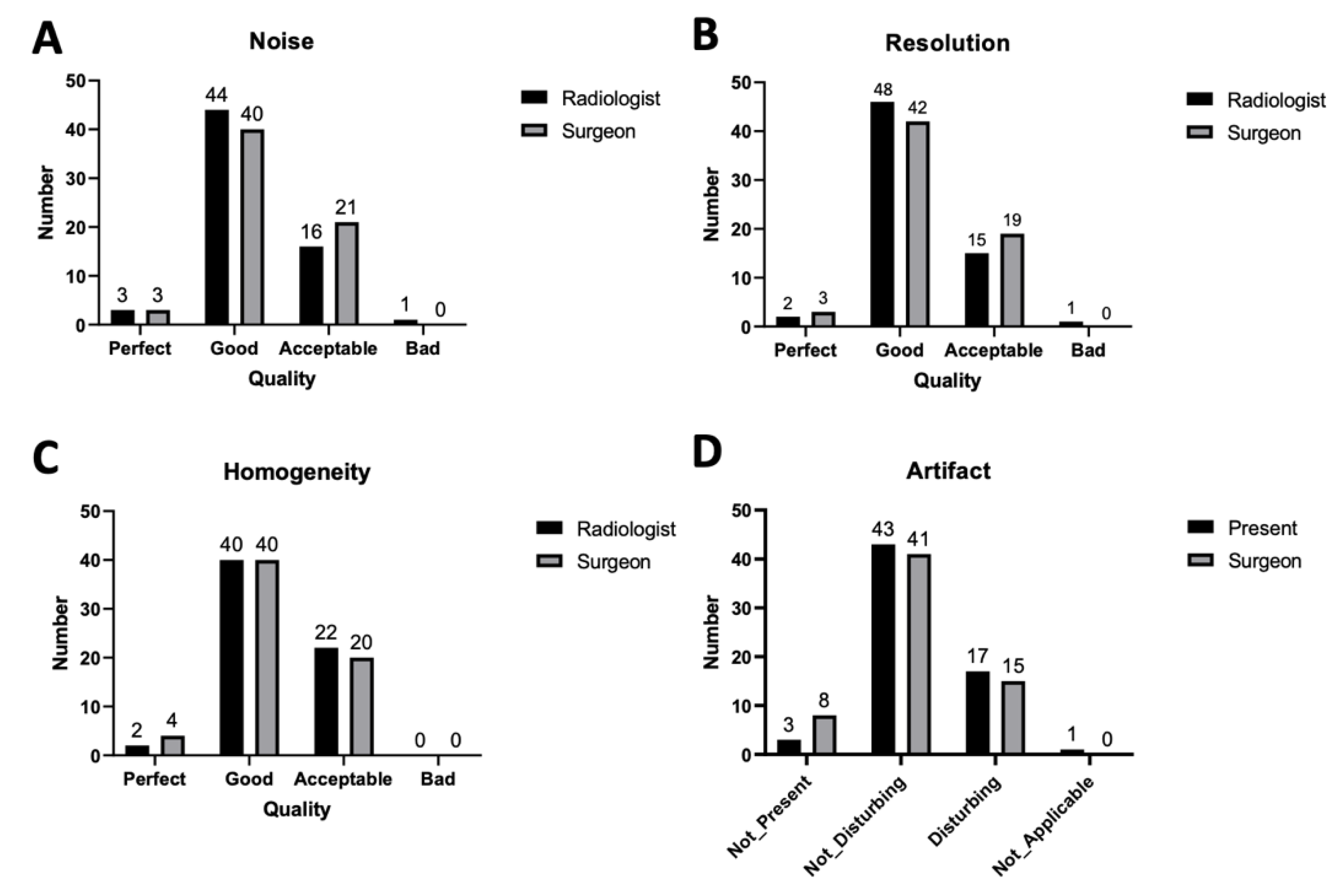
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | three-dimensional |
| CTA | computed tomography angiography |
| DVT | deep venous thrombosis |
| FOV | field of view |
| FV | femoral vein |
| GSV | great saphenous vein |
| IRB | institutional review board |
| MRI | magnetic resonance imaging |
| MRV | magnetic resonance venography |
| OV | occlusive venous disease |
| PV | popliteal vein |
| STIR | short tau inversion recovery |
| TE | echo time |
| TOF | time-of-flight |
| TR | repetition time |
| TRANCE-MRI | triggered angiography non-contrast-enhanced MRI |
| TSE | turbo spin-echo |
| US | ultrasonography |
| VV | varicose vein |
| SU | static venous ulcer |
References
- De Backer, G. Epidemiology of chronic venous insufficiency. Angiology 1997, 48, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Beebe-Dimmer, J.L.; Pfeifer, J.R.; Engle, J.S.; Schottenfeld, D. The epidemiology of chronic venous insufficiency and varicose veins. Ann. Epidemiol. 2005, 15, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.; Evans, C.J.; Lee, A.J. Prevalence and risk factors of chronic venous insufficiency. Angiology 2001, 52 (Suppl. 1), S5–S15. [Google Scholar] [CrossRef]
- Schleimer, K.; Barbati, M.E.; Grommes, J.; Hoeft, K.; Toonder, I.M.; Wittens, C.H.A.; Jalaie, H. Update on diagnosis and treatment strategies in patients with post-thrombotic syndrome due to chronic venous obstruction and role of endovenous recanalization. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, L.; Lawrence, M.; Speicher, M.; Frumkin, K. Emergency Department Management of Suspected Calf-Vein Deep Venous Thrombosis: A Diagnostic Algorithm. West J. Emerg. Med. 2016, 17, 384–390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orbell, J.H.; Smith, A.; Burnand, K.G.; Waltham, M. Imaging of deep vein thrombosis. Br. J. Surg. 2008, 95, 137–146. [Google Scholar] [CrossRef]
- Gaitini, D. Current approaches and controversial issues in the diagnosis of deep vein thrombosis via duplex Doppler ultrasound. J. Clin. Ultrasound 2006, 34, 289–297. [Google Scholar] [CrossRef]
- Tamura, K.; Nakahara, H. MR Venography for the Assessment of Deep Vein Thrombosis in Lower Extremities with Varicose Veins. Ann. Vasc. Dis. 2014, 7, 399–403. [Google Scholar] [CrossRef][Green Version]
- Gutzeit, A.; Sutter, R.; Froehlich, J.M.; Roos, J.E.; Sautter, T.; Schoch, E.; Giger, B.; Wyss, M.; Graf, N.; von Weymarn, C.; et al. ECG-triggered non-contrast-enhanced MR angiography (TRANCE) versus digital subtraction angiography (DSA) in patients with peripheral arterial occlusive disease of the lower extremities. Eur. Radiol. 2011, 21, 1979–1987. [Google Scholar] [CrossRef]
- Schieda, N.; Maralani, P.J.; Hurrell, C.; Tsampalieros, A.K.; Hiremath, S. Updated Clinical Practice Guideline on Use of Gadolinium-Based Contrast Agents in Kidney Disease Issued by the Canadian Association of Radiologists. Can. Assoc. Radiol. J. 2019. [Google Scholar] [CrossRef]
- Osanai, T.; Kazumata, K.; Kobayashi, S.; Fujima, N.; Kurisu, K.; Shimoda, Y.; Houkin, K. Electrocardiogram-Triggered Angiography Non-Contrast-Enhanced (TRANCE) Imaging to Assess Access Route Before Diagnostic Cerebral Angiography. World Neurosurg. 2018, 119, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Suttmeyer, B.; Teichgraber, U.; Rathke, H.; Albrecht, L.; Guttler, F.; Schnackenburg, B.; Hamm, B.; de Bucourt, M. Initial experience with imaging of the lower extremity arteries in an open 1.0 Tesla MRI system using the triggered angiography non-contrast-enhanced sequence (TRANCE) compared to digital subtraction angiography (DSA). Biomed. Tech. 2016, 61, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Huang, Y.K.; Hsu, L.S.; Chen, P.Y.; Chen, C.W. Using non-contrast-enhanced magnetic resonance venography for the evaluation of May-Thurner syndrome in patients with renal insufficiency: A case report. Medicine 2019, 98, e18427. [Google Scholar] [CrossRef]
- Huang, Y.K.; Tseng, Y.H.; Lin, C.H.; Tsai, Y.H.; Hsu, Y.C.; Wang, S.C.; Chen, C.W. Evaluation of venous pathology of the lower extremities with triggered angiography non-contrast-enhanced magnetic resonance imaging. BMC Med. Imaging 2019, 19, 96. [Google Scholar] [CrossRef]
- Radaideh, Q.; Patel, N.M.; Shammas, N.W. Iliac vein compression: Epidemiology, diagnosis and treatment. Vasc. Health Risk Manag. 2019, 15, 115–122. [Google Scholar] [CrossRef]
- Hansrani, V.; Khanbhai, M.; McCollum, C. The Diagnosis and Management of Early Deep Vein Thrombosis. Adv. Exp. Med. Biol. 2017, 906, 23–31. [Google Scholar] [CrossRef]
- Ciccotosto, C.; Goodman, L.R.; Washington, L.; Quiroz, F.A. Indirect CT venography following CT pulmonary angiography: Spectrum of CT findings. J. Thorac. Imaging 2002, 17, 18–27. [Google Scholar]
- Duwe, K.M.; Shiau, M.; Budorick, N.E.; Austin, J.H.; Berkmen, Y.M. Evaluation of the lower extremity veins in patients with suspected pulmonary embolism: A retrospective comparison of helical CT venography and sonography. 2000 ARRS Executive Council Award I. American Roentgen Ray Society. AJR Am. J. Roentgenol. 2000, 175, 1525–1531. [Google Scholar] [CrossRef]
- Suttmeyer, B.; Teichgraber, U.; Thomas, A.; Rathke, H.; Albrecht, L.; Jonczyk, M.; Verba, M.; Guttler, F.; Schnackenburg, B.; Hamm, B.; et al. Non-invasive ECG-triggered 2D TOF MR angiography of the pelvic and leg arteries in an open 1.0-tesla high-field MRI system in comparison to conventional DSA. Biomed. Tech. 2014, 59, 29–37. [Google Scholar] [CrossRef]
- Li, W.; Salanitri, J.; Tutton, S.; Dunkle, E.E.; Schneider, J.R.; Caprini, J.A.; Pierchala, L.N.; Jacobs, P.M.; Edelman, R.R. Lower extremity deep venous thrombosis: Evaluation with ferumoxytol-enhanced MR imaging and dual-contrast mechanism--preliminary experience. Radiology 2007, 242, 873–881. [Google Scholar] [CrossRef]
- Ruehm, S.G.; Zimny, K.; Debatin, J.F. Direct contrast-enhanced 3D MR venography. Eur. Radiol. 2001, 11, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Ruehm, S.G.; Wiesner, W.; Debatin, J.F. Pelvic and lower extremity veins: Contrast-enhanced three-dimensional MR venography with a dedicated vascular coil-initial experience. Radiology 2000, 215, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Semelka, R.C.; Commander, C.W.; Jay, M.; Burke, L.M.; Ramalho, M. Presumed Gadolinium Toxicity in Subjects With Normal Renal Function: A Report of 4 Cases. Investig. Radiol. 2016, 51, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Malikova, H.; Holesta, M. Gadolinium contrast agents-are they really safe? J. Vasc. Access 2017, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Meuli, R.A.; Wedeen, V.J.; Geller, S.C.; Edelman, R.R.; Frank, L.R.; Brady, T.J.; Rosen, B.R. MR gated subtraction angiography: Evaluation of lower extremities. Radiology 1986, 159, 411–418. [Google Scholar] [CrossRef]
- Shimizu, H.; Isoda, H.; Ohno, T.; Yamashita, R.; Kawahara, S.; Furuta, A.; Fujimoto, K.; Kido, A.; Kusahara, H.; Togashi, K. Non-contrast-enhanced MR portography and hepatic venography with time-spatial labeling inversion pulses: Comparison of imaging with the short tau inversion recovery method and the chemical shift selective method. Magn. Reson. Imaging 2015, 33, 81–85. [Google Scholar] [CrossRef]
- Pascarella, L.; Shortell, C.K. Medical management of venous ulcers. Semin. Vasc. Surg. 2015, 28, 21–28. [Google Scholar] [CrossRef]
- Verma, H.; Tripathi, R.K. Algorithm-based approach to management of venous leg ulceration. Semin. Vasc. Surg. 2015, 28, 54–60. [Google Scholar] [CrossRef]
- Chiang, J.T.; Carl, M.; Du, J. Signal and contrast effects due to T2 decay during k-space readout of UTE (ultrashort TE) sequences. Magn. Reson. Imaging 2014, 32, 259–269. [Google Scholar] [CrossRef]



| Occlusive Venous Symptoms (OV) | Venous Static Ulcer (SU) | Symptomatic Varicose Vein (VV) | Sum (%) | |
|---|---|---|---|---|
| Total | 35 | 12 | 16 | 63 |
| Male gender (%) | 19 (54%) | 11 (92%) | 3 (18%) | 33 (52%) |
| Age (year) | 65.5 ± 12.9 | 59.2 ± 11.6 | 52.3 ± 15.9 | 61 ± 14.6 |
| Substance use | ||||
| Smoking | 6 | 3 | 1 | 10 (15.9%) |
| Alcohol | 7 | 2 | 2 | 11 (17.5%) |
| Betel nuts | 3 | 3 | 1 | 7 (11.1%) |
| Comorbidities | ||||
| Hypertension | 18 | 3 | 3 | 24 (38.1%) |
| Diabetes mellitus | 11 | 4 | 2 | 17 (27%) |
| CAD | 2 | 0 | 0 | 2 (3.2%) |
| Stroke | 1 | 0 | 0 | 1 (1.6%) |
| Cancer | 9 | 1 | 0 | 10 (15.9%) |
| Chronic renal failure | 2 | 0 | 1 | 3 (4.8%) |
| Hemodialysis | 2 | 0 | 0 | 2 (3.2%) |
| Previous venous surgery | ||||
| Stripping | 0 | 0 | 2 | 2 (3.2%) |
| GVS ablation | 0 | 3 | 4 | 7 (11.1%) |
| Sclerotherapy | 0 | 1 | 0 | 1 (1.6%) |
| IVC filter | 2 | 0 | 0 | 2 (3.2%) |
| Pelvic/orthopaedic | 6 | 0 | 0 | 6 (9.5%) |
| DVT | Non DVT | p Value | |
|---|---|---|---|
| Subgroup patient numbers | 20 | 15 | |
| Age (years old) | 64.8 ± 10.5 | 66.53 ± 16.2 | |
| Male gender | 12 | 7 | 0.506 |
| Onset less than 8 weeks | 10 | 2 | 0.034 * |
| Duplex in leg suspected for thrombi | 17 | 2 | <0.001 * |
| TRANCE MR in vein | |||
| Deep vein thrombus | 20 | 0 | <0.001 * |
| Congenital anomaly | 2 | 1 | 1 |
| May–Thurner-like (arterial compression) | 10 | 3 | 0.89 |
| Malignant disease in MRI | 4 | 3 | 1 |
| External compression, malignant | 1 | 3 | 0.292 |
| External compression, benign | 2 | 4 | 0.367 |
| Pelvic congestion | 1 | 2 | 0.565 |
| TRANCE MR in artery | |||
| PAOD | 2 | 0 | 0.496 |
| AAA and IAAA | 0 | 1 | 1 |
| CTA and CTV | 3 | 1 | 0.619 |
| Lymphoscintigraphy | 1 | 0 | 1 |
| Intervention | |||
| CDT and EKOS | 2 | 0 | 0.496 |
| Heparinisation in hospital | 11 | 1 | 0.004 * |
| NOAC or warfarin | 19 | 2 | <0.001 * |
| Venous angioplasty | 4 | 1 | 0.365 |
| Venous stenting | 1 | 0 | 1 |
| IVC filter | 2 | 0 | 0.496 |
| Clinical | |||
| New neoplasm diagnosis <1 year | 3 | 3 | 1 |
| Pulmonary emboli | 2 | 0 | 0.496 |
| Age (Years) | 59.3 ± 12.2 |
|---|---|
| Male gender | 11 (92%) |
| Doppler screening | |
| Valvular insufficiency | 2 |
| Venous thrombi | 5 |
| TRANCE MR in vein | |
| Deep vein thrombus | 4 |
| Congenital anomaly | 0 |
| May–Thurner-like picture | 1 |
| Malignant disease | 1 |
| Profound varicose vein | 10 |
| Pelvic congestion | 2 |
| Subcutaneous tissue enhancement without venous pathology | 5 |
| External compression (joint fluid) | 1 |
| TRANCE MR in artery | |
| PAOD | 2 |
| Intervention history | |
| NOAC or warfarin | 5 |
| Skin graft/free flap | 2 |
| Venous ablation/stripping | 4 |
| Age (Years) | 52.3 ± 15.9 |
|---|---|
| Male gender | 3 (18%) |
| Why do the TRANCE MR in the vein? | |
| Rapid progression of the varicose vein | 3 |
| Claudication | 5 |
| Phlebitis, cellulitis, and bleeding episodes | 4 |
| Recurrence of the varicose vein after interventions | 4 |
| Doppler study in leg veins | |
| Superficial venous thrombosis | 3 |
| Deep venous thrombosis | 0 |
| Valve incompetence | 11 |
| TRANCE MR in vein | |
| Thrombus in deep venous system | 1 |
| Congenital anomaly | 1 |
| May–Thurner-like picture | 1 |
| Malignant disease | 1 |
| TRANCE MR in artery | |
| PAOD | 0 |
| Intervention history | |
| NOAC or warfarin | 3 |
| Venous ablation/stripping | 6 |
| Venous operation after TRANCE MR | |
| Truncal ablation with phlebectomy | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-W.; Tseng, Y.-H.; Lin, C.-C.; Kao, C.-C.; Wong, M.Y.; Lin, B.-S.; Huang, Y.-K. Novel Diagnostic Options without Contrast Media or Radiation: Triggered Angiography Non-Contrast-Enhanced Sequence Magnetic Resonance Imaging in Treating Different Leg Venous Diseases. Diagnostics 2020, 10, 355. https://doi.org/10.3390/diagnostics10060355
Chen C-W, Tseng Y-H, Lin C-C, Kao C-C, Wong MY, Lin B-S, Huang Y-K. Novel Diagnostic Options without Contrast Media or Radiation: Triggered Angiography Non-Contrast-Enhanced Sequence Magnetic Resonance Imaging in Treating Different Leg Venous Diseases. Diagnostics. 2020; 10(6):355. https://doi.org/10.3390/diagnostics10060355
Chicago/Turabian StyleChen, Chien-Wei, Yuan-Hsi Tseng, Chien-Chiao Lin, Chih-Chen Kao, Min Yi Wong, Bor-Shyh Lin, and Yao-Kuang Huang. 2020. "Novel Diagnostic Options without Contrast Media or Radiation: Triggered Angiography Non-Contrast-Enhanced Sequence Magnetic Resonance Imaging in Treating Different Leg Venous Diseases" Diagnostics 10, no. 6: 355. https://doi.org/10.3390/diagnostics10060355
APA StyleChen, C.-W., Tseng, Y.-H., Lin, C.-C., Kao, C.-C., Wong, M. Y., Lin, B.-S., & Huang, Y.-K. (2020). Novel Diagnostic Options without Contrast Media or Radiation: Triggered Angiography Non-Contrast-Enhanced Sequence Magnetic Resonance Imaging in Treating Different Leg Venous Diseases. Diagnostics, 10(6), 355. https://doi.org/10.3390/diagnostics10060355





