Cardiovascular Risk and Statin Therapy Considerations in Women
Abstract
1. Introduction
1.1. Smoking
1.2. Sedentary Lifestyle
1.3. Obesity
1.4. Metabolic Syndrome
1.5. High Levels of Cholesterol and Total Lipids
1.6. High Blood Pressure
1.7. Diabetes Mellitus
1.8. Chronic Renal Disease
- Women’s health risks are misunderstood; women are more concerned with the risk of cancer, especially breast cancer [62,63]. It has also been observed that the use of traditional cardiovascular risk and the Framingham score underestimates cardiovascular risk in women [62,63]. It is recommended to use unique risk factors for a better estimation (Table 1) [64].
2. Differences between Sexes Regarding Screening, Diagnosis, and Treatment of Dyslipidemia
3. Statin Metabolism and Peculiarities in Women
- In women, the CYP3A4 enzyme expression is twice as high when compared to men. Consequently, the metabolism of CYP3A4-dependent statins is faster, and their activity is lower than in men.
- In women, muscle mass is lower when compared to men; hence, they are more vulnerable to myopathy.
- In women, the percentage of fat tissue is higher when compared to men. As such, the distribution volume of lipophilic statins, such as simvastatin, is higher, and the maximum serum concentration is lower.
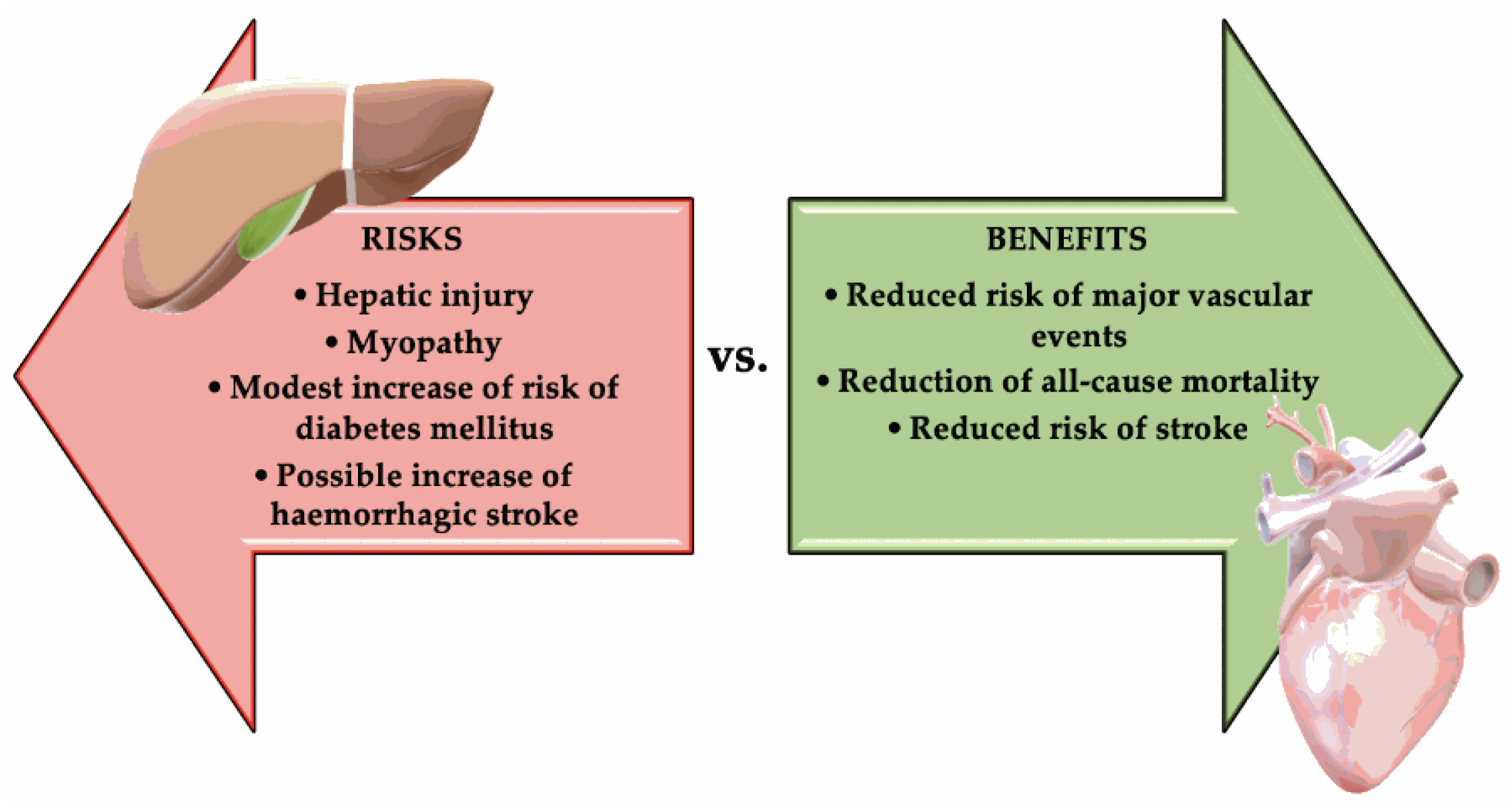
4. Benefits vs. Risks of Statin Treatments
- Modest risk of developing DM (approximately 0.1% annually). In an analysis from the Justification for the Use of Statin in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial, statin therapy accelerated the time to new-onset diabetes by only 5.4 weeks. This is a weak reason not to use statins [88].
- Very rarely: Clear clinically evident hepatic injury, most frequently being asymptomatic, and usually temporary elevation of aminotransferases [91].
- Possible increase of hemorrhagic stroke in a patient with a history of stroke. This risk was suggested by Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial (SPARCL), but it was not confirmed by studies that used computed tomography (CT) with contrast substance [83]. The risk of hemorrhagic stroke in SPARCL correlated with prior history of hemorrhagic stroke and inadequately controlled blood pressure, not with statin or LDL-C reduction per se [92]. Figure 1 summarizes the risks vs. benefits of statins treatment.
- In a patient with myalgia, if the CK levels exceed the reference range limit by 4 times, therapy should be discontinued for 2 to 4 weeks, and after this, the statin dose should be lowered and then titrated to the tolerated dose or another statin can be used [97].
- In a patient with myalgia, if the CK levels are not elevated or small elevations are present, the therapy should be discontinued for 2 weeks. If the symptoms improve, statin therapy can be continued. If the symptoms persist, the statin must be changed; if with the new statin symptoms no longer occur, the same drug can be continued, while if symptoms reoccur, the highest tolerated statin dose should be used, by changing the statin, starting with a low dose, and considering an alternating dosage [97].
- Simvastatin is contraindicated with: itraconazole, ketoconazole, posaconazole, erythromycin, clarithromycin, telithromycin, human immunodeficiency virus protease inhibitors, nefazodone, gemfibrozil, cyclosporine, or danazol [97].
- Do not exceed 10 mg simvastatin daily when taking verapamil or diltiazem [97].
- Do not exceed 20 mg simvastatin daily when taking amlodipine, amiodarone, or ranolazine [97].
5. Statin Treatment in Fertile Women
6. Statin Treatment in Postmenopausal Women
- Treatment costs for the elderly.
- Patient adherence/tolerance to the treatment.
- Hypothyroidism, which can lead to muscle symptoms that can be misinterpreted as secondary effects of statin treatment [99].
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tiffe, T.; Wagner, M.; Rücker, V.; Morbach, C.; Gelbrich, G.; Störk, S.; Heuschmann, P.U. Control of Cardiovascular Risk Factors and Its Determinants in the General Population- Findings From the STAAB Cohort Study. BMC Cardiovasc. Disord. 2017, 17, 276. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Jamal, A.; Phillips, E.; Gentzke, A.S.; Homa, D.M.; Babb, S.D.; King, B.A.; Neff, L.J. Current Cigarette Smoking Among Adults—United States, 2016. Mmwr Morb. Mortal. Wkly. Rep. 2018, 67, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Creamer, M.R.; Wang, T.W.; Babb, S.; Cullen, K.A.; Day, H.; Willis, G.; Jamal, A.; Neff, L. Tobacco Product Use and Cessation Indicators Among Adults—United States, 2018. Mmwr Morb. Mortal. Wkly. Rep. 2019, 68, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Mouhamed, D.H.; Ezzaher, A.; Neffati, F.; Douki, W.; Najjar, M.F.; Gaha, L. Association Between Cigarette Smoking and Dyslipidemia. Immuno-Anal. Biol. Spe. 2013, 28, 195–200. [Google Scholar] [CrossRef]
- Pavanello, C.; Mombelli, G. Considering Gender in Prescribing Statins: What Do Physicians Need To Know? Clin. Lipidol. 2015, 10, 499–512. [Google Scholar] [CrossRef]
- Zhang, H.; Plutzky, J.; Shubina, M.; Turchin, A. Drivers of the Sex Disparity in Statin Therapy in Patients with Coronary Artery Disease: A Cohort Study. PLoS ONE 2016, 11, e0155228. [Google Scholar] [CrossRef]
- Ursoniu, S.; Mikhailidis, D.P.; Serban, M.C.; Penson, P.; Toth, P.P.; Ridker, P.M.; Ray, K.K.; Kees Hovingh, G.; Kastelein, J.J.; Hernandez, A.V.; et al. The Effect of Statins on Cardiovascular Outcomes by Smoking Status: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharm. Res. 2017, 122, 105–117. [Google Scholar] [CrossRef]
- Milionis, H.J.; Rizos, E.; Mikhailidis, D.P. Smoking Diminishes the Beneficial Effect of Statins: Observations from the Landmark Trials. Angiology 2001, 52, 575–587. [Google Scholar] [CrossRef]
- Ussery, E.N.; Fulton, J.E.; Galuska, D.A.; Katzmarzyk, P.T.; Carlson, S.A. Joint Prevalence of Sitting Time and Leisure-Time Physical Activity Among US Adults, 2015-2016. JAMA 2018, 320, 2036–2038. [Google Scholar] [CrossRef]
- Hallal, P.C.; Lee, I.M. Prescription of Physical Activity: An Undervalued Intervention. Lancet 2013, 381, 356–357. [Google Scholar] [CrossRef]
- Kokkinos, P.F.; Faselis, C.; Myers, J.; Panagiotakos, D.; Doumas, M. Interactive Effects of Fitness and Statin Treatment on Mortality Risk in Veterans With Dyslipidaemia: A Cohort Study. Lancet 2013, 381, 394–399. [Google Scholar] [CrossRef]
- Gui, Y.J.; Liao, C.X.; Liu, Q.; Guo, Y.; Yang, T.; Chen, J.Y.; Wang, Y.T.; Hu, J.H.; Xu, D.Y. Efficacy and Safety of Statins and Exercise Combination Therapy Compared to Statin Monotherapy in Patients With Dyslipidaemia: A Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2017, 24, 907–916. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, D. Effects of Aerobic Exercise on Lipids and Lipoproteins. Lipids Health Dis. 2017, 16, 132. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.C.; Hopkins, P.N.; Toth, P.P.; Ballantyne, C.M.; Rader, D.J.; Robinson, J.G.; Daniels, S.R.; Gidding, S.S.; de Ferranti, S.D.; Ito, M.K.; et al. Familial Hypercholesterolemia: Screening, Diagnosis and Management of Pediatric and Adult Patients: Clinical Guidance From the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J. Clin. Lipidol. 2011, 5, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.W.; Crouse, S.F.; Rohack, J.J. Influence of Cholesterol Status on Blood Lipid and Lipoprotein Enzyme Responses to Aerobic Exercise. J. Appl. Physiol. 2000, 89, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Dufaux, B.; Order, U.; Müller, R.; Hollmann, W. Delayed Effects of Prolonged Exercise on Serum Lipoproteins. Metabolism 1986, 35, 105–109. [Google Scholar] [CrossRef]
- Calabresi, L.; Franceschini, G. Lecithin:cholesterol Acyltransferase, High-Density Lipoproteins, and Atheroprotection in Humans. Trends Cardiovasc. Med. 2010, 20, 50–53. [Google Scholar] [CrossRef]
- Bosomworth, N.J. Impediments to Clinical Application of Exercise Interventions in the Treatment of Cardiometabolic Disease. Can. Fam. Physician 2019, 65, 164–170. [Google Scholar]
- Golomb, B.A.; Evans, M.A.; Dimsdale, J.E.; White, H.L. Effects of Statins on Energy and Fatigue With Exertion: Results From a Randomized Controlled Trial. Arch. Intern. Med. 2012, 172, 1180–1182. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Fryar, C.D.; Flegal, K.M. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. Nchs Data Brief. 2015, 1–8. [Google Scholar]
- Dixon, J.B.; O’Brien, P.E. Lipid Profile in the Severely Obese: Changes With Weight Loss After Lap-Band Surgery. Obes. Res. 2002, 10, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Rössner, S.; Björvell, H. Early and Late Effects of Weight Loss on Lipoprotein Metabolism in Severe Obesity. Atherosclerosis 1987, 64, 125–130. [Google Scholar] [CrossRef]
- Vesa, C.M.; Popa, L.; Popa, A.R.; Rus, M.; Zaha, A.A.; Bungau, S.; Tit, D.M.; Corb Aron, R.A.; Zaha, D.C. Current Data Regarding the Relationship between Type 2 Diabetes Mellitus and Cardiovascular Risk Factors. Diagnostics 2020, 10, 314. [Google Scholar] [CrossRef]
- Song, X.; Tabák, A.G.; Zethelius, B.; Yudkin, J.S.; Söderberg, S.; Laatikainen, T.; Stehouwer, C.D.; Dankner, R.; Jousilahti, P.; Onat, A.; et al. Obesity Attenuates Gender Differences in Cardiovascular Mortality. Cardiovasc. Diabetol. 2014, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Ruotolo, G.; Howard, B.V. Dyslipidemia of the Metabolic Syndrome. Curr. Cardiol. Rep. 2002, 4, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; El-Tawil, O.S.; Bungau, S.G.; Atanasov, A.G. Applications of Antioxidants in Metabolic Disorders and Degenerative Diseases: Mechanistic Approach. Oxid. Med. Cell. Longev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Blaton, V. How is the Metabolic Syndrome Related to the Dyslipidemia? EJIFCC 2007, 18, 15–22. [Google Scholar] [CrossRef]
- Niroumand, S.; Khajedaluee, M.; Khadem-Rezaiyan, M.; Abrishami, M.; Juya, M.; Khodaee, G.; Dadgarmoghaddam, M. Atherogenic Index of Plasma (AIP): A marker of cardiovascular disease. Med. J. Islam. Repub. Iran. 2015, 29, 240. [Google Scholar] [PubMed]
- Hedayatnia, M.; Asadi, Z.; Zare-Feyzabadi, R.; Yaghooti-Khorasani, M.; Ghazizadeh, H.; Ghaffarian-Zirak, R.; Nosrati-Tirkani, A.; Mohammadi-Bajgiran, M.; Rohban, M.; Sadabadi, F.; et al. Dyslipidemia and Cardiovascular Disease Risk Among the MASHAD Study Population. Lipids Health Dis. 2020, 19. [Google Scholar] [CrossRef] [PubMed]
- Hadaegh, F.; Harati, H.; Ghanbarian, A.; Azizi, F. Association of Total Cholesterol Versus Other Serum Lipid Parameters With the Short-Term Prediction of Cardiovascular Outcomes: Tehran Lipid and Glucose Study. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 571–577. [Google Scholar] [CrossRef]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy After Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Magkos, F.; Mittendorfer, B. Sex Differences in Lipid and Lipoprotein Metabolism: It’s Not Just About Sex Hormones. J. Clin. Endocrinol. Metab. 2011, 96, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Tit, D.M.; Pallag, A.; Iovan, C.; Furau, G.; Furau, C.; Bungau, S. Somatic-vegetative Symptoms Evolution in Postmenopausal Women Treated with Phytoestrogens and Hormone Replacement Therapy. Iran. J. Public Health 2017, 46, 1528–1534. [Google Scholar] [PubMed]
- Svendsen, O.L.; Hassager, C.; Christiansen, C. Age- And Menopause-Associated Variations in Body Composition and Fat Distribution in Healthy Women as Measured by Dual-Energy X-ray Absorptiometry. Metabolism 1995, 44, 369–373. [Google Scholar] [CrossRef]
- Tit, D.M.; Bungau, S.; Iovan, C.; Cseppento, D.C.N.; Endres, L.; Sava, C.; Sabau, A.M.; Furau, G.; Furau, C. Effects of the Hormone Replacement Therapy and of Soy Isoflavones on Bone Resorption in Postmenopause. J. Clin. Med. 2018, 7, 297. [Google Scholar] [CrossRef]
- Wilmot, K.A.; O’Flaherty, M.; Capewell, S.; Ford, E.S.; Vaccarino, V. Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011: Evidence for Stagnation in Young Adults, Especially Women. Circulation 2015, 132, 997–1002. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Williams, R.R.; Hopkins, P.N.; Hunt, S.C.; Wu, L.L.; Hasstedt, S.J.; Lalouel, J.M.; Ash, K.O.; Stults, B.M.; Kuida, H. Population-based Frequency of Dyslipidemia Syndromes in Coronary-Prone Families in Utah. Arch. Intern. Med. 1990, 150, 582–588. [Google Scholar] [CrossRef]
- Castelli, W.P.; Anderson, K. A Population at Risk. Prevalence of High Cholesterol Levels in Hypertensive Patients in the Framingham Study. Am. J. Med. 1986, 80, 23–32. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Pietz, K.; Battleman, D.S.; Beyth, R.J. Prevalence of Comorbid Hypertension and Dyslipidemia and Associated Cardiovascular Disease. Am. J. Manag. Care 2004, 10, 926–932. [Google Scholar] [PubMed]
- Nickenig, G.; Harrison, D.G. The AT(1)-type Angiotensin Receptor in Oxidative Stress and Atherogenesis: Part I: Oxidative Stress and Atherogenesis. Circulation 2002, 105, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Miettinen, H.; Gaskill, S.P.; Stern, M.P. Metabolic Precursors of Hypertension. The San Antonio Heart Study. Arch. Intern. Med. 1996, 156, 1994–2001. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, G. Should Angiotensin II Receptor Blockers and Statins Be Combined? Circulation 2004, 110, 1013–1020. [Google Scholar] [CrossRef]
- Kannel, W.B.; McGee, D.L. Diabetes and Cardiovascular Disease. The Framingham Study. JAMA 1979, 241, 2035–2038. [Google Scholar] [CrossRef]
- Constantino, M.I.; Molyneaux, L.; Limacher-Gisler, F.; Al-Saeed, A.; Luo, C.; Wu, T.; Twigg, S.M.; Yue, D.K.; Wong, J. Long-Term Complications and Mortality in Young-Onset Diabetes: Type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care 2013, 36, 3863–3869. [Google Scholar] [CrossRef]
- Stoicescu, M.; Csepento, C.; Muţiu, G.; Bungău, S. The Role of Increased Plasmatic Renin Level in the Pathogenesis of Arterial Hypertension in Young Adults. Rom. J. Morphol. Embryol. 2011, 52, 419–423. [Google Scholar]
- de Ferranti, S.D.; de Boer, I.H.; Fonseca, V.; Fox, C.S.; Golden, S.H.; Lavie, C.J.; Magge, S.N.; Marx, N.; McGuire, D.K.; Orchard, T.J.; et al. Type 1 Diabetes Mellitus and Cardiovascular Disease: A Scientific Statement From the American Heart Association and American Diabetes Association. Diabetes Care 2014, 37, 2843–2863. [Google Scholar] [CrossRef]
- Rawshani, A.; Sattar, N.; Franzén, S.; Rawshani, A.; Hattersley, A.T.; Svensson, A.M.; Eliasson, B.; Gudbjörnsdottir, S. Excess Mortality and Cardiovascular Disease in Young Adults With Type 1 Diabetes in Relation to Age at Onset: A Nationwide, Register-Based Cohort Study. Lancet. 2018, 392, 477–486. [Google Scholar] [CrossRef]
- Fox, C.S.; Golden, S.H.; Anderson, C.; Bray, G.A.; Burke, L.E.; de Boer, I.H.; Deedwania, P.; Eckel, R.H.; Ershow, A.G.; Fradkin, J.; et al. Update on Prevention of Cardiovascular Disease in Adults With Type 2 Diabetes Mellitus in Light of Recent Evidence: A Scientific Statement From the American Heart Association and the American Diabetes Association. Diabetes Care 2015, 38, 1777–1803. [Google Scholar] [CrossRef] [PubMed]
- Low Wang, C.C.; Hess, C.N.; Hiatt, W.R.; Goldfine, A.B. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus - Mechanisms, Management, and Clinical Considerations. Circulation 2016, 133, 2459–2502. [Google Scholar] [CrossRef] [PubMed]
- Martín-Timón, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; Del Cañizo-Gómez, F.J. Type 2 Diabetes and Cardiovascular Disease: Have All Risk Factors the Same Strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; MacCallum, P.R. The Obesity, Metabolic Syndrome, and Type 2 Diabetes Mellitus Pandemic: Part I. Increased Cardiovascular Disease Risk and the Importance of Atherogenic Dyslipidemia in Persons With the Metabolic Syndrome and Type 2 Diabetes Mellitus. J. Cardiometab. Syndr. 2009, 4, 113–119. [Google Scholar] [CrossRef]
- Goldberg, I.J. Clinical Review 124: Diabetic Dyslipidemia: Causes and Consequences. J. Clin. Endocrinol. Metab. 2001, 86, 965–971. [Google Scholar] [CrossRef]
- Ferrannini, E.; DeFronzo, R.A. Impact of Glucose-Lowering Drugs on Cardiovascular Disease in Type 2 Diabetes. Eur. Heart J. 2015, 36, 2288–2296. [Google Scholar] [CrossRef]
- Aderibigbe, M.A.; Obafemi, T.O.; Olaleye, M.T.; Akinmoladun, A.C. Effects of Gender, Age and Treatment Duration on Lipid Profile and Renal Function Indices in Diabetic Patients Attending a Teaching Hospital in South-Western Nigeria. Afr. Health Sci. 2018, 18, 900–908. [Google Scholar] [CrossRef]
- Sapna, S.; Alok, M.L. A Study on Lipid Profile Levels of Diabetics and NonDiabetics Among Naini Region of Allahabad, India. Turk. J. Biochem. 2008, 33, 138–141. [Google Scholar]
- Moisi, M.I.; Rus, M.; Bungau, S.; Zaha, D.C.; Uivarosan, D.; Fratila, O.; Tit, D.M.; Endres, L.; Nistor-Cseppento, D.C.; Popescu, M.I. Acute Coronary Syndromes in Chronic Kidney Disease: Clinical and Therapeutic Characteristics. Medicina 2020, 56, 118. [Google Scholar] [CrossRef]
- Tsimihodimos, V.; Dounousi, E.; Siamopoulos, K.C. Dyslipidemia in Chronic Kidney Disease: An Approach to Pathogenesis and Treatment. Am. J. Nephrol. 2008, 28, 958–973. [Google Scholar] [CrossRef]
- Mosca, L.; Benjamin, E.J.; Berra, K.; Bezanson, J.L.; Dolor, R.J.; Lloyd-Jones, D.M.; Newby, L.K.; Piña, I.L.; Roger, V.L.; Shaw, L.J.; et al. Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update: A Guideline From the American Heart Association. Circulation 2011, 123, 1243–1262. [Google Scholar] [CrossRef]
- Monsuez, J.J.; Pham, T.; Karam, N.; Amar, L.; Chicheportiche-Ayache, C.; Menasché, P.; Desnos, M.; Dardel, P.; Weill, I. Awareness of Individual Cardiovascular Risk Factors and Self-Perception of Cardiovascular Risk in Women. Am. J. Med. Sci. 2017, 354, 240–245. [Google Scholar] [CrossRef]
- Douglas, P.S.; Poppas, A. Overview of Cardiovascular Risk Factors in Women. Available online: https://www.uptodate.com/contents/overview-of-cardiovascular-risk-factors-in-women (accessed on 17 March 2020).
- Gretz, N.; Zeier, M.; Geberth, S.; Strauch, M.; Ritz, E. Is Gender a Determinant for Evolution of Renal Failure? A Study in Autosomal Dominant Polycystic Kidney Disease. Am. J. Kidney Dis. 1989, 14, 178–183. [Google Scholar] [CrossRef]
- Silbiger, S.R.; Neugarten, J. The Impact of Gender on the Progression of Chronic Renal Disease. Am. J. Kidney Dis. 1995, 25, 515–533. [Google Scholar] [CrossRef]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Adams, R.J.; Berry, J.D.; Brown, T.M.; Carnethon, M.R.; Dai, S.; de Simone, G.; Ford, E.S.; et al. Heart Disease and Stroke Statistics—2011 Update: A Report From the American Heart Association. Circulation 2011, 123, e18–e209. [Google Scholar] [CrossRef]
- Ford, E.S.; Capewell, S. Coronary Heart Disease Mortality Among Young Adults in the U.S. From 1980 Through 2002: Concealed Leveling of Mortality Rates. J. Am. Coll. Cardiol. 2007, 50, 2128–2132. [Google Scholar] [CrossRef]
- Samad, F.; Agarwal, A.; Samad, Z. Stable Ischemic Heart Disease in Women: Current Perspectives. Int. J. Womens Health 2017, 9, 701–709. [Google Scholar] [CrossRef]
- Hemal, K.; Pagidipati, N.J.; Coles, A.; Dolor, R.J.; Mark, D.B.; Pellikka, P.A.; Hoffmann, U.; Litwin, S.E.; Daubert, M.A.; Shah, S.H.; et al. Sex Differences in Demographics, Risk Factors, Presentation, and Noninvasive Testing in Stable Outpatients with Suspected Coronary Artery Disease: Insights from the PROMISE Trial. Jacc. Cardiovasc. Imaging 2016, 9, 337–346. [Google Scholar] [CrossRef]
- Kragholm, K.; Halim, S.A.; Yang, Q.; Schulte, P.J.; Hochman, J.S.; Melloni, C.; Mahaffey, K.W.; Moliterno, D.J.; Harrington, R.A.; White, H.D.; et al. Sex-Stratified Trends in Enrollment, Patient Characteristics, Treatment, and Outcomes Among Non–ST-Segment Elevation Acute Coronary Syndrome Patients: Insights from Clinical Trials Over 17 Years. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 357–367. [Google Scholar] [CrossRef]
- Peters, S.A.E.; Colantonio, L.D.; Zhao, H.; Bittner, V.; Dai, Y.; Farkouh, M.E.; Monda, K.L.; Safford, M.M.; Muntner, P.; Woodward, M. Sex Differences in High-Intensity Statin Use Following Myocardial Infarction in the United States. J. Am. Coll. Cardiol. 2018, 71, 1729–1737. [Google Scholar] [CrossRef]
- Rodriguez, F.; Lin, S.; Maron, D.J.; Knowles, J.W.; Virani, S.S.; Heidenreich, P.A. Use of High-Intensity Statins for Patients With Atherosclerotic Cardiovascular Disease in the Veterans Affairs Health System: Practice Impact of the New Cholesterol Guidelines. Am. Heart J. 2016, 182, 97–102. [Google Scholar] [CrossRef]
- Nanna, M.G.; Wang, T.Y.; Xiang, Q.; Goldberg, A.C.; Robinson, J.G.; Roger, V.L.; Virani, S.S.; Wilson, P.W.F.; Louie, M.J.; Koren, A.; et al. Sex Differences in the Use of Statins in Community Practice. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005562. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, J.R.; Gerrits, A.J.; Brotons, C.; Duhot, D.; Hollander, M.; Lionis, C.; Macháčová, M.; Marrocco, W.; Oltrogge, J.; Taylor, C.J.; et al. Managing Elevated Lipids in Primary Care. Available online: https://cvgk.nl/2018/09/04/consensusdocument-europese-huisartsen-over-lipidenmanagement-in-de-eerste-lijn//download-consensusdocument-europese-huisartsen-over-lipidenmanagement-in-de-eerste-lijn.pdf (accessed on 20 May 2020).
- Wei, M.Y.; Ito, M.K.; Cohen, J.D.; Brinton, E.A.; Jacobson, T.A. Predictors of Statin Adherence, Switching, and Discontinuation in the USAGE Survey: Understanding the Use of Statins in America and Gaps in Patient Education. J. Clin. Lipidol. 2013, 7, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Cuevas, A.; Cafferata, A. Diagnosis and Management of Statin Intolerance. J. Atheroscler. Thromb. 2019, 26, 207–215. [Google Scholar] [CrossRef]
- Salami, J.A.; Warraich, H.J.; Valero-Elizondo, J.; Spatz, E.S.; Desai, N.R.; Rana, J.S.; Virani, S.S.; Blankstein, R.; Khera, A.; Blaha, M.J.; et al. National Trends in Nonstatin Use and Expenditures Among the US Adult Population From 2002 to 2013: Insights From Medical Expenditure Panel Survey. J. Am. Heart Assoc. 2018, 7, e007132. [Google Scholar] [CrossRef]
- Meor Anuar Shuhaili, M.F.R.; Samsudin, I.N.; Stanslas, J.; Hasan, S.; Thambiah, S.C. Effects of Different Types of Statins on Lipid Profile: A Perspective on Asians. Int. J. Endocrinol. Metab. 2017, 15, e43319. [Google Scholar] [CrossRef]
- Schachter, M. Chemical, Pharmacokinetic and Pharmacodynamic Properties of Statins: An Update. Fundam. Clin. Pharm. 2005, 19, 117–125. [Google Scholar] [CrossRef]
- Mombelli, G.; Bosisio, R.; Calabresi, L.; Magni, P.; Pavanello, C.; Pazzucconi, F.; Sirtori, C.R. Gender-related Lipid and/or Lipoprotein Responses to Statins in Subjects in Primary and Secondary Prevention. J. Clin. Lipidol. 2015, 9, 226–233. [Google Scholar] [CrossRef]
- García, M.J.; Reinoso, R.F.; Sánchez Navarro, A.; Prous, J.R. Clinical Pharmacokinetics of Statins. Methods Find. Exp. Clin. Pharm. 2003, 25, 457–481. [Google Scholar] [CrossRef]
- Mach, F.; Ray, K.K.; Wiklund, O.; Corsini, A.; Catapano, A.L.; Bruckert, E.; De Backer, G.; Hegele, R.A.; Hovingh, G.K.; Jacobson, T.A.; et al. Adverse Effects of Statin Therapy: Perception vs. The Evidence-Focus on Glucose Homeostasis, Cognitive, Renal and Hepatic Function, Haemorrhagic Stroke and Cataract. Eur. Heart J. 2018, 39, 2526–2539. [Google Scholar] [CrossRef]
- Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of Cardiovascular Events and Death With Pravastatin in Patients With Coronary Heart Disease and a Broad Range of Initial Cholesterol Levels. N. Engl. J. Med. 1998, 339, 1349–1357. [Google Scholar] [CrossRef]
- Wilt, T.J.; Bloomfield, H.E.; MacDonald, R.; Nelson, D.; Rutks, I.; Ho, M.; Larsen, G.; McCall, A.; Pineros, S.; Sales, A. Effectiveness of Statin Therapy in Adults With Coronary Heart Disease. Arch. Intern. Med. 2004, 164, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Fulcher, J.; O’Connell, R.; Voysey, M.; Emberson, J.; Blackwell, L.; Mihaylova, B.; Simes, J.; Collins, R.; Kirby, A.; et al. Efficacy and Safety of LDL-lowering Therapy Among Men and Women: Meta-Analysis of Individual Data From 174,000 Participants in 27 Randomised Trials. Lancet 2015, 385, 1397–1405. [Google Scholar] [CrossRef]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B Stat. Methodol 1972, 34, 187–220. [Google Scholar] [CrossRef]
- Ridker, P.M. The JUPITER Trial: Results, Controversies, and Implications for Prevention. Circ. Cardiovasc. Qual. Outcomes 2009, 2, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Brinton, E.A.; Maki, K.C.; Jacobson, T.A.; Sponseller, C.A.; Cohen, J.D. Metabolic Syndrome Is Associated With Muscle Symptoms Among Statin Users. J. Clin. Lipidol. 2016, 10, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.; Pedro-Botet, J.; Climent, E.; Millán, J.; Rius, J. Statin Associated Myopathy in Clinical Practice. Results of DAMA Study. Clin. Investig. Arter. 2017, 29, 7–12. [Google Scholar] [CrossRef]
- Ihle, P.; Dippel, F.W.; Schubert, I. Statin-associated Myopathy. Assessment of Frequency Based on Data of All Statutory Health Insurance Funds in Germany. Pharm. Res. Perspect. 2018, 6, e00404. [Google Scholar] [CrossRef]
- Jose, J. Statins and Its Hepatic Effects: Newer Data, Implications, and Changing Recommendations. J. Pharm. Bioallied Sci. 2016, 8, 23–28. [Google Scholar] [CrossRef]
- Goldstein, L.B.; Amarenco, P.; Szarek, M.; Callahan, A.; Hennerici, M.; Sillesen, H.; Zivin, J.A.; Welch, K.M. Hemorrhagic Stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels Study. Neurology 2008, 70, 2364–2370. [Google Scholar] [CrossRef]
- Ramkumar, S.; Raghunath, A.; Raghunath, S. Statin Therapy: Review of Safety and Potential Side Effects. Acta Cardiol. Sin. 2016, 32, 631–639. [Google Scholar] [CrossRef]
- Selva-O’Callaghan, A.; Alvarado-Cardenas, M.; Pinal-Fernández, I.; Trallero-Araguás, E.; Milisenda, J.C.; Martínez, M.; Marín, A.; Labrador-Horrillo, M.; Juárez, C.; Grau-Junyent, J.M. Statin-induced Myalgia and Myositis: An Update on Pathogenesis and Clinical Recommendations. Expert Rev. Clin. Immunol. 2018, 14, 215–224. [Google Scholar] [CrossRef]
- Barry, A.R.; Beach, J.E.; Pearson, G.J. Prevention and Management of Statin Adverse Effects: A Practical Approach for Pharmacists. Can. Pharm. J. (Ott.) 2018, 151, 179–188. [Google Scholar] [CrossRef]
- Pasternak, R.C.; Smith, S.C.; Bairey-Merz, C.N.; Grundy, S.M.; Cleeman, J.I.; Lenfant, C. ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins. J. Am. Coll. Cardiol. 2002, 40, 567–572. [Google Scholar] [CrossRef]
- Laufs, U.; Scharnagl, H.; Halle, M.; Windler, E.; Endres, M.; März, W. Treatment Options for Statin-Associated Muscle Symptoms. Dtsch. Arztebl. Int. 2015, 112, 748–755. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Khan, A.; Maki, K.C.; Brinton, E.A.; Cohen, J.D. Provider Recommendations for Patient-Reported Muscle Symptoms on Statin Therapy: Insights From the Understanding Statin Use in America and Gaps in Patient Education Survey. J. Clin. Lipidol. 2018, 12, 78–88. [Google Scholar] [CrossRef]
- Bahamondes, L.; Bahamondes, M.V. New and Emerging Contraceptives: A State-Of-The-Art Review. Int. J. Womens Health 2014, 6, 221–234. [Google Scholar] [CrossRef][Green Version]
- Darroch, J.E.; Singh, S. Trends in Contraceptive Need and Use in Developing Countries in 2003, 2008, and 2012: An Analysis of National Surveys. Lancet 2013, 381, 1756–1762. [Google Scholar] [CrossRef]
- Asare, G.A.; Santa, S.; Ngala, R.A.; Asiedu, B.; Afriyie, D.; Amoah, A.G. Effect of Hormonal Contraceptives on Lipid Profile and the Risk Indices for Cardiovascular Disease in a Ghanaian Community. Int. J. Womens Health 2014, 6, 597–603. [Google Scholar] [CrossRef]
- Godfrey, L.M.; Erramouspe, J.; Cleveland, K.W. Teratogenic Risk of Statins in Pregnancy. Ann. Pharm. 2012, 46, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Zarek, J.; Koren, G. The Fetal Safety of Statins: A Systematic Review and Meta-Analysis. J. Obs. Gynaecol. Can. 2014, 36, 506–509. [Google Scholar] [CrossRef]
- Bateman, B.T.; Hernandez-Diaz, S.; Fischer, M.A.; Seely, E.W.; Ecker, J.L.; Franklin, J.M.; Desai, R.J.; Allen-Coleman, C.; Mogun, H.; Avorn, J.; et al. Statins and congenital malformations: Cohort study. BMJ 2015, 350, h1035. [Google Scholar] [CrossRef] [PubMed]
- Lecarpentier, E.; Morel, O.; Fournier, T.; Elefant, E.; Chavatte-Palmer, P.; Tsatsaris, V. Statins and Pregnancy: Between Supposed Risks and Theoretical Benefits. Drugs 2012, 72, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Women’s Health Stats and Facts 2011. Available online: https://www.acog.org/-/media/NewsRoom/MediaKit.pdf (accessed on 21 May 2020).
- Forman, D.; Wenger, N.K. What Do the Recent American Heart Association/American College of Cardiology Foundation Clinical Practice Guidelines Tell Us About the Evolving Management of Coronary Heart Disease in Older Adults? J. Geriatr. Cardiol. 2013, 10, 123–128. [Google Scholar] [CrossRef]
- Roberts, C.G.; Guallar, E.; Rodriguez, A. Efficacy and Safety of Statin Monotherapy in Older Adults: A Meta-Analysis. J. Gerontol. A Biol. Sci. Med. Sci 2007, 62, 879–887. [Google Scholar] [CrossRef]
- Newman, C.B.; Preiss, D.; Tobert, J.A.; Jacobson, T.A.; Page, R.L.; Goldstein, L.B.; Chin, C.; Tannock, L.R.; Miller, M.; Raghuveer, G.; et al. Statin Safety and Associated Adverse Events: A Scientific Statement From the American Heart Association. Arter. Thromb. Vasc. Biol. 2019, 39, e38–e81. [Google Scholar] [CrossRef]
- Crismaru, I.; Pantea Stoian, A.; Bratu, O.G.; Gaman, M.A.; Stanescu, A.M.A.; Bacalbasa, N.; Diaconu, C.C. Low-density Lipoprotein Cholesterol Lowering Treatment: The Current Approach. Lipids Health Dis. 2020, 19, 85. [Google Scholar] [CrossRef]
- Hung, Y.T.; Yeung, V.T. Hypothyroidism Presenting as Hypercholesterolaemia and Simvastatin-Induced Myositis. Hong Kong Med. J. 2000, 6, 423–424. [Google Scholar]
- Hamilton-Craig, I. Statin-associated Myopathy. Med. J. Aust. 2001, 175, 486–489. [Google Scholar] [CrossRef]
- Duyff, R.F.; Van den Bosch, J.; Laman, D.M.; van Loon, B.J.; Linssen, W.H. Neuromuscular Findings in Thyroid Dysfunction: A Prospective Clinical and Electrodiagnostic Study. J. Neurol. Neurosurg. Psychiatry 2000, 68, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Bar, S.L.; Holmes, D.T.; Frohlich, J. Asymptomatic Hypothyroidism and Statin-Induced Myopathy. Can. Fam. Physician 2007, 53, 428–431. [Google Scholar] [PubMed]
- Tokinaga, K.; Oeda, T.; Suzuki, Y.; Matsushima, Y. HMG-CoA Reductase Inhibitors (Statins) Might Cause High Elevations of Creatine Phosphokinase (CK) in Patients With Unnoticed Hypothyroidism. Endocr. J. 2006, 53, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Jbara, Y.; Bricker, D. Rhabdomyolysis in the Setting of Induced Hypothyroidism and Statin Therapy: A Case Report. Eur. Thyroid J. 2015, 4, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.K.; Maki, K.C.; Brinton, E.A.; Cohen, J.D.; Jacobson, T.A. Muscle Symptoms in Statin Users, Associations With Cytochrome P450, and Membrane Transporter Inhibitor Use: A Subanalysis of the USAGE Study. J. Clin. Lipidol. 2014, 8, 69–76. [Google Scholar] [CrossRef]
- Colantonio, L.D.; Huang, L.; Monda, K.L.; Bittner, V.; Serban, M.C.; Taylor, B.; Brown, T.M.; Glasser, S.P.; Muntner, P.; Rosenson, R.S. Adherence to High-Intensity Statins Following a Myocardial Infarction Hospitalization Among Medicare Beneficiaries. Jama Cardiol. 2017, 2, 890–895. [Google Scholar] [CrossRef]
- Lin, I.; Sung, J.; Sanchez, R.J.; Mallya, U.G.; Friedman, M.; Panaccio, M.; Koren, A.; Neumann, P.; Menzin, J. Patterns of Statin Use in a Real-World Population of Patients at High Cardiovascular Risk. J. Manag. Care Spec. Pharm. 2016, 22, 685–698. [Google Scholar] [CrossRef]
| Cardiovascular Risk Factors Unique to Women |
|---|
| Early menarche Premenstrual syndrome Menopause Hysterectomy Combined estrogen-progestin oral contraceptives Polycystic ovary syndrome Pregnancy complications (hypertension and diabetes mellitus, spontaneous pregnancy loss, placental abruption, and others) |
| Statin | Solubility | Effect of Food | CYP450 Metabolism and Isoenzyme | Active Metabolites | Renal Excretion (%) | Elimination Half-Life (h) |
|---|---|---|---|---|---|---|
| Fungal-derived statin | ||||||
| Lovastatin | Lp | BA increased | 3A4 | yes | 10 | 3 |
| Pravastatin | Hp | BA decreased | – | no | 20 | 1.8 |
| Simvastatin | Lp | no effect | 3A4 | yes | 13 | 2 |
| Fully synthetic compounds | ||||||
| Atorvastatin | Lp | BA decreased | 3A4 | yes | <5 | 14 |
| Cerivastatin | Lp | no effect | 3A4, 2C8 | yes | 30 | 2.5 |
| Fluvastatin | Lp | BA decreased | 2C9 | no | 6 | 1.2 |
| Pitavastatin | Lp | not available | limited | minor | not available | 11 |
| Rosuvastatin | Hp | no effect | limited | minor | 10 | 19 |
| General Factors Associated with the Highest Risk of Myopathy | Specific Factors for Statin-Induced Myopathy |
|---|---|
| Elderly, especially >80-year old Female sex Low body mass index Hypothyroidism Renal or hepatic impairment Diabetes mellitus Recent surgery | Advanced age (>80-year old) The genetic risk factor for simvastatin myopathy (SLCO1B1 rs4149056) Hypothyroidism Severe chronic kidney disease Impaired liver function Perioperative period |
| Interacting medications (macrolide antibiotics, fibrates, cyclosporine, amiodarone, verapamil, antifungals, protease inhibitors) | Alcohol abuse Consumption of large quantities of grapefruit juice Interacting medications |
| Contraceptive | LDL-C | HDL-C | Triglycerides * |
|---|---|---|---|
| Combination estrogen/progestin pills |  |  |  |
| Estrogen component |  |  |  |
| Progestin component |  |  |  |
| Transdermal patch |  |  |  |
| Vaginal ring |  |  | No change |
| Depot medroxyprogesterone acetate |  |  | No change |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghe, G.; Toth, P.P.; Bungau, S.; Behl, T.; Ilie, M.; Pantea Stoian, A.; Bratu, O.G.; Bacalbasa, N.; Rus, M.; Diaconu, C.C. Cardiovascular Risk and Statin Therapy Considerations in Women. Diagnostics 2020, 10, 483. https://doi.org/10.3390/diagnostics10070483
Gheorghe G, Toth PP, Bungau S, Behl T, Ilie M, Pantea Stoian A, Bratu OG, Bacalbasa N, Rus M, Diaconu CC. Cardiovascular Risk and Statin Therapy Considerations in Women. Diagnostics. 2020; 10(7):483. https://doi.org/10.3390/diagnostics10070483
Chicago/Turabian StyleGheorghe, Gina, Peter P. Toth, Simona Bungau, Tapan Behl, Madalina Ilie, Anca Pantea Stoian, Ovidiu Gabriel Bratu, Nicolae Bacalbasa, Marius Rus, and Camelia Cristina Diaconu. 2020. "Cardiovascular Risk and Statin Therapy Considerations in Women" Diagnostics 10, no. 7: 483. https://doi.org/10.3390/diagnostics10070483
APA StyleGheorghe, G., Toth, P. P., Bungau, S., Behl, T., Ilie, M., Pantea Stoian, A., Bratu, O. G., Bacalbasa, N., Rus, M., & Diaconu, C. C. (2020). Cardiovascular Risk and Statin Therapy Considerations in Women. Diagnostics, 10(7), 483. https://doi.org/10.3390/diagnostics10070483










