Screen-Printed Electrodes (SPE) for In Vitro Diagnostic Purpose
Abstract
1. Introduction
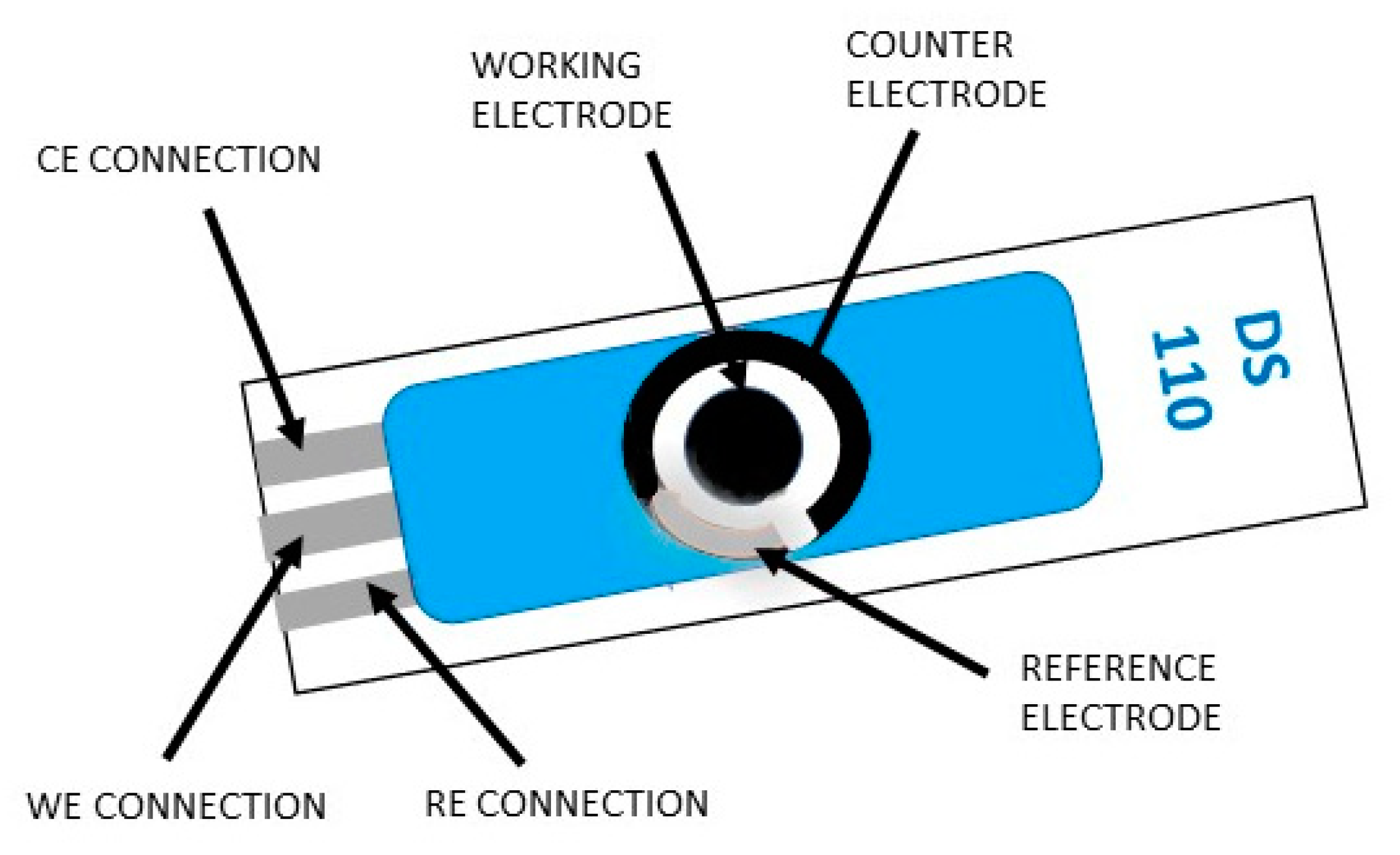
2. The Basic Fabrication Principles, the Configuration Designs of the Screen-Printed Electrodes
3. Screen-Printed Electrodes to detect Pathogens
3.1. Detection and Quantification of Food-Borne Pathogens Using Screen-Printed Electrodes
3.2. Detection and Quantification of Infectious Diseases Etiological Agents Using Screen-Printed Electrodes
4. Illicit Drug Detection Using Screen-Printed Electrodes
5. Screen-Printed Electrodes for Early Cancer Diagnostic
5.1. Aptasensors for Cancer Detection
5.2. Immunosensors for Cancer Detection
5.3. Aptasensors versus Immunosensors for Cancer Detection
6. Metabolic Syndrome Specific Biomarker C-Reactive Protein (CRP) Detection Based on Screen-printed Electrodes
Screen-Printed Electrodes for CRP Detection
7. Evaluation of the Performance of SPE-Based Sensors versus Other Diagnosis Methods
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Taleat, Z.; Khoshroo, A.; Ardakani, M.M. Screen-printed electrodes for biosensing: A review. Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- Du, C.X.; Han, V.; Dong, S.L.; Li, L.H.; Wei, Y. A novel procedure for fabricating flexible screen-printed electrodes with improved electrochemical performance. IOP Conf. Ser. Mater. Sci. Eng. 2016, 137, 1–7. [Google Scholar] [CrossRef]
- Yean, C.Y.; Kamarudin, B.; Ozkan, D.A.; Yin, L.S.; Lalitha, P.; Ismail, A.; Ozsoz, M.; Ravichandran, M. Enzyme-linked amperometric electrochemical genosensor assay for the detection of PCR amplicons on a streptavidin-treated screen-printed carbon electrode. Anal. Chem. 2008, 80, 2774–2779. [Google Scholar] [CrossRef]
- Pereira, S.V.; Bertolino, F.A.; Fernández-Baldo, M.A.; Messina, G.A.; Salinas, E.; Sanz, M.I.; Raba, J. A microfluidic device based on a screen-printed carbon electrode with electrodeposited gold nanoparticles for the detection of IgG anti-Trypanosoma cruzi antibodies. Analyst 2011, 136, 4745–4751. [Google Scholar] [CrossRef]
- Serafín, V.; Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. A novel hybrid platform for the preparation of disposable enzyme biosensors based on poly (3, 4-ethylenedioxythiophene) electrodeposition in an ionic liquid medium onto gold nanoparticles-modified screen-printed electrodes. J. Electroanal. Chem. 2011, 656, 152–158. [Google Scholar] [CrossRef]
- Carapuça, H.M.; Monterroso, S.C.; Rocha, L.S.; Duarte, A.C. Simultaneous determination of copper and lead in seawater using optimised thin-mercury film electrodes in situ plated in thiocyanate media. Talanta 2004, 64, 566–569. [Google Scholar] [CrossRef]
- Del Carlo, M.; Di Marcello, M.; Perugini, M.; Ponzielli, V.; Sergi, M.; Mascini, M.; Compagnone, D. Electrochemical DNA biosensor for polycyclic aromatic hydrocarbon detection. Microchim. Acta 2008, 163, 163–169. [Google Scholar] [CrossRef]
- Lucarelli, F.; Authier, L.; Bagni, G. Graphene–PEDOT: PSS on screen-printed carbon electrode for enzymatic biosensing. Anal. Lett. 2003, 36, 1887–1901. [Google Scholar] [CrossRef]
- Viswanathana, S.; Rani, C.; Ho, J.A. Electrochemical immunosensor for multiplexed detection of food-borne pathogens using nanocrystal bioconjugates and MWCNT screen-printed electrode. Talanta 2012, 94, 315–319. [Google Scholar] [CrossRef]
- Salam, F.; Tothill, I.E. Detection of Salmonella typhimurium using an electrochemical immunosensor. Biosens. Bioelectron. 2009, 24, 2630–2636. [Google Scholar] [CrossRef]
- Fei, J.; Dou, W.; Zhao, G. Amperometric immunoassay for the detection of Salmonella pullorum using a screen-printed carbon electrode modified with gold nanoparticle-coated reduced graphene oxide and immunomagnetic beads. Microchim. Acta 2016, 183, 757–764. [Google Scholar] [CrossRef]
- Escamilla-Gómez, V.; Campuzano, S.; Pedrero, M.; Pingarrón, J.M. Gold screen-printed-based impedimetric immunobiosensors for direct and sensitive Escherichia coli quantisation. Biosens. Bioelectron. 2009, 24, 3365–3371. [Google Scholar] [CrossRef] [PubMed]
- Garcíaa, T.; Revenga-Parraa, M.; Anorgac, L.; Aranac, S.; Parientea, F.; Lorenzoa, E. Disposable DNA biosensor based on thin-film gold electrodes for selective Salmonella detection. Sens. Actuators B 2012, 161, 1030–1037. [Google Scholar] [CrossRef]
- Dou, W.; Tang, W.; Zhao, G. A disposable electrochemical immunosensor arrays using 4-channel screen-printed carbon electrode for simultaneous detection of Escherichia coli O157: H7 and Enterobacter sakazakii. Electrochim. Acta 2013, 97, 79–85. [Google Scholar] [CrossRef]
- Davis, D.; Guo, X.; Musavi, L.; Lin, C.S.; Chen, S.H.; Wu, V.C.H. Gold nanoparticle-modified carbon electrode biosensor for the detection of listeria monocytogenes. Ind. Biotechnol. 2013, 9, 31–36. [Google Scholar] [CrossRef]
- Loaiza, O.A.; Campuzano, S.; Pedrero, M.; Pingaron, J.M. Designs of enterobacteriaceae Lac Z gene DNA gold screen-printed biosensors. Electroanalysis 2008, 20, 1397–1405. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Q.; Huo, H.; Bai, S.; Cai, G.; Lai, W.; Lin, J. Efficient separation and quantitative detection of Listeria monocytogenes based on screen-printed interdigitated electrode, urease and magnetic nanoparticles. Food Control. 2017, 73, 555–561. [Google Scholar] [CrossRef]
- Escamilla-Gómez, V.; Campuzano, S.; Pedrero, M.; Pingarrón, J.M. Electrochemical immunosensor designs for the determination of Staphylococcus aureus using 3, 3-dithiodipropionic acid di(N-succinimidyl ester)-modified gold electrodes. Talanta 2008, 77, 876–881. [Google Scholar] [CrossRef]
- Ward, A.C.; Hannah, A.J.; Kendrick, S.L.; Tucker, N.P.; MacGregor, G.; Connolly, P. Identification and characterisation of Staphylococcus aureus on low-cost screen-printed carbon electrodes using impedance spectroscopy. Biosens. Bioelectron. 2018, 110, 65–70. [Google Scholar] [CrossRef]
- Zhao, G.; Xing, F.; Deng, S. A disposable amperometric enzyme immunosensor for rapid detection of Vibrio parahaemolyticus in food based on agarose/Nano-Au membrane and screen-printed electrode. Electrochem. Commun. 2007, 9, 1263–1268. [Google Scholar] [CrossRef]
- Joint United Nations, Programme on HIV/AIDS, UNAIDS DATA. 2018. Available online: https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf (accessed on 23 July 2020).
- Tran, L.D.; Nguyen, B.H.; Hieu, N.V.; Tran, H.V.; Nguyen, H.L.; Nguyen, P.X. Electrochemical detection of short HIV sequences on chitosan/Fe3O4 nanoparticle-based screen-printed electrodes. Mater. Sci. Eng. C 2011, 31, 477–485. [Google Scholar] [CrossRef]
- Adam, V.; Huska, D.; Hubalek, J.; Kizek, R. Easy to use and rapid isolation and detection of a viral nucleic acid by using paramagnetic microparticles and carbon nanotubes-based screen-printed electrodes. Microfluid. Nanofluid. 2010, 8, 329–339. [Google Scholar] [CrossRef]
- El Goumi, Y. Electrochemical genosensors: Definition and fields of application. Int. J. Biosens. Bioelectron. 2017, 3, 353–355. [Google Scholar] [CrossRef][Green Version]
- Martinez-Paredes, G.; Gonzalez-Garcia, B.M.; Costa-Garcia, A. Genosensor for SARS virus detection based on gold nanostructured screen-printed carbon electrodes. Electroanalysis 2009, 21, 379–385. [Google Scholar] [CrossRef]
- World Health Organization, Disease Information, SARS (Severe Acute Respiratory Syndrome). Available online: https://www.who.int/ith/diseases/sars/en/ (accessed on 23 July 2020).
- Wang, J.; Cai, X.; Rivas, G.; Shiraishi, H.; Farias, P.A.M.; Dontha, N. DNA electrochemical biosensor for the detection of short DNA sequences related to the human immunodeficiency virus. Anal. Chem. 1996, 68, 2629–2634. [Google Scholar] [CrossRef]
- Escosura-Muniz, A.; Maltez-da Costa, M.; Sánchez-Espinel, C.; Díaz-Freitas, B.; Fernández-Suarez, J.; González-Fernández, A.; Merkoçi, A. Gold nanoparticle-based electrochemical magnetoimmunosensor for rapid detection of anti-hepatitis B virus antibodies in human serum. Biosens. Bioelectron. 2010, 26, 1710–1714. [Google Scholar] [CrossRef]
- Valipour, A.; Roushani, M. TiO2 nanoparticles doped with celestine blue as a label in a sandwich immunoassay for the hepatitis C virus core antigen using a screen-printed electrode. Microchim. Acta 2017, 184, 2015–2022. [Google Scholar] [CrossRef]
- Silva, M.M.S.; Dias, A.C.M.S.; Cordeiro, M.T.; Marques, E.; Goulart, M.O.F.; Dutra, R.F. A thiophene-modified screen-printed electrode for detection of dengue virus NS1 protein. Talanta 2014, 128, 505–510. [Google Scholar] [CrossRef]
- Faruque, S.M.; Albert, M.J.; Mekalanos, J.J. Epidemiology, genetics, and ecology of toxigenic Vibrio cholera. Microbiol. Mol. Biol. 1998, 62, 1301–1314. [Google Scholar] [CrossRef]
- Rao, V.K.; Sharma, M.K.; Goel, A.K.; Singh, L.; Sekhar, K. Amperometric immunosensor for the detection of vibrio cholerae O1 using disposable screen-printed electrodes. Anal. Sci. 2006, 22, 1207–1211. [Google Scholar] [CrossRef]
- Mabey, D.; Peeling, R.W.; Ustianowski, A.; Perkins, M.D. Diagnostics for the developing world. Nat. Rev. Microbiol. 2004, 2, 231–240. [Google Scholar] [CrossRef]
- Grewal, Y.S.; Shiddiky, M.J.A.; Spadafora, L.J.; Cangelosi, G.A.; Trau, M. Nano-yeast–scFv probeson screen-printed gold electrodes for detection of Entamoeba histolytica antigens in a biological matrix. Biosens. Bioelectron. 2014, 55, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Rushworth, J.V.; Wright, J.D.; Millner, P.A. Novel impedimetric immunosensor for detection of Pathogenic Bacteria Streptococcus pyogenes in human saliva. Anal. Chem. 2013, 85, 12118–12125. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime (UNODC), World Drug Report 2019: 35 Million People Worldwide Suffer from Drug Use Disorders While Only 1 in 7 People Receive Treatment. 2019. Available online: https://wdr.unodc.org/wdr2019/ (accessed on 23 July 2020).
- Helzer, J.E.; Robins, L.N.; McEvoy, L.T.; Spitznagel, E.A. Comparison of clinical and diagnostic interview schedule diagnoses. Arch. Gen. Psychiatry 1985, 42, 657–666. [Google Scholar] [CrossRef]
- Koob, G.F.; Ahmed, S.H.; Boutrel, B.; Chen, S.A.; Kenny, P.J.; Markou, A.; O’Dell, L.E.; Parsons, L.H.; Sanna, P.P. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci. Biobehav. R. 2004, 27, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.; Pacheco, J.G.; Rebelo, P.; Delerue-Matos, C. Molecularly imprinted electrochemical sensor prepared on a screen-printed carbon electrode for naloxone detection. Sens. Actuators B 2017, 243, 745–752. [Google Scholar] [CrossRef]
- Beitollahi, H.; Mohammadi, S.Z.; Tajik, S. Electrochemical behavior of Morphine at the surface of magnetic core shell manganese Ferrite nanoparticles modified screen-printed electrode and its determination in real samples. Int. J. Nano Dimens. 2019, 10, 304–312. [Google Scholar]
- Wanklyn, C.; Burton, D.; Enston, E.; Bartlett, C.A.; Taylor, S.; Raniczkowska, A.; Black, M.; Murphy, L. Disposable screen-printed sensor for the electrochemical detection of delta-9-tetrahydrocannabinol in undiluted saliva. Chem. Cent. J. 2016, 10, 1. [Google Scholar] [CrossRef]
- Bartlett, C.A.; Taylor, S.; Fernandez, C.; Wanklyn, C.; Burton, D.; Enston, E.; Raniczkowska, A.; Black, M.; Murphy, L. Disposable screen-printed sensor for the electrochemical detection of methamphetamine in undiluted saliva. Chem. Cent. J. 2016, 10, 3. [Google Scholar] [CrossRef]
- Butler, D.; Pravda, M.; Guilbault, G.G. Development of a disposable amperometric immunosensor for the detection of ecstasy and its analogues using screen-printed electrodes. Anal. Chim. Acta 2006, 556, 333–339. [Google Scholar] [CrossRef]
- Zang, D.; Yan, M.; Ge, S.; Ge, L.; Yu, J. A disposable simultaneous electrochemical sensor array based on a molecularly imprinted film at a NH2-graphene modified screen-printed electrode for determination of psychotropic drugs. Analyst 2013, 138, 2704–2711. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.; Cowen, T.; Piletskyb, S.; De Wael, K. Electrochemical sensing of cocaine in real samples based on electrodeposited biomimetic affinity ligands. Analyst 2019, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kwak, J.; Park, J. Nanotechnology for early cancer detection. Sensors 2010, 10, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Rauf, S.; Catanante, G.; Nawaz, M.; Nunes, G.; Marty, J.; Hayat, A. One step assembly of thin films of carbon nanotubes on screen-printed interface for electrochemical aptasensing of breast cancer biomarker. Sensors 2016, 16, 1651. [Google Scholar] [CrossRef]
- Florea, A.; Taleat, Z.; Cristea, C.; Mazloum-Ardakani, M.; Săndulescu, R. Label free MUC1 aptasensors based on electrodeposition of gold nanoparticles on screen-printed electrodes. Electrochem. Commun. 2013, 33, 127–130. [Google Scholar] [CrossRef]
- Piro, B.; Reisberg, S. Recent advances in electrochemical immunosensors. Sensors 2017, 17, 794. [Google Scholar] [CrossRef]
- de Oliveira, R.; Materon, E.; Melendez, M.; Carvalho, A.; Faria, R. Disposable microfluidic immunoarray device for sensitive breast cancer biomarker detection. ACS Appl. Mater. Interfaces 2017, 9, 27433–27440. [Google Scholar] [CrossRef]
- Zhao, L.; Han, H.; Ma, Z. Improved screen-printed carbon electrode for multiplexed label-free amperometric immuniosensor: Addressing its conductivity and reproducibility challenges. Biosens. Bioelectron. 2018, 101, 304–310. [Google Scholar] [CrossRef]
- Srivastava, M.; Nirala, N.; Srivastava, S.; Prakash, R. A comparative study of aptasensor vs. immunosensor for label-free PSA cancer detection on GQDs-aunrs modified screen-printed electrodes. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Cattafesta, M.; Bissoli, S.N.; Salaroli, L.B. Metabolic syndrome and C-reactive protein in bank employees. Diabetes Metab. Syndr. Obes. 2016, 9, 137–144. [Google Scholar]
- Alsehri, A.M. Metabolic syndrome and cardiovascular risk. J. Fam. Community Med. 2010, 17, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Scholze, J.; Alegria, E.; Ferri, C.; Langham, S.; Stevens, W.; Jeffries, D.; Uhl-Hochgraeber, K. Epidemiological and economic burden of metabolic syndrome and its consequences in patients with hypertension in Germany, Spain and Italy; a prevalence-based model. BMC Public Health 2010, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Prasad, D.; Kabir, Z.; Dash, A.; Das, B. Prevalence and risk factors for diabetes and impaired glucose tolerance in Asian Indians: A community survey from urban Eastern India. Diabetes Metab. Syndr. 2012, 6, 96–101. [Google Scholar] [CrossRef]
- Kamath, D.Y.; Xavier, D.; Sigamani, A.; Pais, P. High sensitivity C-reactive protein (hsCRP) & cardiovascular disease: An Indian perspective. Indian J. Med. Res. 2015, 142, 261–268. [Google Scholar]
- Devaraj, S.; Singh, U.; Jialal, I. Human C-reactive protein and the metabolic syndrome. Curr. Opin. Lipidol. 2009, 20, 182–189. [Google Scholar] [CrossRef]
- Boonkaew, S.; Chaiyo, S.; Jampasa, S.; Rengpipat, S.; Siangproh, W.; Chailapakul, O. An origami paper-based electrochemical immunoassay for the C-reactive protein using a screen-printed carbon electrode modified with graphene and gold nanoparticles. Microchim. Acta 2019, 186, 1–10. [Google Scholar] [CrossRef]
- Kumar, D.; Prasad, B. Multiwalled carbon nanotubes embedded molecularly imprinted polymer-modified screen-printed carbon electrode for the quantitative analysis of C-reactive protein. Sensor. Actuat. B Chem. 2012, 171–172, 1141–1150. [Google Scholar] [CrossRef]
- Kokkinos, C.; Prodromidis, M.; Economou, A.; Petrou, P.; Kakabakos, S. Disposable integrated bismuth citrate-modified screen-printed immunosensor for ultrasensitive quantum dot-based electrochemical assay of C-reactive protein in human serum. Anal. Chim. Acta 2015, 886, 29–36. [Google Scholar] [CrossRef]
- Iha, K.; Inada, M.; Kawada, N.; Nakaishi, K.; Watabe, S.; Tan, Y.H.; Shen, C.; Ke, L.Y.; Yoshimura, T.; Ito, E. Ultrasensitive elisa developed for diagnosis. Diagnostics 2019, 9, 78. [Google Scholar] [CrossRef]
- Woan-Fei, L.J.; Nurul-Syakima, A.M.; Kok-Gan, C.; Learn-Han, L. Rapid methods for the detection of foodborne bacterial pathogens: Principles, applications, advantages and limitation. Front. Microbiol. 2015, 5, 1–19. [Google Scholar]
- Shen, Z.; Hou, N.; Jin, M.; Qiu, Z.; Wang, J.; Zhang, B.; Wang, X.; Wang, J.; Zhou, D.; Li, J. A novel enzyme-linked immunosorbent assay for detection of Escherichia coli O157: H7 using immunomagnetic and beacon gold nanoparticles. Gut Pathog. 2014, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Garcia-D’Angeli, A.; Brennand, J.P.; Huo, Q. Predicting detection limits of enzyme-linked immunosorbent assay (ELISA) and bioanalytical techniques in general. Analyst 2014, 139, 439–445. [Google Scholar] [CrossRef]
- Abdelshafi, N.A.; Bell, J.; Rurack, K.; Schneider, R.J. Microfluidic electrochemical immunosensor for the trace analysis of cocaine in water and body fluids. Drug Test. Anal. 2019, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]



| SPE Type | Activation/Functionalization Patterns | Compounds | Immobilization | Electrochemical Methods | Reference |
|---|---|---|---|---|---|
| Screen-Printed Carbon Electrode | Surface modified using multiwalled carbon nanotubes | Carbon nanotube-polyallylamine | NC 1-antibodies conjugates (metal-based nanocrystals) | Square-wave voltammetry | [9] |
| Sandwich-like immunoassay | Reduced graphene oxide gold nanoparticles silica immunomagnetic beads | Ab1 conjugation to silica beads Ab2 linked to rGO 2/AuNPs 3 | Differential pulse voltammetry | [11] | |
| Surface modified by composite films | Multi-walled carbon nanotubes (MWCNTs)/sodium alginate (SA)/carboxymethyl chitosan (CMC) composite | HRP 4-labeled antibodies immobilized on MWCNTs/SA/CMC complex | Cyclic voltammetry | [14] | |
| Surface modified with gold nanoparticles using glutaraldehyde as cross-linker | Glutaraldehyde Gold nanoparticles 1,1′-ferrocene-dicarboxylic acid (FeDC) (mediator) | Immobilization of Ab on the gold nanoparticles | Cyclic voltammetry and amperometry measurements | [15] | |
| Surface modified with agarose-Nano-Au membrane | Gold nanoparticles Agarose solution | HRP-labeled antibody immobilized on agarose-Nano-Au membrane | Cyclic voltammetry | [20] | |
| Gold modified SPCE 5 for hybridization-based genosensor | Gold nanoparticles Synthetic oligonucleotides | Oligonucleotide probes were fixed on the Au-NP through a thiol group attached to the 3′-end | Cyclic voltammetry | [25] | |
| Sandwich-like immunoassay | Tosyl-activated Magnetic Beads Gold nanoparticles | Magnetic Beads coated with target antigen, followed by binding of Ab1 | Chronoamperometry | [28] | |
| Nanocomposite modified surface | Nafion/TiO2 rhodium nanoparticles | Ab 6 immobilization on rhodium nanoparticles | Differential pulse voltammetry EIS 7 | [29] | |
| Carbon ink and thiophene on a polyethylene terephthalate and AuNP-Ptn A | Thiophene Protein A Gold nanoparticles | Ab immobilization on the gold nanoparticles | Cyclic voltammetry | [30] | |
| Sandwich-like immunoassay using home-made SPE made from silver and carbon ink on a polystyrene substrate | Polystyrene Mesitylene graphite particles | Incubation of home-made immunosensor with capturing antibody for 1 h (physical adsorption) | Cyclic voltammetry | [31] | |
| Surface coated with agarose/Nano-Au membrane and horseradish peroxidase (HRP) labeled antibody (HRP-antibody) | Agarose HRP-antibody Nano-Au | HRP-antibody Agarose-Nano-Au immobilized on polyethylene | Cyclic voltammetry | [32] | |
| Screen-Printed Gold Electrode | Amine coupling of carboxymethyl dextran to the gold surface | Carboxymethyl dextran N-ethyl-N′-(3-dimethylaminopropyl)-carbodiimide (EDC) N-hydroxysuccinimide (NHS) | Binding of the Ab to carboxylic groups of carboxymethyl dextran | Chronoamperometry | [10] |
| 3,3 dithiodipropionic acid di(Nsuccinimidyl ester) (DTSP)-based self-assembled monolayers (SAMs) | DTSP | Ab binding through primary amino groups to ester groups of DTSP Or by Thiolated antibodies | Amperometric measurements | [12] | |
| Genosensor obtained by immobilization of thiolated capture sequence on thin-film gold electrodes | Thiolated capture synthetic oligonucleotides | Thiol-functionalized oligonucleotide probes were bind via gold-sulfur interaction | Differential pulse voltammetry | [13] | |
| Surface functionalization with biotinylated bovine serum albumin (BSA) solution, streptavidin, and nano-Yeast scFv | Biotinylated BSA streptavidin | Ab binding via biotin-streptavidin complex | Differential pulse voltammetry | [34] | |
| Surface modification with polymers-polytyramine (Ptyr) | Polytyramine NeutrAvidin | Ab binding via biotin-NeutrAvidin coupling | Cyclic voltammetry Electrochemical Impedance Spectroscopy | [35] |
| SPE Type | Activation/Functionalization Patterns | Compounds | Immobilization | Electrochemical Methods | Reference |
|---|---|---|---|---|---|
| Screen-Printed Carbon Electrode | Functionalization via molecularly imprinted polymer-4-aminobenzoic acid and MWCNT | 4-aminobenzoic monomers MWCNT Naloxone (template) | Electropolymerization of the polymeric film on the surface of the electrode modified with MWCNT | Differential pulse voltammetry | [39] |
| Surface modification by mediators that once oxidized react with drugs such as MAMP 1 or Δ9-THC 2. | N-(4-amino-3-methoxyphenyl)-methanesulfonamide) N,N′-(1,4-phenylene)-dibenzenesulfonamide | NA | Cyclic voltammetry | [41,42] | |
| Direct immobilization of Ab on SPCE by passive absorbtion | Antibodies HRP marked target sample | Passive absorbtion of Ab under humidity conditions | Amperometric measurements | [43] | |
| Deposition of molecularly imprinted polymer–polypirrole on SPE modified by NH2-graphene | Pyrrole monomer target drug templates (methcathinone and cathinone) | Electropolymerization of the polymeric film on the surface of the electrode modified with NH2-graphene | Cyclic voltammetry Electrochemical impedance spectroscopy | [44] |
| Sensors Type | Activation/Functionalization Patterns | Compounds | Immobilization | Electrochemical Analysis Technics | Reference |
|---|---|---|---|---|---|
| Aptasensors | Covalently functionalized CNTs 1 on screen-printed carbon electrodes in the construction of an electrochemical aptasensor | MWCNTs, orthodichlorobenzen, 4-aminobenzoicacid, acetonitrile | The terminal benzoic acid groups on SPCE surface were activated by immersing the SPCE into a solution of 100 mM N-(3-dimethylaminopropyl)-N0-ethylcarbodiimide hydrochloride (EDC) Aptamer solution was incubated onto the activated SPCE. | Electrochemical impedance spectroscopy | [47] |
| The surface of working electrode was modified with nanoparticle (AuNPs) by electrodeposition from solution. | A solution of HAuCl4 0.6 M in H2SO4 0.5 M Electrodeposition of gold on SPEs, after electrochemical cleaning with 0.5 M H2SO4 by potential scanning within a range of −0.2 to 1.2 V | Electrochemical impedance spectroscopy Differential pulse voltammetry Cyclic voltammetry | [48] | ||
| WE surface was modified by graphene quantum dots and gold nanorod on a thin film of chitosan. | Chitosan Graphite powder H2SO4 HNO3 N-cetyl-N, N, N-Trimethyl Ammonium Bromide (CTAB) HAuCl4 NaBH4 | Aptamer solution was added to the WE and then it was stored overnight in a humid chamber | Cyclic voltammetry Electrochemical impedance spectroscopy Differential pulse voltammetry | [52] | |
| Immunosensors | Microfluidic immunoarray device (Dyμ ID) was based on the use of a double-sided adhesive polystyrene card with the microfluidic channel used for sealing the device and a screen-printed array with eight electrodes as working electrodes, one counter electrode, and one reference electrode The immunoarray was modified using the layer-by-layer technique aiming at immobilizing the primary antibody | The volume of the magnetic particles modified with the polyclonal antibody and horseradish peroxidase (MP 2-Ab2-HRPs) added in the capturing step and incubation and capture time along with the flow rate | Polyclonal antibodies were bound to (MPs) and peroxidase enzymes were used as a strategy for capture, separation, and preconcentration of the biomarker, in addition to amplification of the electroanalytical signal | Cyclic voltammetry Electrochemical impedance spectroscopy | [50] |
| Four different solutions were prepared for the individual functionalization of each WE 3: poly (o-phemylenediamine)-Au/Pd (PoPD-Au/Pd), poly (methylene blue)-Au/Pd (PMB-Au/Pd), poly (N, N′-diphenyl-p-phenylediamine)-Au/Pd (PPPD-Au/Pd) and poly (3, 3′, 5, 5′-tetramethylbenzidine)-Au/Pd (PTMB-Au/Pd). After preparation each solution was mixed with sodium alginate and gold nanoparticles, and then 10 μL of each solution was added to each of the 4 WE | Poly (o-phemylenediamine) Poly (methylene blue) Poly (N, N′-diphenyl-p-phenylediamine) Poly (3, 3′, 5, 5′-tetramethylbenzidine) | Antibody solutions were added on each WE and incubated for 12 h | Square wave voltammetry | [51] | |
| WE surface was modified by graphene quantum dots and gold nanorod on a thin film of chitosan. | Chitosan Graphite powderH2SO4 HNO3 N-cetyl-N, N, N-Trimethyl Ammonium Bromide (CTAB) HAuCl4 NaBH4 | Antibody solution was added to the WE and then it was stored overnight in a humid chamber. | Cyclic voltammetry Electrochemical impedance spectroscopy Differential pulse voltammetry | [52] |
| Electrode Type | Activation/Functionalization Patterns | Compounds | Immobilization | Electrochemical Analysis Technics | Reference |
|---|---|---|---|---|---|
| Carbon Screen-Printed Electrode | First of all, the WE was modified with graphene to enhance sensitivity. Then, gold nanoparticles were electrodeposited on the modified surface and self-assembled monolayer of L-cysteine | L-cysteine Graphene powder Potassium tetrachloroaurate (III) | The specific antibody was covalently immobilized, after the activation of the carboxyl groups with EDC/NHS solution | Electrochemical impedance spectroscopy | [60] |
| Surface modification through MIP technique using 2-Acryl amidoethyldihydrogen phosphate (AEDP) and N-(4-dimethylaminophenyl)-acrylamide (DMAA). The sensitivity of the designed model was enhanced by the addition of multiwalled carbon nanotubes (MWCNTs) | 2-Acryl amidoethyldihydrogen phosphate N-(4-dimethylaminophenyl)-acrylamide MWCNTs | No capture molecule was required | Cyclic voltammetry Differential pulse voltammetry | [61] | |
| Surface modification with Bismuth Citrate for the development of a sandwich-type assay | Graphene Bismuth citrate Bovine serum albumin | Capture antibody was immobilized by physical absorption onto the surface of the WE. The second biotinylated Ab tied to streptavidin-conjugated quantum dots. | Anodic stripping voltammetry (ASV) | [62] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mincu, N.-B.; Lazar, V.; Stan, D.; Mihailescu, C.M.; Iosub, R.; Mateescu, A.L. Screen-Printed Electrodes (SPE) for In Vitro Diagnostic Purpose. Diagnostics 2020, 10, 517. https://doi.org/10.3390/diagnostics10080517
Mincu N-B, Lazar V, Stan D, Mihailescu CM, Iosub R, Mateescu AL. Screen-Printed Electrodes (SPE) for In Vitro Diagnostic Purpose. Diagnostics. 2020; 10(8):517. https://doi.org/10.3390/diagnostics10080517
Chicago/Turabian StyleMincu, Nicolae-Bogdan, Veronica Lazar, Dana Stan, Carmen Marinela Mihailescu, Rodica Iosub, and Andreea Lorena Mateescu. 2020. "Screen-Printed Electrodes (SPE) for In Vitro Diagnostic Purpose" Diagnostics 10, no. 8: 517. https://doi.org/10.3390/diagnostics10080517
APA StyleMincu, N.-B., Lazar, V., Stan, D., Mihailescu, C. M., Iosub, R., & Mateescu, A. L. (2020). Screen-Printed Electrodes (SPE) for In Vitro Diagnostic Purpose. Diagnostics, 10(8), 517. https://doi.org/10.3390/diagnostics10080517






