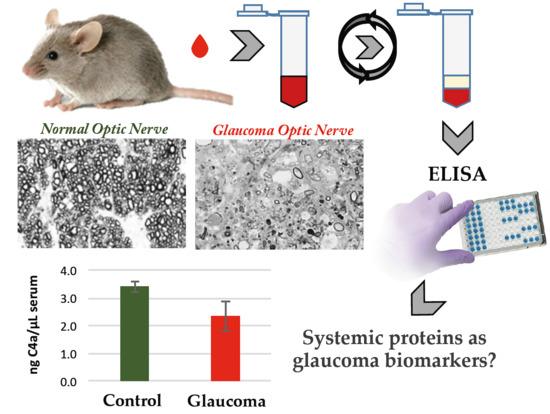
Systemic Alterations of Immune Response-Related Proteins during Glaucoma Development in the Murine Model DBA/2J
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Strains, Breeding, and Husbandry
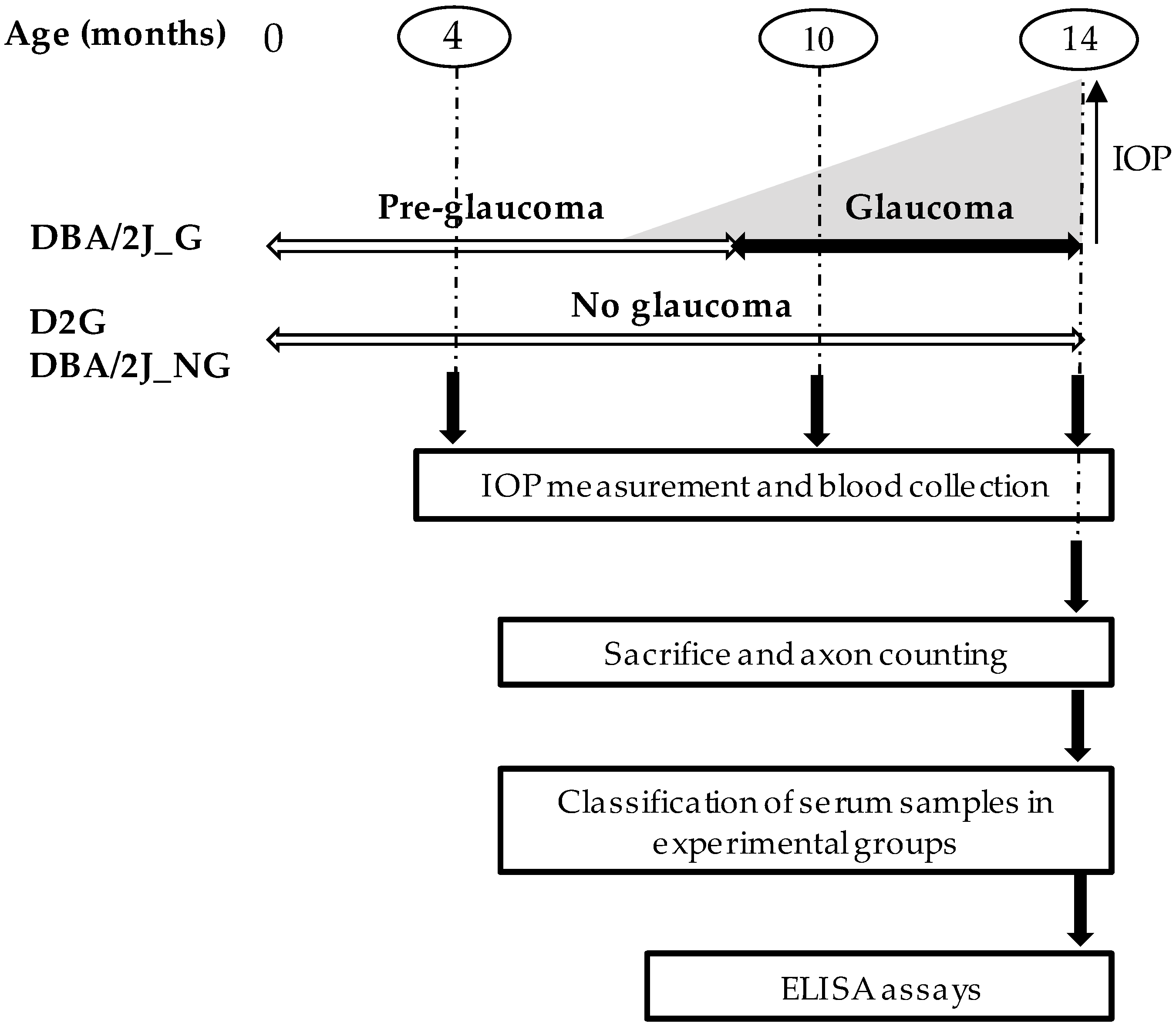
2.2. Experimental Design
2.3. Intraocular Pressure (IOP) Measurement
2.4. Blood Sampling Collection
2.5. Optic Nerves Dissection, Fixation, and Staining
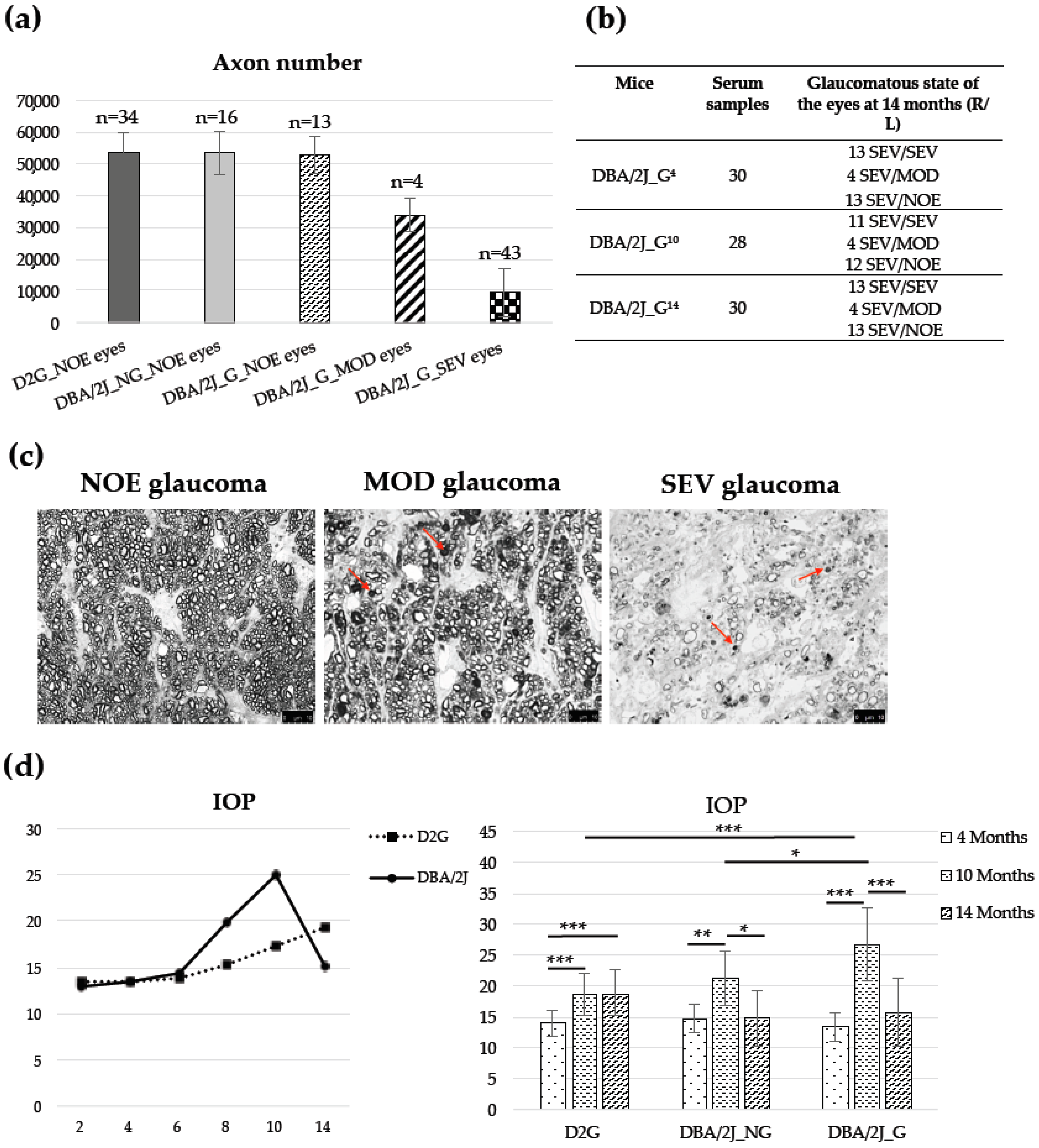
2.6. Optic Nerve Damage and Mice Stratification
2.7. Experimental Groups
2.8. ELISA Assays
2.9. Statistical Analysis
3. Results
3.1. Animals, Optic Nerve Axons Number, and IOP
3.2. Quantification of Serum Proteins by ELISA Analysis
3.3. Machine Learning Models
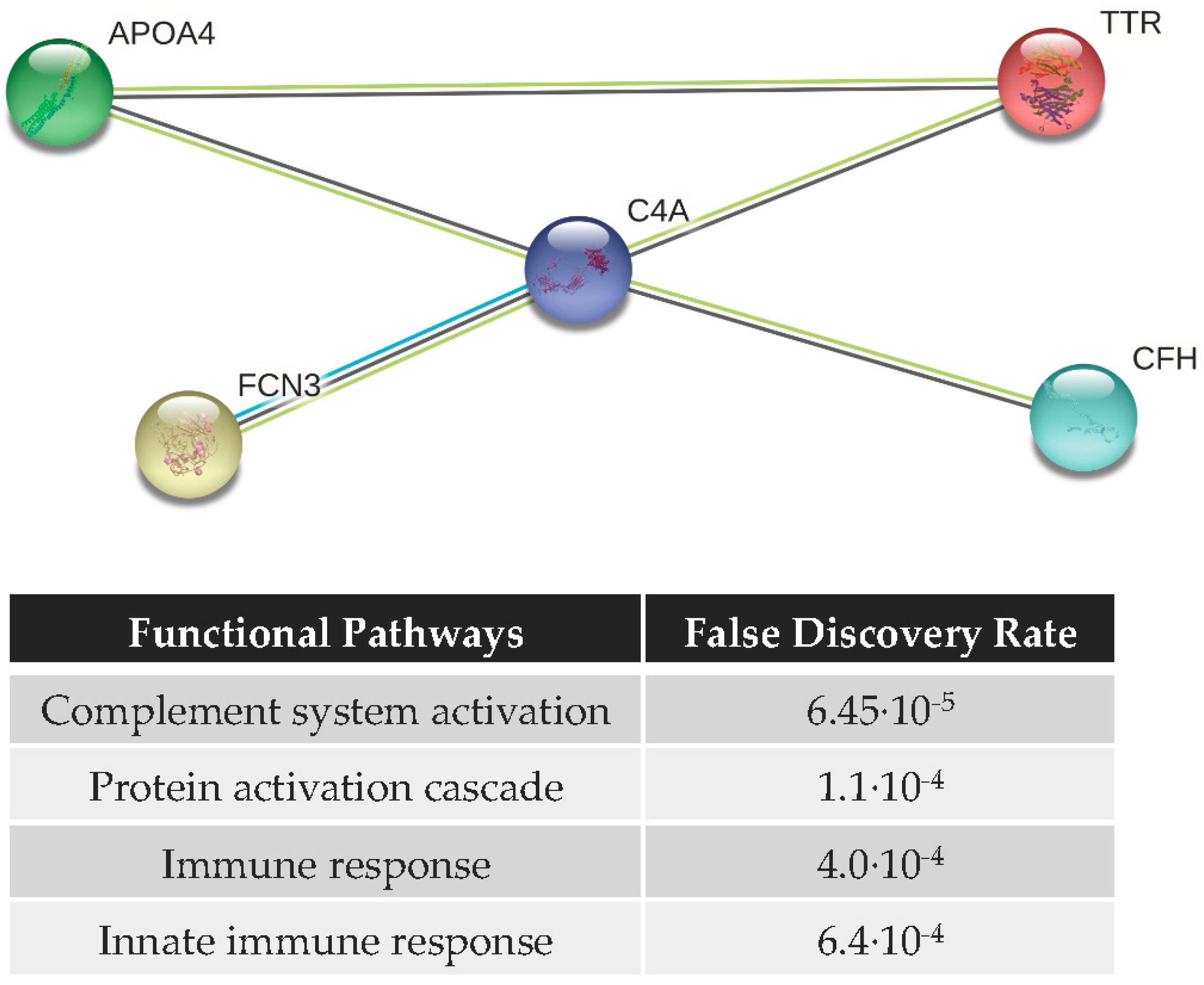
3.4. Functional Analysis and Protein–Protein Interaction (PPI) Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| a1AT | alpha 1-antitrypsin |
| APOA1 | apolipoprotein A1 |
| APOA4 | apolipoprotein A4 |
| APOL1 | apolipoprotein L1 |
| AUC | area under the curve |
| C3 | complement C3 |
| C4a | complement C4a |
| C5 | complement component 5 |
| CA | correct assignment |
| CFH | complement factor H |
| D2G | DBA/2J-Gpnmb+/SjJ mice strain without signs of glaucoma |
| DBA/2J_G | DBA/2J mice that developed glaucoma with the age |
| DBA/2J_NG | DBA/2J mice that did not develop glaucoma |
| FCN3 | ficolin-3 |
| GPNMB | transmembrane glycoprotein NMB |
| IOP | intraocular pressure |
| IPD | iris pigment dispersion |
| ISA | iris stromal atrophy |
| ITIH4 | inter-alpha-trypsin inhibitor heavy chain H4 |
| MOD | nerves with moderate damage |
| NOE | nerves with neither or early damage |
| PEXG | pseudoexfoliation glaucoma |
| POAG | Primary open-angle glaucoma |
| PPD | paraphenylenediamine |
| PPI | protein–protein interaction |
| Prec. | precision |
| RGC | retinal ganglion cell |
| Sens. | sensitivity |
| SEV | nerves with severe glaucoma |
| Spec. | specificity |
| TTR | transthyretin |
References
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2010, 90, 262–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Gottanka, J.; Kuhlmann, A.; Scholz, M.; Johnson, D.H.; Lütjen-Drecoll, E. Pathophysiologic changes in the optic nerves of eyes with primary open angle and pseudoexfoliation glaucoma. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4170–4181. [Google Scholar] [CrossRef] [PubMed]
- Jeng, S.M.; Karger, R.A.; Hodge, D.O.; Burke, J.P.; Johnson, D.H.; Good, M.S. The risk of glaucoma in pseudoexfoliation syndrome. J. Glaucoma 2007, 16, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Gabelt, B.T.; Kaufman, P.L. Changes in aqueous humor dynamics with age and glaucoma. Prog. Retin. Eye Res. 2005, 24, 612–637. [Google Scholar] [CrossRef]
- Braunger, B.M.; Fuchshofer, R.; Tamm, E.R. The aqueous humor outflow pathways in glaucoma: A unifying concept of disease mechanisms and causative treatment. Eur. J. Pharm Biopharm. 2015, 95, 173–181. [Google Scholar] [CrossRef]
- Kerrigan-Baumrind, L.A.; Quigley, H.A.; Pease, M.E.; Kerrigan, D.F.; Mitchell, R.S. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Investig. Ophthalmol. Vis. Sci. 2000, 41, 741–748. [Google Scholar]
- Beykin, G.; Goldberg, J.L. Molecular Biomarkers for Glaucoma. Curr. Ophthalmol. Rep. 2019, 7, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Agnifili, L.; Pieragostino, D.; Mastropasqua, A.; Fasanella, V.; Brescia, L.; Tosi, G.M.; Sacchetta, P.; Mastropasqua, L. Molecular biomarkers in primary open-angle glaucoma: From noninvasive to invasive. Prog. Brain Res. 2015, 221, 1–32. [Google Scholar] [CrossRef]
- Lauwen, S.; de Jong, E.; Lefeber, D.J.; den Hollander, A. Omics Biomarkers in Ophthalmology. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO88–BIO98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medeiros, F.A. Biomarkers and Surrogate Endpoints: Lessons Learned From Glaucoma. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO20–BIO26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hindle, A.G.; Thoonen, R.; Jasien, J.V.; Grange, R.M.H.; Amin, K.; Wise, J.; Ozaki, M.; Ritch, R.; Malhotra, R.; Buys, E.S. Identification of Candidate miRNA Biomarkers for Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 134–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Iglesias, H.; Alvarez, L.; García, M.; Escribano, J.; Rodríguez-Calvo, P.P.; Fernández-Vega, L.; Coca-Prados, M. Comparative proteomic study in serum of patients with primary open-angle glaucoma and pseudoexfoliation glaucoma. J. Proteom. 2014, 16, 65–78. [Google Scholar] [CrossRef]
- John, S.W.; Smith, R.S.; Savinova, O.V.; Hawes, N.L.; Chang, B.; Turnbull, D.; Davisson, M.; Roderick, T.H.; Heckenlively, J.R. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Investig. Ophthalmol. Vis. Sci. 1998, 39, 951–962. [Google Scholar]
- Libby, R.T.; Anderson, M.G.; Pang, I.H.; Robinson, Z.H.; Savinova, O.V.; Cosma, I.M.; Snow, A.; Wilson, L.A.; Smith, R.S.; Clark, A.F.; et al. Inherited glaucoma in DBA/2J mice: Pertinent disease features for studying the neurodegeneration. Vis. Neurosci. 2005, 22, 637–648. [Google Scholar] [CrossRef]
- Chang, B.; Smith, R.S.; Hawes, N.L.; Anderson, M.G.; Zabaleta, A.; Savinova, O.; Roderick, T.H.; Heckenlively, J.R.; Davisson, M.T.; John, S.W. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat. Genet. 1999, 21, 405–409. [Google Scholar] [CrossRef]
- Howell, G.R.; Libby, R.T.; Jakobs, T.C.; Smith, R.S.; Phalan, F.C.; Barter, J.W.; Barbay, J.M.; Marchant, J.K.; Mahesh, N.; Porciatti, V.; et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell Biol. 2007, 179, 1523–1537. [Google Scholar] [CrossRef] [Green Version]
- Burroughs, S.L.; Kaja, S.; Koulen, P. Quantification of deficits in spatial visual function of mouse models for glaucoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3654–3659. [Google Scholar] [CrossRef] [Green Version]
- Howell, G.R.; Libby, R.T.; Marchant, J.K.; Wilson, L.A.; Cosma, I.M.; Smith, R.S.; Anderson, M.G.; John, S.W. Absence of glaucoma in DBA/2J mice homozygous for wild-type versions of Gpnmb and Tyrp1. BMC Genet. 2007, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G.; NC3Rs Reporting Guidelines Working Group. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharm. 2010, 160, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Millar, J.C.; Pang, I.H.; Wax, M.B.; Clark, A.F. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4617–4621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeki, T.; Aihara, M.; Ohashi, M.; Araie, M. The efficacy of TonoLab in detecting physiological and pharmacological changes of mouse intraocular pressure-comparison with TonoPen and microneedle manometry. Curr. Eye Res. 2008, 33, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.C.; Pang, I.H. Non-continuous measurement of intraocular pressure in laboratory animals. Exp. Eye Res. 2015, 141, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Savinova, O.V.; Sugiyama, F.; Martin, J.E.; Tomarev, S.I.; Paigen, B.J.; Smith, R.S.; John, S.W. Intraocular pressure in genetically distinct mice: An update and strain survey. BMC Genet. 2001, 2, 12. [Google Scholar] [CrossRef]
- Aihara, M.; Lindsey, J.D.; Weinreb, R.N. Twenty-four-hour pattern of mouse intraocular pressure. Exp. Eye Res. 2003, 77, 681–686. [Google Scholar] [CrossRef]
- Drouyer, E.; Dkhissi-Benyahya, O.; Chiquet, C.; WoldeMussie, E.; Ruiz, G.; Wheeler, L.A.; Denis, P.; Cooper, H.M. Glaucoma alters the circadian timing system. PLoS ONE 2008, 3, e3931. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.S.; Zabaleta, A.; John, S.W.M.; Bechtold, L.S.; Ikeda, S.; Relyea, M.J.; Sundberg, J.P.; Kao, W.W.-Y.; Liu, C. General and Special Histopathology. In Systematic Evaluation of the Mouse Eye: Anatomy, Pathology and Biomethods; Smith, R.S., John, S.W.M., Nishina, P.M., Sundberg, J.P., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 265–276. [Google Scholar]
- Anderson, M.G.; Libby, R.T.; Gould, D.B.; Smith, R.S.; John, S.W.M. High-dose radiation with bone marrow transfer prevents neurodegeneration in an inherited glaucoma. Proc. Natl. Acad. Sci. USA 2005, 102, 4566–4571. [Google Scholar] [CrossRef] [Green Version]
- Libby, R.T.; Li, Y.; Savinova, O.V.; Barter, J.; Smith, R.S.; Nickells, R.W.; John, S.W. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005, 1, e4. [Google Scholar] [CrossRef] [Green Version]
- Sadun, A.A.; Smith, L.E.; Kenyon, K.R. Paraphenylenediamine: A new method for tracing human visual pathways. J. Neuropathol. Exp. Neurol. 1983, 42, 200–206. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, G.R.; Macalinao, D.G.; Sousa, G.L.; Walden, M.; Soto, I.; Kneeland, S.C.; Barbay, J.M.; King, B.L.; Marchant, J.K.; Hibbs, M.; et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J. Clin. Investig. 2011, 121, 1429–1444. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Howell, G.R.; Barbay, J.M.; King, B.L.; Marchant, J.K.; Hibbs, M.; Stevens, B.; Barres, B.A.; Clark, A.F.; Libby, R.T.; et al. Retinal Ganglion Cell Dendritic Atrophy in DBA/2J Glaucoma. PLoS ONE 2013, 8, e72282. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.M.; Braine, C.E.; Williams, P.A.; Zhu, X.; MacNicoll, K.H.; Sousa, G.L.; Buchanan, R.A.; Smith, R.S.; Libby, R.T.; Howell, G.R.; et al. Early immune responses are independent of RGC dysfunction in glaucoma with complement component C3 being protective. Proc. Natl. Acad. Sci. USA 2017, 114, E3839–E3848. [Google Scholar] [CrossRef] [Green Version]
- Curk, T.; Demsar, J.; Xu, Q.; Leban, G.; Petrovic, U.; Bratko, I.; Shaulsky, G.; Zupan, B. Microarray data mining with visual programming. Bioinformatics 2005, 21, 396–398. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [Green Version]
- Panagis, L.; Zhao, X.; Ge, Y.; Ren, L.; Mittag, T.W.; Danias, J. Retinal Gene Expression Changes Related to IOP Exposure and Axonal Loss in DBA/2J Mice. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7807–7816. [Google Scholar] [CrossRef] [Green Version]
- Tezel, G. A proteomics view of the molecular mechanisms and biomarkers of glaucomatous neurodegeneration. Prog. Retin. Eye Res. 2013, 35, 18–43. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Li, X.; Wang, W.; Mo, J.S.; Kaplan, H.; Cooper, N.G. Early Involvement of Immune/Inflammatory Response Genes in Retinal Degeneration in DBA/2J Mice. Ophthalmol. Eye Dis. 2010, 1, 23–41. [Google Scholar] [CrossRef] [Green Version]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007, 131, 1164–1178. [Google Scholar] [CrossRef] [Green Version]
- Silverman, S.M.; Ma, W.; Wang, A.; Zhao, L.; Wong, W.T. C3- and CR3-dependent microglial clearance protects photoreceptors in retinitis pigmentosa. J. Exp. Med. 2019, 216, 1925–1943. [Google Scholar] [CrossRef] [Green Version]
- Bosco, A.; Anderson, S.R.; Breen, K.T.; Romero, C.O.; Steele, M.R.; Chiodo, V.A.; Boye, S.L.; Hauswirth, W.W.; Tomlinson, S.; Vetter, M.L. Complement C3-Targeted Gene Therapy Restricts Onset and Progression of Neurodegeneration in Chronic Mouse Glaucoma. Mol. Ther. 2018, 26, 2379–2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, G.R.; Soto, I.; Ryan, M.; Graham, L.C.; Smith, R.S.; John, S.W. Deficiency of complement component 5 ameliorates glaucoma in DBA/2J mice. J. Neuroinflam. 2013, 10, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janeway, C.A.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Pieragostino, D.; Bucci, S.; Agnifili, L.; Fasanella, V.; D’Aguanno, S.; Mastropasqua, A.; Ciancaglini, M.; Mastropasqua, L.; Di Ilio, C.; Sacchetta, P.; et al. Differential protein expression in tears of patients with primary open angle and pseudoexfoliative glaucoma. Mol. Biosyst. 2012, 8, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bollinger, K.E.; Kodeboyina, S.K.; Zhi, W.; Patton, J.; Bai, S.; Edwards, B.; Ulrich, L.; Bogorad, D.; Sharma, A. Proteomic alterations in aqueous humor from patients with primary open angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2635–2643. [Google Scholar] [CrossRef] [Green Version]
- Nesargikar, P.N.; Spiller, B.; Chavez, R. The complement system: History, pathways, cascade and inhibitors. Eur. J. Microbiol. Immunol. 2012, 2, 103–111. [Google Scholar] [CrossRef]
- Grus, F.H.; Joachim, S.C.; Sandmann, S.; Thiel, U.; Bruns, K.; Lackner, K.J.; Pfeiffer, N. Transthyretin and complex protein pattern in aqueous humor of patients with primary open-angle glaucoma. Mol. Vis. 2008, 14, 437–445. [Google Scholar]
- Duan, X.; Xue, P.; Wang, N.; Dong, Z.; Lu, Q.; Yang, F. Proteomic analysis of aqueous humor from patients with primary open angle glaucoma. Mol. Vis. 2010, 16, 2839–2846. [Google Scholar]
- Sharma, S.; Chataway, T.; Klebe, S.; Griggs, K.; Martin, S.; Chegeni, N.; Dave, A.; Zhou, T.; Ronci, M.; Voelcker, N.H.; et al. Novel protein constituents of pathological ocular pseudoexfoliation syndrome deposits identified with mass spectrometry. Mol. Vis. 2018, 24, 801–817. [Google Scholar] [PubMed]
- Prapunpoj, P.; Leelawatwattana, L. Evolutionary changes to transthyretin: Structure-function relationships. FEBS J. 2009, 276, 5330–5341. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, L.; Kimata, K. Structure and function of inter-alpha-trypsin inhibitor heavy chains. Connect. Tissue Res. 2008, 49, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, V.M.; Irvine, K.M.; Ravasi, T.; Sweet, M.J.; Hume, D.A. Gpnmb is induced in macrophages by IFN-γ and lipopolysaccharide and acts as a feedback regulator of Proinflammatory responses. J. Immunol. 2007, 178, 6557–6566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Zhuo, H.; Ouyang, H.; Liu, Y.; Yuan, F.; Sun, L.; Liu, F.; Liu, H. Glycoprotein non-metastatic melanoma protein b (Gpnmb) is highly expressed in macrophages of acute injured kidney and promotes M2 macrophages polarization. Cell Immunol. 2017, 316, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Alboslemy, T.; Safadi, F.; Kim, M.H. Glycoprotein nonmelanoma clone B regulates the crosstalk between macrophages and mesenchymal stem cells toward wound repair. J. Investig. Dermatol. 2018, 138, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hüttenrauch, M.; Ogorek, I.; Klafki, H.; Otto, M.; Stadelmann, C.; Weggen, S.; Wiltfang, J.; Wirths, O. Glycoprotein NMB: A novel Alzheimer’s disease associated marker expressed in a subset of activated microglia. Acta Neuropathol. Commun. 2018, 6, 108. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.K.; Lee, R.K.; Grus, F.H. Seventh ARVO/Pfizer Ophthalmics Research Institute Conference Working Group. Molecular biomarkers in glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 121–131. [Google Scholar] [CrossRef] [Green Version]
- De Moraes, C.G.; Liebmann, J.M.; Levin, L.A. Detection and measurement of clinically meaningful visual field progression in clinical trials for glaucoma. Prog. Retin. Eye Res. 2017, 56, 107–147. [Google Scholar] [CrossRef] [Green Version]


| Glaucoma Group | Control Group | |||||||
| Protein | Conc. | DBA/2J_G4 | DBA/2J_G10 | DBA/2J_G14 | DBA/2J_NG4 | DBA/2J_NG10 | DBA/2J_NG14 | |
| APOA1 | n | 22 (14F/8M) | 21 (13F/8M) | 20 (12F/8M) | 5 (4F/1M) | 7 (6F/1M) | 7 (6F/1M) | |
| ng/µL | 764.74 | 716.27 | 735.00 | 695.39 | 651.12 | 662.65 | ||
| SD | 197.97 | 174.58 | 203.06 | 246.05 | 120.66 | 109.21 | ||
| APOA4 | n | 19 (11F/8M) | 19 (11F/8M) | 19 (11F/8M) | 7 (6F/1M) | 7 (5F/2M) | 7 (4F/3M) | |
| ng/µL | 22.26 | 17.88 | 12.38 | 24.94 | 26.64 | 16.46 | ||
| SD | 4.82 | 5.84 | 1.87 | 3.20 | 4.32 | 3.00 | ||
| C3 | n | 20 (13F/7M) | 19 (13F/6M) | 20 (13F/7M) | 6 (5F/1M) | 6 (5F/1M) | 8 (6F/2M) | |
| ng/µL | 137.06 | 120.7 | 140.88 | 161.06 | 112.26 | 155.27 | ||
| SD | 47.82 | 19.26 | 46.24 | 44.71 | 21.51 | 47.24 | ||
| C4a | n | 22 (14F/8M) | 20 (13F/7M) | 20 (12F/8M) | 4 (3F/1M) | 7 (5F/2M) | 7 (5F/2M) | |
| ng/µL | 3.29 | 2.45 | 2.34 | 3.43 | 3.14 | 3.41 | ||
| SD | 0.63 | 0.81 | 0.54 | 0.20 | 0.53 | 0.68 | ||
| TTR | n | 19 (12F/7M) | 20 (13F/7M) | 20 (13F/7M) | 5 (4F/1M) | 8 (6F/2M) | 8 (6F/2M) | |
| ng/µL | 22.94 | 28.59 | 27.53 | 24.58 | 22.36 | 26.45 | ||
| SD | 3.76 | 3.90 | 7.80 | 4.59 | 8.83 | 6.44 | ||
| Glaucoma Group | Control Group | |||||||
| Protein | Conc. | DBA/2J_G4 | DBA/2J_G10 | DBA/2J_G14 | D2G4 | D2G14 | ||
| a1AT | n | 20 (12F/8M) | 19 (11F/8M) | 20 (12F/8M) | 12 (11F/1M) | 12 (11F/1M) | ||
| ng/µL | 557.87 | 521.57 | 530.55 | 475.14 | 507.40 | |||
| SD | 227.17 | 186.89 | 214.91 | 161.21 | 81.90 | |||
| APOL1 | n | 20 (12F/8M) | 19 (11F/8M) | 20 (12F/8M) | 14 (11F/3M) | 14 (11F/3M) | ||
| ng/µL | 2.51 | 2.19 | 2.25 | 2.09 | 2.53 | |||
| SD | 0.41 | 0.50 | 0.48 | 0.39 | 0.41 | |||
| CFH | n | 18 (11F/7M) | 19 (11F/8M) | 20 (12F/8M) | 14 (11F/3M) | 14 (11F/3M) | ||
| ng/µL | 68.31 | 89.67 | 89.67 | 84.04 | 88.02 | |||
| SD | 10.36 | 9.38 | 12.87 | 10.46 | 9.04 | |||
| ITIH4 | n | 18 (11F/7M) | 19 (11F/8M) | 19 (13F/6M) | 14 (11F/3M) | 14 (11F/3M) | ||
| ng/µL | 73.41 | 112.99 | 112.04 | 98.98 | 126.71 | |||
| SD | 23.73 | 27.56 | 26.68 | 31.27 | 25.39 | |||
| FCN3 | n | 20 (12F/8M) | 18 (10F/8M) | 19 (11F/8M) | 14 (11F/3M) | 14 (11F/3M) | ||
| ng/µL | 442.00 | 530.20 | 460.10 | 352.71 | 479.07 | |||
| SD | 177.63 | 225.59 | 178.87 | 134.78 | 210.25 | |||
| Comparison | Method | CA | Sens. | Spec. | AUC | Prec. |
|---|---|---|---|---|---|---|
| DBA/2J_G4 vs. DBA/2J_G10 vs. DBA/2J_G14 | Random Forest | 0.62 | 0.74 | 0.90 | 0.84 | 0.80 |
| DBA/2J_G4 vs. DBA/2J_G14 | Naive Bayes | 0.89 | 0.93 | 0.85 | 0.96 | 0.86 |
| DBA/2J_G4 vs. DBA/2J_G10 | Naive Bayes | 0.78 | 0.80 | 0.77 | 0.88 | 0.76 |
| DBA/2J_G10 vs. DBA/2J_G14 | Random Forest | 0.65 | 0.43 | 0.85 | 0.68 | 0.72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Vega Cueto, A.; Álvarez, L.; García, M.; Artime, E.; Álvarez Barrios, A.; Rodríguez-Uña, I.; Coca-Prados, M.; González-Iglesias, H. Systemic Alterations of Immune Response-Related Proteins during Glaucoma Development in the Murine Model DBA/2J. Diagnostics 2020, 10, 425. https://doi.org/10.3390/diagnostics10060425
Fernández-Vega Cueto A, Álvarez L, García M, Artime E, Álvarez Barrios A, Rodríguez-Uña I, Coca-Prados M, González-Iglesias H. Systemic Alterations of Immune Response-Related Proteins during Glaucoma Development in the Murine Model DBA/2J. Diagnostics. 2020; 10(6):425. https://doi.org/10.3390/diagnostics10060425
Chicago/Turabian StyleFernández-Vega Cueto, Andrés, Lydia Álvarez, Montserrat García, Enol Artime, Ana Álvarez Barrios, Ignacio Rodríguez-Uña, Miguel Coca-Prados, and Héctor González-Iglesias. 2020. "Systemic Alterations of Immune Response-Related Proteins during Glaucoma Development in the Murine Model DBA/2J" Diagnostics 10, no. 6: 425. https://doi.org/10.3390/diagnostics10060425
APA StyleFernández-Vega Cueto, A., Álvarez, L., García, M., Artime, E., Álvarez Barrios, A., Rodríguez-Uña, I., Coca-Prados, M., & González-Iglesias, H. (2020). Systemic Alterations of Immune Response-Related Proteins during Glaucoma Development in the Murine Model DBA/2J. Diagnostics, 10(6), 425. https://doi.org/10.3390/diagnostics10060425