Performance of a Noninvasive Time-Harmonic Elastography Technique for Liver Fibrosis Evaluation Using Vibration Controlled Transient Elastography as Reference Method
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design and Population
2.2. Clinical Assessment
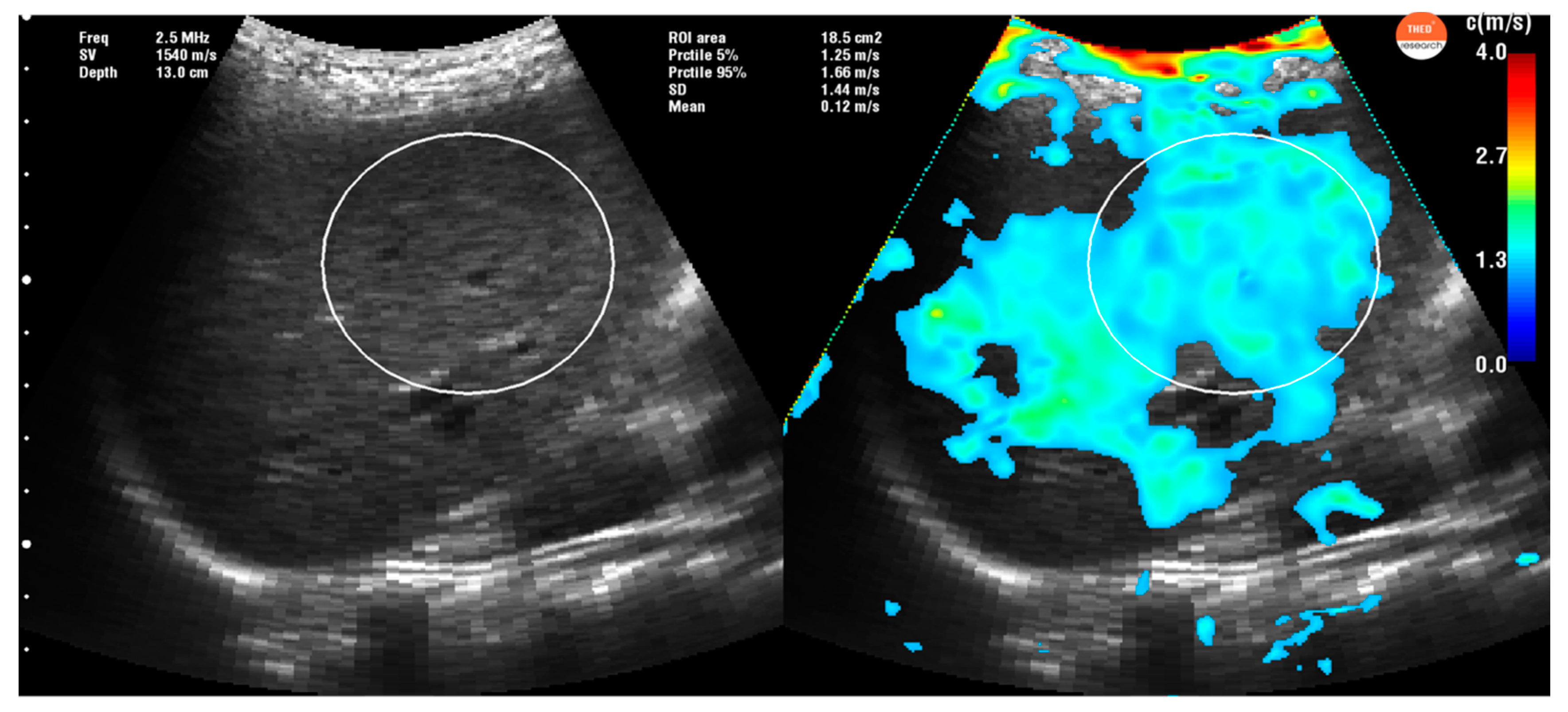
2.3. Time Harmonic Elastography Evaluation
2.4. Vibration-Controlled Transient Elastography (VCTE)
2.5. Inter and Intra-Reproducibility Assessment
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. The Cut-Off Values for Predicting Different Stages ofLiver Fibrosis
3.3. Parameters That Influenced the THE Results.
3.4. Intra and Interobserver Reproducibility of LS Measurements by the THE System
4. Discussions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferraioli, G.; Wong, V.W.S.; Castera, L.; Berzigotti, A.; Sporea, I.; Dietrich, C.F.; Choi, B.I.; Wilson, S.R.; Kudo, M.; Barr, R.G. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med. Biol. 2018, 44, 2419–2440. [Google Scholar] [CrossRef] [Green Version]
- Van Beers, B.E.; Daire, J.L.; Garteiser, P. New imaging techniques for liver diseases. J. Hepatol. 2015, 62, 690–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeff, L.B.; Everson, G.T.; Morgan, T.R.; Curto, T.M.; Lee, W.M.; Ghany, M.G.; Shiffman, M.L.; Fontana, R.J.; Di Bisceglie, A.M.; Bonkovsky, H.L.; et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin. Gastroenterol. Hepatol. 2010, 8, 877–883. [Google Scholar] [CrossRef]
- Sporea, I. Is there a real future for liver elastography? J. Gastrointest. Liver Dis. 2012, 21, 129–131. [Google Scholar]
- Dietrich, C.F.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraiolli, G.; Fridrich-Rust, M.; Gilja, O.H.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017, 38, e16–e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraioli, G.; Filice, C.; Castera, L.; Choi, B.I.; Sporea, I.; Wilson, S.R.; Cosgrove, D.; Dietrich, C.F.; Amy, D.; Bamber, J.C.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: Liver. Ultrasound Med. Biol. 2015, 41, 1161–1179. [Google Scholar] [CrossRef] [Green Version]
- European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 2015, 63, 237–264. [Google Scholar] [CrossRef] [Green Version]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2004, 51, 396–409. [Google Scholar] [CrossRef]
- Tzschätzsch, H.; Ipek-Ugay, S.; Guo, J.; Streitberger, K.J.; Gentz, E.; Fischer, T.; Klaua, R.; Schultz, M.; Braun, J.; Sack, I. In vivo time-harmonic multifrequency elastography of the human liver. Phys. Med. Biol. 2014, 59, 1641–1654. [Google Scholar] [CrossRef] [Green Version]
- Tzschätzsch, H.; Nguyen Trong, M.; Scheuermann, T.; Ipek-Ugay, S.; Fischer, T.; Schultz, M.; Braun, J.; Sack, I. Two-Dimensional Time-Harmonic Elastography of the Human Liver and Spleen. Ultrasound Med. Biol. 2016, 42, 2562–2571. [Google Scholar] [CrossRef]
- De Lédinghen, V.; Wong, V.W.; Vergniol, J.; Wong, G.L.; Foucher, J.; Chu, S.H.; Le Bail, B.; Choi, P.C.; Chermak, F.; Yiu, K.K.; et al. Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: Comparison between M and XL probe of FibroScan®. J. Hepatol. 2012, 56, 833–839. [Google Scholar] [CrossRef]
- Castéra, L.; Foucher, J.; Bernard, P.H.; Carvalho, F.; Allaix, D.; Merrouche, W.; Couzigou, P.; de Lédinghen, V. Pitfalls of liver stiffness measurement: A 5-year prospective study of 13,369examinations. Hepatology 2010, 51, 828–835. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Tzschatzsch, H.; Ipek-Ugay, S.; Nguyen Trong, M.; Fischer, T.; Schultz, M.; Braun, J.; Sack, I. Multifrequency time-harmonic elastography for the measurement of liver viscoelasticity in large tissue windows. Ultrasound Med. Biol. 2015, 41, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Lucidarme, D.; Foucher, J.; Le Bail, B.; Vergniol, J.; Castera, L.; Duburque, C.; Forzy, G.; Filoche, B.; Couzigou, P.; de Lédinghen, V. Factors of accuracy of transient elastography (fibroscan) for the diagnosis of liver fibrosis in chronic hepatitis C. Hepatology 2009, 49, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Zarski, J.P.; de Ledinghen, V.; Rousselet, M.C.; Sturm, N.; Lebail, B.; Fouchard-Hubert, I.; Gallois, Y.; Oberti, F.; Bertrais, S.; et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013, 57, 1182–1191. [Google Scholar] [CrossRef] [Green Version]
- Myers, R.P.; Crotty, P.; Pomier-Layrargues, G.; Ma, M.; Urbanski, S.J.; Elkashab, M. Prevalence, risk factors and causes of discordance in fibrosis staging by transient elastography and liver biopsy. Liver Int. 2010, 30, 1471–1480. [Google Scholar] [CrossRef]
- Tsochatzis, E.A.; Gurusamy, K.S.; Ntaoula, S.; Cholongitas, E.; Davidson, B.R.; Burroughs, A.K. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: A meta-analysis of diagnostic accuracy. J. Hepatol. 2011, 54, 650–659. [Google Scholar] [CrossRef]
- Ipek-Ugay, S.; Tzschätzsch, H.; Hudert, C.; Marticorena Garcia, S.R.; Fischer, T.; Braun, J.; Althoff, C.; Sack, I. Time Harmonic Elastography Reveals Sensitivity of Liver Stiffness to Water Ingestion. Ultrasound Med. Biol. 2016, 42, 1289–1294. [Google Scholar] [CrossRef]
- Arena, U.; Lupsor Platon, M.; Stasi, C.; Moscarella, S.; Assarat, A.; Bedogni, G.; Piazzolla, V.; Badea, R.; Laffi, G.; Marra, F.; et al. Liver stiffness is influenced by a standardized meal in patients with chronic hepatitis C virus at different stages of fibrotic evolution. Hepatology 2013, 58, 65–72. [Google Scholar] [CrossRef]
- Hudert, C.A.; Tzschätzsch, H.; Guo, J.; Rudolph, B.; Bläker, H.; Loddenkemper, C.; Luck, W.; Müller, H.-P.; Baumgart, D.C.; Hamm, B.; et al. US Time-Harmonic Elastography: Detection of Liver Fibrosis in Adolescents with Extreme Obesity with Nonalcoholic Fatty Liver Disease. Radiology 2018, 288, 99–106. [Google Scholar] [CrossRef]
- Bota, S.; Herkner, H.; Sporea, I.; Salzl, P.; Sirli, R.; Neghina, A.M.; Peck-Radosavljevic, M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013, 33, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Friedrich-Rust, M.; Nierhoff, J.; Lupsor, M.; Sporea, I.; Fierbinteanu-Braticevici, C.; Strobel, D.; Takahashi, H.; Yoneda, M.; Suda, T.; Zeuzem, S.; et al. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: A pooled meta-analysis. J. Viral Hepat. 2012, 19, e212–e219. [Google Scholar] [CrossRef] [PubMed]
- Sporea, I.; Bota, S.; Gradinaru-Tascau, O.; Sirli, R.; Popescu, A. Comparative study between two point shear wave elastographic techniques: Acoustic radiation force impulse (ARFI) elastography and ElastPQ. Med. Ultrason. 2014, 16, 309–314. [Google Scholar] [CrossRef]
- Moga, T.V.; Stepan, A.M.; Pienar, C.; Bende, F.; Popescu, A.; Șirli, R.; Dănilă, M.; Sporea, I. Intra- and Inter-Observer Reproducibility of a 2-D Shear Wave Elastography Technique and the Impact of Ultrasound Experience in Achieving Reliable Data. Ultrasound Med. Biol. 2018, 44, 1627–1637. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | n = 165 |
|---|---|
| Age, years (mean ± SD) | 53.5 ± 16.1 |
| Gender, male (%) | 96 (58.1%) |
| BMI, kg/m2 (mean ± SD) | 27.4 ± 5.25 |
| AST, IU/L (median, 25th pct–75th pct) | 31 (6–78) |
| ALT, IU/L (median, 25th pct–75th pct) | 30 (10–190) |
| GGT, IU/L (median, 25th pct–75th pct) | 42.5 (20–800) |
| ALP, IU/L (median, 25th pct–75th pct) | 80 (6.7–315) |
| Platelet count, 103/mm3 (median, 25th pct–75th pct) | 182 (25–574) |
| Fibrosis stage (assessed with VCTE) (%) | |
| (F0-F1) | 82 (49.6%) |
| (F2) | 26 (15.7%) |
| (F3) | 11 (6.6%) |
| (F4) | 46 (28.1%) |
| Etiology of chronic liver diseases (%) | |
| Hepatitis B virus (HBV) | 35 (21.2%) |
| Hepatitis C virus (HCV) | 46 (27.9%) |
| Non-alcoholic fatty liver disease (NAFLD) | 31 (18.7%) |
| Alcohol liver disease | 25 (15.2%) |
| Healthy subjects | 28 (17%) |
| Fibrosis Stage (n = 137) | VCTE, kPa | THE, kPa | p-Value |
|---|---|---|---|
| F0-F1 (n = 54) | 5.08 ± 1.01 | 7.05 ± 0.74 | <0.0001 |
| F2-F3 (n = 37) | 8.89 ± 1.13 | 8.21 ± 1.07 | 0.006 |
| F4 (n = 46) | 26.57 ± 13.50 | 11.81 ± 3.02 | <0.0001 |
| F0-1 vs. F2-4 | F0-3 vs. F4 | ||
|---|---|---|---|
| AUROC (95% CI) | 0.88 (0.65–0.94) | 0.90 (0.82–0.93) | |
| p-value | <0.0001 | <0.0001 | |
| Youden criteria | Cut-off | 1.59 m/s (7.58 kPa) | 1.75 m/s (9.18 kPa) |
| Se (95% CI) | 81.8% (71.4–89.7%) | 79% (64–90%) | |
| Sp (95% CI) | 77.9% (65.3–87.7%) | 91.4% (83.9–96.2%) | |
| PPV (95% CI) | 82.9% (72.5–89.7%) | 81% (65.9–91.4%) | |
| NPV (95% CI) | 76.7% (64–86.6%) | 90.4% (82.6–95.5%) | |
| LR+ (95% CI) | 3.71 (2.3–6.1) | 9.19 (4.7–18.1) | |
| LR− (95% CI) | 0.23 (0.1–0.4) | 0.23 (0.1–0.4) | |
| Rule out Se > 90%, NPV > 90% | Cut-off | 1.49 m/s (6.66 kPa) | 1.61 m/s (7.77 kPa) |
| Se (95% CI) | 97.4% (90.9–99.7%) | 90.7% (77.9–97.4%) | |
| Sp (95% CI) | 32.2% (20.6–45.6%) | 66.6% (56.3–76.1%) | |
| PPV (95% CI) | 65.8% (55.8–73.9%) | 55.7% (43.3–67.6%) | |
| NPV (95% CI) | 90.0% (69.6–98.9%) | 93.9% (85.2–98.3%) | |
| LR+ (95% CI) | 1.44 (1.2–1.7) | 2.72 (2–3.7) | |
| LR− (95% CI) | 0.08 (0.02–0.3) | 0.17 (0.07–0.4) | |
| Rule in Sp > 90%, PPV > 90% | Cut-off | 1.65 m/s (8.16 kPa) | 1.83 m/s (10.04 kPa) |
| Se (95% CI) | 67.5% (55.9–77.8%) | 65.1% (49.1–79%) | |
| Sp (95% CI) | 91.5% (81.3–97.2%) | 96.7% (90.9–99.3%) | |
| PPV (95% CI) | 91.2% (80.7–97.1%) | 90.3% (74.2–98%) | |
| NPV (95% CI) | 68.4% (57.4–78.7%) | 85.7% (77.5–91.8%) | |
| LR+ (95% CI) | 7.97 (3.4–18.7) | 20.1 (6.5–62.8) | |
| LR− (95% CI) | 0.35 (0.3–0.5) | 0.36 (0.2–0.5) |
| Parameter | Univariate Analysis p-Value | Multivariate Analysis p-Value |
|---|---|---|
| AST | 0.0004 | 0.18 |
| ALT | 0.64 | NA |
| BMI | 0.88 | NA |
| AGE > 60 years | <0.0001 | 0.1 |
| ALP | 0.04 | 0.27 |
| GGT | 0.002 | 0.56 |
| Female gender | <0.0001 | 0.51 |
| Platelets | 0.54 | NA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moga, T.V.; Sporea, I.; Lupușoru, R.; Popescu, A.; Popa, A.; Bota, S.; Șirli, R.; Danilă, M.; Schlesinger, A.; Tzschätzsch, H. Performance of a Noninvasive Time-Harmonic Elastography Technique for Liver Fibrosis Evaluation Using Vibration Controlled Transient Elastography as Reference Method. Diagnostics 2020, 10, 653. https://doi.org/10.3390/diagnostics10090653
Moga TV, Sporea I, Lupușoru R, Popescu A, Popa A, Bota S, Șirli R, Danilă M, Schlesinger A, Tzschätzsch H. Performance of a Noninvasive Time-Harmonic Elastography Technique for Liver Fibrosis Evaluation Using Vibration Controlled Transient Elastography as Reference Method. Diagnostics. 2020; 10(9):653. https://doi.org/10.3390/diagnostics10090653
Chicago/Turabian StyleMoga, Tudor Voicu, Ioan Sporea, Raluca Lupușoru, Alina Popescu, Alexandru Popa, Simona Bota, Roxana Șirli, Mirela Danilă, Anton Schlesinger, and Heiko Tzschätzsch. 2020. "Performance of a Noninvasive Time-Harmonic Elastography Technique for Liver Fibrosis Evaluation Using Vibration Controlled Transient Elastography as Reference Method" Diagnostics 10, no. 9: 653. https://doi.org/10.3390/diagnostics10090653









