Peripheral Nerve Imaging Aids in the Diagnosis of Immune-Mediated Neuropathies—A Case Series
Abstract
:1. Introduction
1.1. Case Presentation
1.1.1. Patient 1
1.1.2. Patient 2
2. Materials and Methods
2.1. High-Resolution Nerve Ultrasound
2.2. MR Neurography
3. Results
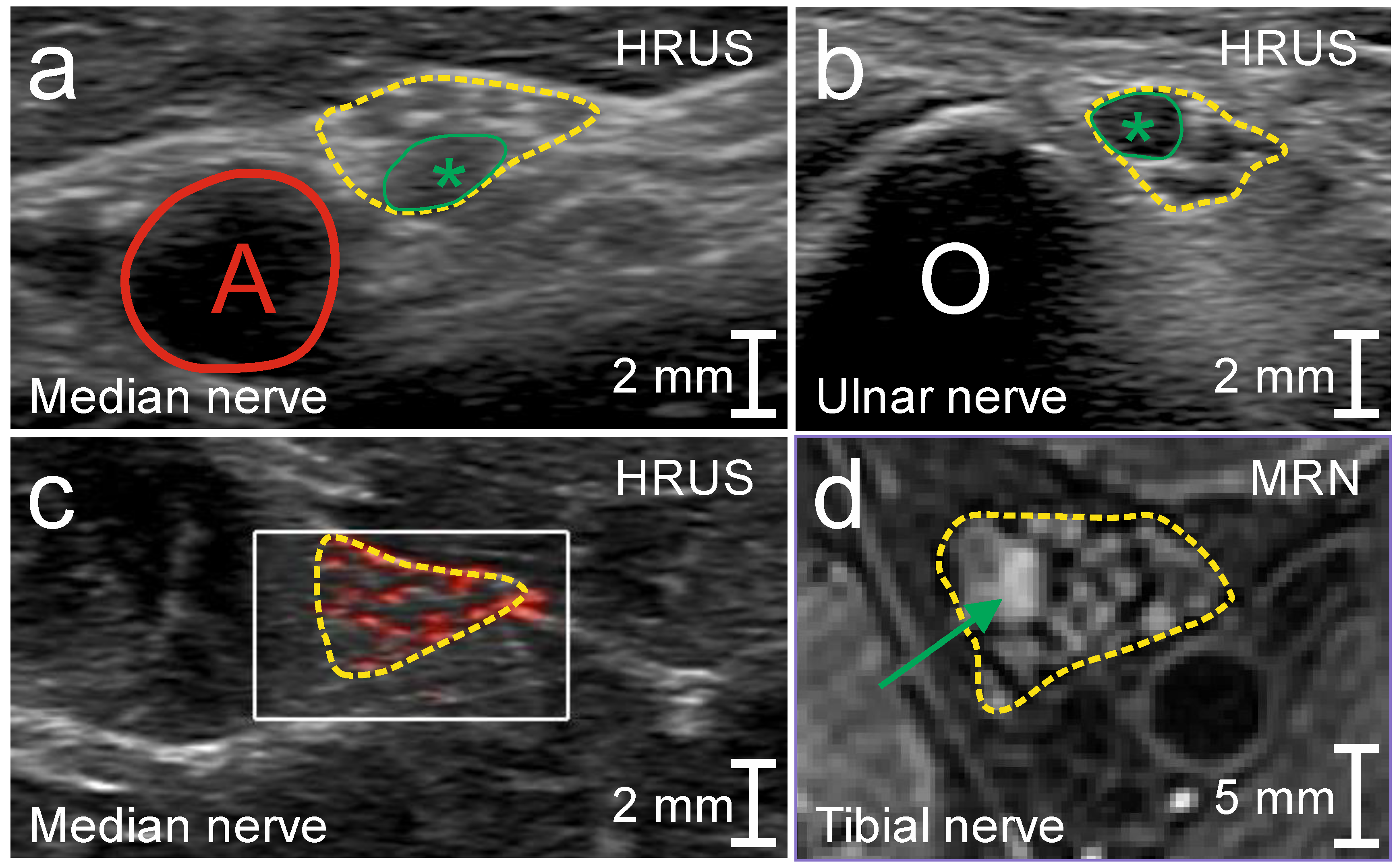
3.1. Patient 1
3.2. Patient 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kieseier, B.C.; Mathey, E.K.; Sommer, C.; Hartung, H.P. Immune-mediated neuropathies. Nat. Rev. Dis. Primers 2018, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Saperstein, D.S.; Katz, J.S.; Amato, A.A.; Barohn, R.J. Clinical Spectrum of Chronic Acquired Demyelinating Polyneuropathies. Muscle Nerve 2001, 24, 311–324. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, A.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic Lateral Sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef]
- Latov, N. Diagnosis and treatment of chronic acquired demyelinating polyneuropathies. Nat. Rev. Neurol. 2014, 10, 435–446. [Google Scholar] [CrossRef]
- De Carvalho, M.; Dengler, R.; Eisen, A.; England, J.D.; Kaji, R.; Kimura, J.; Mills, K.; Mitsumoto, H.; Nodera, H.; Shefner, J.; et al. Electrodiagnostic criteria for diagnosis of ALS. Clin. Neurophysiol. 2008, 119, 497–503. [Google Scholar] [CrossRef]
- De Carvalho, M.; Swash, M. Awaji diagnostic algorithm increases sensitivity of El Escorial criteria for ALS diagnosis. Amyotroph. Lateral Scler. 2009, 10, 53–57. [Google Scholar] [CrossRef]
- Van den Bergh, P.Y.K.; Hadden, R.D.M.; Bouche, P.; Cornblath, D.R.; Hahn, A.; Illa, I.; Koski, C.L.; Léger, J.-M.; Nobile-Orazio, E.; Pollard, J.; et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—First Revision. Eur. J. Neurol. 2010, 17, 356–363. [Google Scholar]
- Van den Bergh, P.Y.K.; Hadden, R.D.M.; Bouche, P.; Cornblath, D.R.; Hahn, A.; Illa, I.; Koski, C.L.; Léger, J.-M.; Nobile-Orazio, E.; Pollard, J.; et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of multifocal motor neuropathy. Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society—First revision. J. Peripher. Nerv. Syst. 2010, 15, 295–301. [Google Scholar]
- Turner, M.R.; Talbot, K. Mimics and chameleons in motor neurone disease. Pract. Neurol. 2013, 13, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, R.D.; Goutman, S.A.; Callaghan, B.C. Pearls & Oy-sters: The importance of atypical features and tracking progression in patients misdiagnosed with ALS. Neurology 2016, 86, e136–e139. [Google Scholar] [PubMed] [Green Version]
- Grimm, A.; Décard, B.F.; Athanasopoulou, I.; Schweikert, K.; Sinnreich, M.; Axer, H. Nerve ultrasound for differentiation between amyotrophic lateral sclerosis and multifocal motor neuropathy. J. Neurol. 2015, 262, 870–880. [Google Scholar] [CrossRef]
- Loewenbrück, K.F.; Liesenberg, J.; Dittrich, M.; Schäfer, J.; Patzner, B.; Trausch, B.; Machetanz, J.; Hermann, A.; Storch, A. Nerve ultrasound in the differentiation of multifocal motor neuropathy (MMN) and amyotrophic lateral sclerosis with predominant lower motor neuron disease (ALS/LMND). J. Neurol. 2016, 263, 35–44. [Google Scholar] [CrossRef]
- Telleman, J.A.; Grimm, A.; Goedee, S.; Visser, L.H.; Zaidman, C.M. Nerve Ultrasound in Polyneuopathies. Muscle Nerve 2018, 57, 716–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, S.; Vielhaber, S.; Schreiber, F.; Cartwright, M.S. Peripheral nerve imaging in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.A.; Pincus, M.R. Henry’s Clinical Diagnosis and Management by Laboratory Methods; 22nd W.B.; Saunders, Inc.: Philadelphia, PA, USA, 2011; pp. 480–509. [Google Scholar]
- Schreiber, S.; Spotorno, N.; Schreiber, F.; Acosta-Cabronero, J.; Kaufmann, J.; Machts, J.; Debska-Vielhaber, G.; Garz, C.; Bittner, D.; Hensiek, N.; et al. Significane of CSF Nfl and tau in ALS. J. Neurol. 2018, 265, 2633–2645. [Google Scholar] [CrossRef] [PubMed]
- Schumann, G.; Klauke, R. New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: Preliminary upper reference limits obtained in hospitalized subjects. Clin. Chim. Acta 2003, 327, 69–79. [Google Scholar] [CrossRef]
- Grimm, A.; Axer, H.; Heiling, B.; Winter, N. Nerve ultrasound normal values—Readjustment of the ultrasound pattern sum score UPSS. Clin. Neurophysiol. 2018, 129, 1403–1409. [Google Scholar] [CrossRef]
- Schreiber, S.; Abdulla, S.; Debska-Vielhaber, G.; Machts, J.; Danhardt-Stieger, V.; Feistner, H.; Oldag, A.; Goertler, M.; Petri, S.; Kollewe, K.; et al. Peripheral Nerve Ultrasound In Amyotrophic Lateral Sclerosis Phenotypes. Muscle Nerve 2015, 51, 669–975. [Google Scholar] [CrossRef]
- Schreiber, S.; Dannhardt-Stieger, V.; Henkel, D.; Debska-Vielhaber, G.; Machts, J.; Abdulla, S.; Kropf, S.; Kollewe, K.; Petri, S.; Heinze, H.-J.; et al. Quantifiying Disease Progression In Amyotrophic Lateral Sclerosis Using Peripheral Nerve Sonography. Muscle Nerve 2016, 54, 391–397. [Google Scholar] [CrossRef]
- Grimm, A.; Winter, N.; Rattay, T.W.; Härtig, F.; Dammeier, N.M.; Auffenberg, E.; Koch, M.; Axer, H. A look inside the nerve—Morphology of nerve fascicles in healthy controls and patients with polyneuropathy. Clin. Neurophysiol. 2017, 128, 2521–2526. [Google Scholar] [CrossRef]
- Karahan, A.Y.; Arslan, S.; Ordahan, B.; Bakdik, S. Superb Microvascular Imaging of the Median Nerve in Carpal Tunnel Syndrome. An Electrodiagnostic and Ultrasonographic Study. J. Ultrasound Med. 2018, 37, 2855–2861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aseem, F.; Williams, J.W.; Walker, F.O.; Cartwright, M.S. Neuromuscular Ultrasound in Patients with Carpal Tunnel Syndrome And Normal Nerve Conduction Studies. Muscle Nerve 2017, 55, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, M.S.; Walker, F.O. Neuromuscular Ultrasound in Common Entrapment Neuropathies. Muscle Nerve 2013, 48, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Gasparotti, R.; Padua, L.; Briani, C.; Lauria, G. New technologies for the assessment of neuropathies. Nat. Rev. Neurol. 2017, 13, 203–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaidman, C.M.; Harms, M.B.; Pestronk, A. Ultrasound of inherited vs. acquired demyelinating polyneuropathies. J. Neurol. 2013, 260, 2580–2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, A.; Vittore, D.; Schubert, V.; Lipski, C.; Heiling, B.; Décard, B.F.; Axer, H. Ultrasound pattern sum score, homogeneity score and regional nerve enlargement index for differentiation of demyelinating inflammatory and hereditary neuropathies. Clin. Neurophysiol. 2016, 127, 2618–2624. [Google Scholar] [CrossRef] [PubMed]
- Décard, B.F.; Pham, M.; Grimm, A. Ultrasound and MRI of nerves for monitoring disease activity and treatment effects in chronic dysimmune neuropathies—Current concepts and future directions. Clin. Neurophysiol. 2018, 129, 155–167. [Google Scholar] [CrossRef]
- Lawson, V.H.; Arnold, W.D. Multifocal motor neuropathy: A review of pathogenesis, diagnosis, and treatment. Neuropsychiatr. Dis. Treat. 2014, 10, 567–576. [Google Scholar]
- Schreiber, S.; Schreiber, F.; Garz, C.; Debska-Vielhaber, G.; Assmann, A.; Perosa, V.; Petri, S.; Dengler, R.; Nestor, P.; Vielhaber, S. Toward In Vivo Determination of Peripheral Nervous System Immune Activity in Amyotrophic Lateral Sclerosis. Muscle Nerve 2019, 59, 567–576. [Google Scholar] [CrossRef]
- De Leeuw, C.; Wijntjes, J.; Lassche, S.; van Alfen, N. Nerve Ultrasound for Distinguishing Inflammatory Neuropathy From Amyotrophic Lateral Sclerosis: Not Black and White. Muscle Nerve 2020, 61, E33–E37. [Google Scholar] [CrossRef]
- Riva, N.; Iannaccone, S.; Corbo, M.; Casellato, C.; Sferrazza, B.; Lazzerini, A.; Scarlato, M.; Cerri, F.; Previtali, S.C.; Nobile-Orazio, E.; et al. Motor Nerve Biopsy: Clinical Usefulness and Histopathological Criteria. Ann. Neruol. 2011, 69, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Goedee, H.S.; van der Pol, W.L.; van Asseldonk, J.-T.H.; Franssen, H.; Notermans, N.C.; Vrancken, A.J.F.E.; van Es, M.A.; Nikolakopoulos, S.; Visser, L.H.; van den Berg, L.H. Diagnostic value of sonography in treatment-naive chronic inflammatory neuropathies. Neurology 2017, 88, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Kronlage, M.; Knop, K.C.; Schwarz, D.; Godel, T.; Heiland, S.; Bendszus, M.; Bäumer, P. Amyotrophic Lateral Sclerosis versus Multifocal Motor Neuropathy: Utility of MR Neurography. Radiology 2019, 292, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rajabally, Y.A.; Jacob, S. Chronic Inflammatory Demyelinating Polyneuropathy-Like Disorder Associated With Amyotrophic Lateral Sclerosis. Muscle Nerve 2008, 38, 855–860. [Google Scholar] [CrossRef]
- Gerevini, S.; Agosta, F.; Riva, N.; Spinelli, E.G.; Pagani, E.; Caliendo, G.; Chaabane, L.; Copetti, M.; Quattrini, A.; Comi, G.; et al. MR Imaging of Brachial Plexus and Limb-Girdle Muscles in Patients with Amyotrophiy Lateral Sclerosis. Radiology 2016, 279, 553–561. [Google Scholar] [CrossRef]
- Mariotto, S.; Farinazzo, A.; Magliozzi, R.; Alberti, D.; Monaco, S.; Ferrari, S. Serum and cerebrospinal neurofilament light chain levels in patients with acquired peripheral neuropathies. J. Peripher. Nerv. Syst. 2018, 23, 174–177. [Google Scholar] [CrossRef]
- Herraets, I.J.T.; Goedee, H.S.; Telleman, J.A.; van Eijk, R.P.A.; van Asseldonk, J.T.; Visser, L.H.; van den Berg, L.H.; van der Pol, W.L. Nerve ultrasound improves detection of treatment-responsive chronic inflammatory neuropathies. Neurology 2020, 94, e1470–e1479. [Google Scholar] [CrossRef]
- Hobson-Webb, L.D.; Grimm, A. Quantifiying neuromuscular ultrasound in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2017, 128, 1030–1031. [Google Scholar] [CrossRef]
- Schreiber, S.; Schreiber, F.; Peter, A.; Isler, E.; Dörner, M.; Heinze, H.-J.; Petri, S.; Tempelmann, C.; Nestor, P.J.; Grimm, A.; et al. 7T MR neurography-ultrasound fusion for peripheral nerve imaging. Muscle Nerve 2020, 4, 521–526. [Google Scholar] [CrossRef]
- Pitarokoili, K.; Gold, R.; Yoon, M.-Y. Nerve Ultrasound in A Case of Multifocal Motor Neuropathy Without Conduction Block. Muscle Nerve 2015, 53, 294–299. [Google Scholar] [CrossRef]
- Rattay, T.W.; Winter, N.; Décard, B.F.; Dammeier, N.M.; Härtig, F.; Ceanga, M.; Axer, H.; Grimm, A. Nerve ultrasound as follow-up tool in treated multifocal motor neuropathy. Eur. J. Neurol. 2017, 24, 1125–1134. [Google Scholar] [CrossRef]
- Staff, N.P.; Amrami, K.K.; Howe, B.M. MRI abnormalities of peripheral nerve and muscle are common in amyotrophic lateral sclerosis and share features with multifocal motor neuropathy. Muscle Nerve 2015, 52, 137–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haakma, W.; Jongbloed, B.A.; Froeling, M.; Goedee, H.S.; Bos, C.; Leemans, A.; van den Berg, L.H.; Hendrikse, J.; van der Pol, W.L. MRI shows thickening and altered diffusion in the median and ulnar nerves in multifocal motor neuropathy. Eur. Radiol. 2017, 27, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- Oudeman, J.; Eftomiv, F.; Strijkers, G.J.; Schneiders, J.J.; Roosendaal, S.D.; Engbersen, M.P.; Froeling, M.; Goedee, H.S.; van Doorn, P.A.; Caan, M.W.A.; et al. Diagnostic accuracy of MRI and ultrasound in chronic immune-mediated neuropathies. Neurology 2020, 94, e62–e74. [Google Scholar] [CrossRef] [PubMed]
- Winter, N.; Dammeier, N.; Schäffer, E.; Bornemann, A.; Stahl, J.-H.; Herlan, S.; Schuhmann, M.U.; Grimm, A. Nerve Ultrasonography as an Additive Tool to Clinical Examination and Electrodiagnostics in Sporadic Mononeuritis—Imaging is the Key. Ultraschall Med. 2019, 40, 465–472. [Google Scholar] [CrossRef]

| Patient | 1 | 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time Point | M 2 | M 13 | M 18 | Y 5 | ||||||||
| Nerve (Stimulation) | DML (ms) | CMAP (mV) | MCV (m/s) | DML (ms) | CMAP (mV) | MCV (m/s) | DML (ms) | CMAP (mV) | MCV (m/s) | DML (ms) | CMAP (mV) | MCV (m/s) |
| Motor | ||||||||||||
| Peroneal (ankle) R | 4.3 (5.0) | 1.0 (2.1) | 6.4 (5.0) | 0.4 (2.1) | 3.9 (5.0) | 4.2 (2.1) | ||||||
| L | 4.7 (5.0) | 2.3 (2.1) | ||||||||||
| (Fibula head) R | 1.0 (2.1) | 36.4 (41) | 0.5 (2.1) | 36 (41) | 2.7 b (2.1) | 44.5 (41) | ||||||
| L | 2.1 (2.1) | 49.3 (41) | ||||||||||
| Tibial (ankle) R | 4.7 (5.1) | 0.6 (2.9) | ||||||||||
| L | 5.2 (5.1) | 2.0 (2.9) | 6.2 (5.1) | 0.1 (2.9) | ||||||||
| (popliteal fossa) R | 0.5 (2.9) | 39.8 (40) | ||||||||||
| L | 2.0 (2.9) | 49.9 (40) | 0.2 (2.9) | 34.7 (40) | ||||||||
| Median (wrist) R | 2.9 (4.5) | 7.9 (2.9) | 2.8 (4.5) | 9.9 (2.9) | 2.9 (4.5) | 9.4 (2.9) | ||||||
| L | 3.1 (4.5) | 6.2 (2.9) | 3.0 (4.5) | 8.7 (2.9) | ||||||||
| (elbow) R | 7.1 (2.9) | 59.1 (47) | 6.4 a (2.9) | 54.3 (47) | 8.7 (2.9) | 56.2 (47) | ||||||
| (axilla) L | 5.5 (2.9) | 56 (47) | 5.0 c (2.9) | 33.1 d (47) | ||||||||
| Ulnar (wrist) R | 2.5 (3.5) | 10.9 (2.5) | 2.7 (3.5) | 11.0 (2.5) | 2.5 (3.5) | 9.3 (2.5) | ||||||
| L | 2.8 (3.5) | 9 (2.5) | 2.6 (3.5) | 7.7 (2.5) | ||||||||
| (elbow) R | 10.8 (2.5) | 67.9 (48) | 10.3 (2.5) | 69.8 (48) | 8.9 (2.5) | 66.5 (48) | ||||||
| L | 6.9 (2.5) | 66.2 (48) | 5.6 (2.5) | 64.4 (48) | ||||||||
| Sensory | SNAP (µV) | SCV (m/s) | SNAP (µV) | SCV (m/s) | SNAP (µV) | SCV (m/s) | SNAP (µV) | SCV (m/s) | ||||
| Sural (ankle) R | 3.7 (3.5) | 45.1 (38) | ||||||||||
| L | 21.8 (3.5) | 48.8 (38) | 6.0 (3.5) | 41.8 (38) | 14.8 (3.5) | 48.5 (38) | ||||||
| Median (dig. II) R | 5.7 (2) | 52.7 (44) | 8.3 (2) | 52.4 (44) | 8.5 (2) | 55.2 (44) | ||||||
| L | 9.4 (2) | 60.7 (44) | 10.8 (2) | 56.8 (44) | 6.3 (2) | 58.3 (44) | 0.9 (2) | 46.6 (44) | ||||
| Ulnar (dig. V) R | 4.8 (2) | 60.6 (43) | 5.2 (2) | 58.1 (43) | ||||||||
| L | 4.8 (2) | 59.9 (43) | 7.4 (2) | 55 (43) | 2.0 (2) | 48.0 (43) |
| Patient | 1 | 2 | |||
|---|---|---|---|---|---|
| Time Point | M 2 | M 8 | M 18 | Y 6 | |
| Median nerve wrist | R | 17 (13 *) | 13 (13 *) | 13 (13 *) | 8 (13 *) |
| L | 13 (13 *) | 16 (13 *) | 14 (13 *) | 11 (13 *) | |
| Median nerve forearm | R | 12 (10) | 11 (10) | 13 (10) | 7 (10) |
| L | 16 (10) | 11 (10) | 12 (10) | 7 (10) | |
| Median nerve elbow | R | 9–11 (12.5) | |||
| L | 17 (12.5) | ||||
| Median nerve upper arm | R | 17 (12) | 14 (12) | 14 (12) | 9 (12) |
| L | 18 (12) | 17 (12) | 18 (12) | 12–32 (12) | |
| Ulnar nerve wrist | R | 7 (8 **) | |||
| L | 9 (8 **) | 7 (8 **) | |||
| Ulnar nerve forearm | R | 11 (8.5) | 9 (8.5) | 12 (8.5) | 4 (8.5) |
| L | 13 (8.5) | 10 (8.5) | 12 (8.5) | 6 (8.5) | |
| Ulnar nerve cubital tunnel | R | 9 (9–10 **) | |||
| L | 13 (9–10 **) | 6 (9–10 **) | |||
| Ulnar nerve upper arm | R | 15 (9.5) | |||
| L | 16 (9.5) | 7 (9.5) | |||
| Tibial nerve distal | R | 21 (14) | 20 (14) | 24 (14) | |
| L | 20 (14) | 23 (14) | 15 (14) | ||
| Tibial nerve proximal | R | 35 (33) | |||
| L | 28 (33) | ||||
| Peroneal nerve proximal | R | 5 (11.5) | |||
| L | 5 (11.5) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dörner, M.; Schreiber, F.; Stephanik, H.; Tempelmann, C.; Winter, N.; Stahl, J.-H.; Wittlinger, J.; Willikens, S.; Kramer, M.; Heinze, H.-J.; et al. Peripheral Nerve Imaging Aids in the Diagnosis of Immune-Mediated Neuropathies—A Case Series. Diagnostics 2020, 10, 535. https://doi.org/10.3390/diagnostics10080535
Dörner M, Schreiber F, Stephanik H, Tempelmann C, Winter N, Stahl J-H, Wittlinger J, Willikens S, Kramer M, Heinze H-J, et al. Peripheral Nerve Imaging Aids in the Diagnosis of Immune-Mediated Neuropathies—A Case Series. Diagnostics. 2020; 10(8):535. https://doi.org/10.3390/diagnostics10080535
Chicago/Turabian StyleDörner, Marc, Frank Schreiber, Heike Stephanik, Claus Tempelmann, Natalie Winter, Jan-Hendrik Stahl, Julia Wittlinger, Sophia Willikens, Magdalena Kramer, Hans-Jochen Heinze, and et al. 2020. "Peripheral Nerve Imaging Aids in the Diagnosis of Immune-Mediated Neuropathies—A Case Series" Diagnostics 10, no. 8: 535. https://doi.org/10.3390/diagnostics10080535





