Detection of Recurrent Cervical Cancer and Prediction of Its Patient Survival with Serum Squamous-Cell Carcinoma-Antigen and 2-[18F] Fluoro-2-Deoxy-d-Glucose-Positron Emission Tomography/Computed Tomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Serum SCC-Ag Measurement
2.3. FDG-PET/CT Imaging
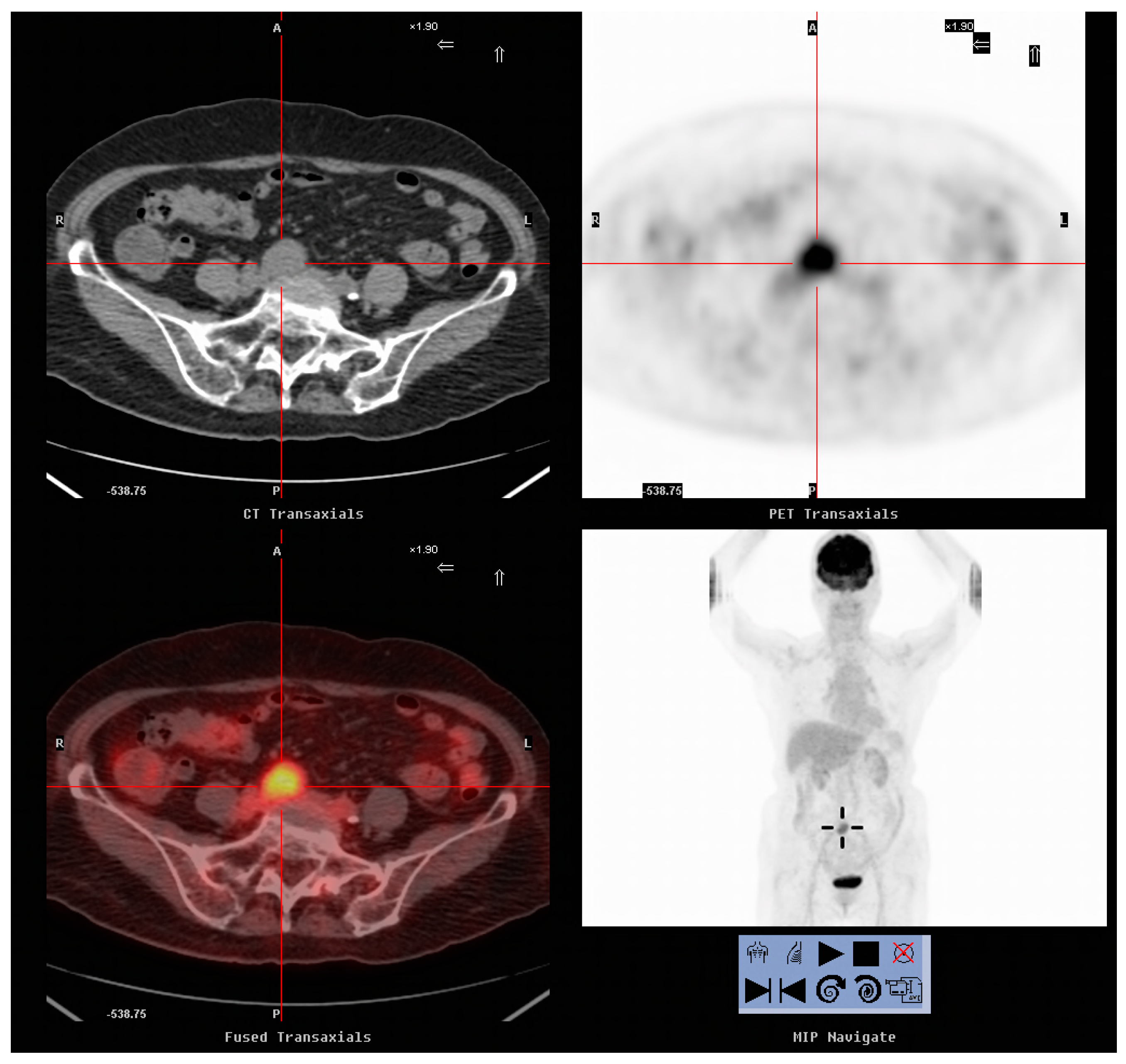
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [Green Version]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [Green Version]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjose, S.; Saraiya, M.; Ferlay, J. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.C.; Hung, Y.C.; Kung, P.T.; Yang, W.H.; Wang, Y.H.; Tsai, W.C. Factors involved in the delay of treatment initiation for cervical cancer patients: A nationwide population-based study. Medicine (Baltimore) 2016, 95, e4568. [Google Scholar] [CrossRef]
- Waggoner, S.E. Cervical cancer. Lancet 2003, 361, 2217–2225. [Google Scholar] [CrossRef]
- Bodurka-Bevers, D.; Morris, M.; Eifel, P.J.; Levenback, C.; Bevers, M.W.; Lucas, K.R.; Wharton, J.T. Posttherapy surveillance of women with cervical cancer: An outcomes analysis. Gynecol. Oncol. 2000, 78, 187–193. [Google Scholar] [CrossRef]
- Sakurai, H.; Suzuki, Y.; Nonaka, T.; Ishikawa, H.; Shioya, M.; Kiyohara, H.; Katoh, H.; Nakayama, Y.; Hasegawa, M.; Nakano, T. Fdg-pet in the detection of recurrence of uterine cervical carcinoma following radiation therapy—Tumor volume and fdg uptake value. Gynecol. Oncol. 2006, 100, 601–607. [Google Scholar] [CrossRef]
- Gadducci, A.; Tana, R.; Cosio, S.; Genazzani, A.R. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: A review of the literature. Crit. Rev. Oncol. Hematol. 2008, 66, 10–20. [Google Scholar] [CrossRef]
- Reesink-Peters, N.; van der Velden, J.; Ten Hoor, K.A.; Boezen, H.M.; de Vries, E.G.; Schilthuis, M.S.; Mourits, M.J.; Nijman, H.W.; Aalders, J.G.; Hollema, H.; et al. Preoperative serum squamous cell carcinoma antigen levels in clinical decision making for patients with early-stage cervical cancer. J. Clin. Oncol. 2005, 23, 1455–1462. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, T.; Du, L.; Xu, X.; Tian, B.; Sun, T.; Han, C.; Zhao, X.; Jing, J. The correlation between the serum squamous carcinoma antigen and the prognosis of recurrent cervical squamous carcinoma. J. Clin. Lab. Anal. 2017, 31, e22020. [Google Scholar] [CrossRef]
- Bolli, J.A.; Doering, D.L.; Bosscher, J.R.; Day, T.G., Jr.; Rao, C.V.; Owens, K.; Kelly, B.; Goldsmith, J. Squamous cell carcinoma antigen: Clinical utility in squamous cell carcinoma of the uterine cervix. Gynecol. Oncol. 1994, 55, 169–173. [Google Scholar] [CrossRef]
- Esajas, M.D.; Duk, J.M.; de Bruijn, H.W.; Aalders, J.G.; Willemse, P.H.; Sluiter, W.; Pras, B.; ten Hoor, K.; Hollema, H.; van der Zee, A.G. Clinical value of routine serum squamous cell carcinoma antigen in follow-up of patients with early-stage cervical cancer. J. Clin. Oncol. 2001, 19, 3960–3966. [Google Scholar] [CrossRef]
- Belhocine, T. An appraisal of 18f-fdg pet imaging in post-therapy surveillance of uterine cancers: Clinical evidence and a research proposal. Int. J. Gynecol. Cancer 2003, 13, 228–233. [Google Scholar] [CrossRef]
- Yen, T.C.; Ng, K.K.; Ma, S.Y.; Chou, H.H.; Tsai, C.S.; Hsueh, S.; Chang, T.C.; Hong, J.H.; See, L.C.; Lin, W.J.; et al. Value of dual-phase 2-fluoro-2-deoxy-d-glucose positron emission tomography in cervical cancer. J. Clin. Oncol. 2003, 21, 3651–3658. [Google Scholar] [CrossRef]
- Grigsby, P.W.; Siegel, B.A.; Dehdashti, F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J. Clin. Oncol. 2001, 19, 3745–3749. [Google Scholar] [CrossRef]
- Kidd, E.A.; Siegel, B.A.; Dehdashti, F.; Grigsby, P.W. The standardized uptake value for f-18 fluorodeoxyglucose is a sensitive predictive biomarker for cervical cancer treatment response and survival. Cancer 2007, 110, 1738–1744. [Google Scholar] [CrossRef]
- Nakamura, K.; Okumura, Y.; Kodama, J.; Hongo, A.; Kanazawa, S.; Hiramatsu, Y. The predictive value of measurement of suvmax and scc-antigen in patients with pretreatment of primary squamous cell carcinoma of cervix. Gynecol. Oncol. 2010, 119, 81–86. [Google Scholar] [CrossRef]
- Yen, T.C.; See, L.C.; Chang, T.C.; Huang, K.G.; Ng, K.K.; Tang, S.G.; Chang, Y.C.; Hsueh, S.; Tsai, C.S.; Hong, J.H.; et al. Defining the priority of using 18f-fdg pet for recurrent cervical cancer. J. Nucl. Med. 2004, 45, 1632–1639. [Google Scholar]
- Havrilesky, L.J.; Wong, T.Z.; Secord, A.A.; Berchuck, A.; Clarke-Pearson, D.L.; Jones, E.L. The role of pet scanning in the detection of recurrent cervical cancer. Gynecol. Oncol. 2003, 90, 186–190. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, P.; Yu, L. The volume-metabolic combined parameters from 18F-FDG PET/CT may help predict the outcomes of cervical carcinoma. Acad. Radiol. 2016, 23, 605–610. [Google Scholar] [CrossRef]
- Han, S.; Kim, H.; Kim, Y.J.; Suh, C.H.; Woo, S. Prognostic value of volume-based metabolic parameters of 18F-FDG PET/CT in uterine cervical cancer: A systematic review and meta-analysis. AJR 2018, 211, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.N.; Rath, G.K.; Kumar, R.; Malhotra, A.; Kumar, S.; Pandjatcharam, J.; Maharjan, S. Positron emission tomography scan for predicting clinical outcome of patients with recurrent cervical carcinoma following readiation therapy. J. Cancer Res. Ther. 2012, 8, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, S.; Sharma, P.; Patel, C.D.; Sharma, D.N.; Dhull, V.S.; Jain, S.K.; Thulkar, S.; Malhotra, A.; Kumar, R. Prospective evaluation of qualitative and quantitative 18F-FDG PET-CT parameters for predicting survival in recurrent carcinoma of cervix. Nucl. Med. Commun. 2013, 34, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Yokoi, K.; Nakagawa, K.; Fujisawa, T.; Nakajima, J.; Akiyama, H.; Nishimura, Y.; Kobayashi, K. Pulmonary metastases from uterine malignancies: Results of surgical resection in 133 patients. J. Thorac. Cardiovasc. Surg. 2004, 127, 1107–1112. [Google Scholar] [CrossRef] [Green Version]
- Ki, E.Y.; Lee, K.H.; Park, J.S.; Hur, S.Y. A clinicopathological review of pulmonary metastasis from uterine cervical cancer. Cancer Res. Treat. 2016, 48, 266–272. [Google Scholar] [CrossRef]
- Oh, J.; Bae, J.Y. Optimal cutoff level of serum squamous cell carcinoma antigen to detect recurrent cervical squamous cell carcinoma during post-treatment surveillance. Obstet. Gynecol. Sci. 2018, 61, 337–343. [Google Scholar] [CrossRef]
- Duk, J.M.; van Voorst Vader, P.C.; ten Hoor, K.A.; Hollema, H.; Doeglas, H.M.; de Bruijn, H.W. Elevated levels of squamous cell carcinoma antigen in patients with a benign disease of the skin. Cancer 1989, 64, 1652–1656. [Google Scholar] [CrossRef]
- Jao, M.S.; Chang, T.C.; Chang, H.P.; Wu, T.I.; Chao, A.; Lai, C.H. Long-term follow up of cervical cancer patients with unexplained squamous cell carcinoma antigen elevation after post-therapy surveillance using positron emission tomography. J. Obstet. Gynaecol. Res. 2010, 36, 1003–1008. [Google Scholar] [CrossRef]
- Sookha, R.R.; Zhi, W.; Shen, Y.; Lazare, C.; Wang, L.; Meng, Y.; Cao, C.; Hu, J.; Wu, P. Clinical value of combining (18)f-fdg pet/ct and routine serum tumor markers in the early detection of recurrence among follow-up patients treated for cervical squamous cell carcinoma. J. Cancer 2018, 9, 3101–3108. [Google Scholar] [CrossRef]
- Salvatici, M.; Achilarre, M.T.; Sandri, M.T.; Boveri, S.; Vanna, Z.; Landoni, F. Squamous cell carcinoma antigen (scc-ag) during follow-up of cervical cancer patients: Role in the early diagnosis of recurrence. Gynecol. Oncol. 2016, 142, 115–119. [Google Scholar] [CrossRef]
- Hong, J.H.; Tsai, C.S.; Lai, C.H.; Chang, T.C.; Wang, C.C.; Chou, H.H.; Lee, S.P.; Hsueh, S. Recurrent squamous cell carcinoma of cervix after definitive radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, H. Prognostic role of squamous cell carcinoma antigen in cervical cancer: A meta-analysis. Dis. Markers 2019, 2019, 6710352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Liu, X.; Hu, K.; Zhang, F. The clinical value of pet and pet/ct in the diagnosis and management of suspected cervical cancer recurrence. Nucl. Med. Commun. 2018, 39, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Ogino, I.; Nakayama, H.; Kitamura, T.; Okamoto, N.; Inoue, T. The curative role of radiotherapy in patients with isolated para-aortic node recurrence from cervical cancer and value of squamous cell carcinoma antigen for early detection. Int. J. Gynecol. Cancer 2005, 15, 630–638. [Google Scholar] [CrossRef]
- Micco, M.; Varga, H.A.; Burger, I.A.; Kollmeier, M.A.; Goldman, D.A.; Park, K.J.; Abu-Rustum, N.R.; Hricak, H.; Sala, E. Combined pre-treatment MRI and 18F-FDG PET/CT parameters as prognostic biomarkers in patients with cervical cancer. Eur. J. Radiol. 2014, 83, 1169–1176. [Google Scholar] [CrossRef]
- Chung, H.H.; Kim, J.W.; Han, K.H.; Eo, J.S.; Kang, K.W.; Park, N.H.; Song, Y.S.; Chung, J.K.; Kang, S.B. Prognostic value of metabolic tumor volume measured by FDG-PET/CT in patients with cervical cancer. Gynecol. Oncol. 2011, 120, 270–274. [Google Scholar] [CrossRef]
- Rahman, T.; Tsujikawa, T.; Yamamoto, M.; Chino, Y.; Shinagawa, A.; Kurokawa, T.; Tsuchida, T.; Kimura, H.; Yoshida, Y.; Okazawa, H. Different prognostic implications of 18F-FDG PET between histological subtypes in patients with cervical cancer. Medicine 2016, 95, e3017. [Google Scholar] [CrossRef]
- Schoder, H.; Gonen, M. Screening for cancer with pet and pet/ct: Potential and limitations. J. Nucl. Med. 2007, 48 (Suppl. 1), 4S–18S. [Google Scholar]
- Chen, Y.K.; Ding, H.J.; Su, C.T.; Shen, Y.Y.; Chen, L.K.; Liao, A.C.; Hung, T.Z.; Hu, F.L.; Kao, C.H. Application of pet and pet/ct imaging for cancer screening. Anticancer Res. 2004, 24, 4103–4108. [Google Scholar]
- Ide, M. Cancer screening with fdg-pet. Q. J. Nucl. Med. Mol. Imaging 2006, 50, 23–27. [Google Scholar]



| Group 1 (n = 62) | Group 2 (n = 26) | p | ||
|---|---|---|---|---|
| Age | 57.9 ± 10.53 | 55.54 ± 8.78 | 0.283 | |
| Stage | FIGO | |||
| CIS | 4 (6.5) | 2 (7.7) | 1.000 | |
| IA | 5 (8.1) | 0 (0) | 0.316 | |
| IB | 18 (29) | 6 (23.1) | 0.567 | |
| IIA | 2 (3.2) | 1 (3.8) | 1.000 | |
| IIB | 10 (16.1) | 4 (15.4) | 1.000 | |
| IIIA | 0 (0) | 1 (3.8) | 0.295 | |
| IIIB | 10 (16.1) | 9 (34.6) | 0.054 | |
| IVA | 2 (3.2) | 0 (0) | 1.000 | |
| IVB | 8 (12.9) | 1 (3.8) | 0.271 | |
| Unknown | 3 (4.8) | 2 (7.7) | 0.630 | |
| Treatment | ||||
| OP | 8 (12.9) | 6 (23.1) | 0.234 | |
| C/T | 4 (6.5) | 0 (0) | 0.315 | |
| R/T | 4 (6.5) | 1 (3.8) | 1.000 | |
| CCRT | 11 (17.7) | 13 (50) | 0.002 | |
| OP + C/T | 6 (9.7) | 0 (0) | 0.174 | |
| OP + R/T | 9 (14.5) | 3 (11.5) | 1.000 | |
| OP + CCRT | 15 (24.2) | 2 (7.7) | 0.084 | |
| OP + C/T + R/T | 5 (8.1) | 1 (3.8) | 0.665 | |
| Group 1 (n = 62) | Group 2 (n = 26) | p | |
|---|---|---|---|
| Time from diagnosis of cancer, months (range) | 61.39 (4–432) | 64.69 (4–360) | 0.894 |
| Mean SCC-Ag level, ng/mL (range) | 10.3 (1.5–64.2) | 0.8 (0.1–1.4) | 0.004 |
| Mean follow-up time, months (range) | 37.74 (1–147) | 53.08 (14–123) | 0.059 |
| Recurrence, N (%) * | 48 (77.4%) | 7 (26.9%) | <0.001 |
| Time to recurrence, months (range) | 50.65 (5–432) | 98.86 (7–360) | 0.517 |
| Histology, N (%) * | 26 (54.2%) | 3 (42.9%) | 0.696 |
| Surgical resection, N (%) * | 13 (27.1%) | 3 (42.9%) | 0.402 |
| Death, N (%) * | 37 (59.7%) | 3 (11.5%) | <0.001 |
| TP | FP | TN | FN | Sensitivity | Specificity | Accuracy | |
|---|---|---|---|---|---|---|---|
| Serum SCC-Ag (n = 88) | 48 | 14 | 19 | 7 | 87.3% | 57.6% * | 76.1% † |
| FDG-PET/CT (n = 88) | 54 | 3 | 30 | 1 | 98.2% | 90.9% * | 95.5% † |
| Group 1 FDG-PET/CT (n = 62) | 47 | 1 | 13 | 1 | 97.9% | 92.9% | 96.8% |
| Group 2 FDG-PET/CT (n = 26) | 7 | 2 | 17 | 0 | 100% | 89.5% | 92.3% |
| Case | Age (Year) | SCC-Ag (ng/mL) | Stage | FDG-PET/CT Findings | SUVmax | Pathology | Follow-Up Time (Month) |
|---|---|---|---|---|---|---|---|
| 1 | 78 | 6.7 | 0 | A 3 cm nodule over right pre-sacral area | 5.2 | SqCC | 147 |
| 2 | 58 | 1.9 | IA | A 2.6 cm pelvic nodule | 7.1 | SqCC | 20 |
| 3 | 68 | 50.3 | IVA | Pelvic masses | 11.2 | SqCC | 10 |
| 4 | 57 | 1.8 | 0 | A 2 cm nodule in vaginal stump | 8.1 | SqCC | 95 |
| 5 | 56 | 3.9 | IVA | A 3.2 cm nodule at posterior pelvic region | 12.7 | SqCC | 37 |
| 6 | 42 | 4.4 | IB | RML nodule | 13.8 | SqCC * | 36 |
| 7 | 48 | 2.8 | IB1 | Bilateral inguinal nodes | 8.4 | SqCC | 65 |
| 8 | 61 | 3.7 | IIA1 | Left para-aortic and inguinal nodes | 6.7 | SqCC | 64 |
| 9 | 56 | 9.5 | 0 | Vaginal cuff and left pelvic nodes | 4.9 | SqCC | 48 |
| 10 | 55 | 2.2 | 0 | A 3.3 cm LUL nodule | 16.9 | SqCC * | 56 |
| 11 | 45 | 2.8 | 0 | A 3.3 cm left pelvic nodule | 9.8 | SqCC | 37 |
| 12 | 56 | 1.8 | IB1 | A 11 cm mass in liver | 22.2 | SqCC * | 29 |
| 13 | 49 | 11.4 | IVB | A 4.1 cm mass in lower vagina to vulva | 11.1 | SqCC | 30 |
| 14 | 56 | 0.7 | II | Uterine cervix | 3.1 | SqCC | 29 |
| 15 | 49 | 0.9 | IB1 | RUL and RLL nodules | 4.2 | SqCC * | 42 |
| 16 | 46 | 0.1 | IB | A 1.3 cm left para-aortic nodule | 5.3 | SqCC | 123 |
| 17 | 69 | 28 | IIA | A 6 cm mass involving lower right kidney and psoas muscle | 16.4 | UCC | 24 |
| 18 | 45 | 1.0 | IIB | A 1.5 cm nodule in cervix uteri | 7.2 | Abscess | 73 |
| Surgical Resection (n = 16) | Non-Surgical Resection (n = 39) | p | |
|---|---|---|---|
| Mean SCC-Ag level, ng/mL (range) | 6.63 (0.7–50.3) | 12 (0.4–70) | 0.208 |
| Mean SUVmax (range) | 9.88 (3.1–22.2) | 11.85 (4.8–60.4) | 0.298 |
| Time to recurrence, months (range) | 71.63 (8–360) | 50.69 (5–432) | 0.416 |
| Mean follow-up time, months (range) | 56.06 (10–147) | 21.18 (1–105) | 0.002 |
| Histology, N (%) * | 16 (100%) | 13 (33.3%) | <0.001 |
| Death, N (%) * | 6 (37.5%) | 32 (82.1%) | 0.001 |
| Time from recurrence to death, months (range) | 28.2 (10–37) | 12.3 (2–32) | 0.015 |
| Madian (Range) | Cutoff | OS (%) | p | DFS (%) | p | |
|---|---|---|---|---|---|---|
| SCC-Ag (ng/mL) | 4.2 (0.1–70) | ≤4.2 | 11 (40.7) | 0.143 | 8 (29.6) | 0.327 |
| >4.2 | 6 (22.2) | 4 (14.8) | ||||
| SUVmax | 10.1 (3.1–60.4) | ≤10.1 | 13 (48.1) | 0.018 | 9 (33.3) | 0.099 |
| >10.1 | 4 (14.8) | 3 (11.1) | ||||
| MTV(cm3) | 30 (1–1493.0) | ≤30 | 14 (51.9) | 0.003 | 11 (40.7) | 0.002 |
| >30 | 3 (11.1) | 1 (3.7) | ||||
| TLG(mlxcm3) | 139.6 (4.6–4196.5) | ≤139.6 | 16 (59.3) | <0.001 | 11 (40.7) | 0.002 |
| >139.6 | 1 (3.7) | 1 (3.7) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, N.-J.; Hu, C.; Chiu, Y.-L.; Yu, C.-C.; Li, C.-J.; Sheu, J.J.-C.; Chiang, A.-J. Detection of Recurrent Cervical Cancer and Prediction of Its Patient Survival with Serum Squamous-Cell Carcinoma-Antigen and 2-[18F] Fluoro-2-Deoxy-d-Glucose-Positron Emission Tomography/Computed Tomography. Diagnostics 2020, 10, 657. https://doi.org/10.3390/diagnostics10090657
Peng N-J, Hu C, Chiu Y-L, Yu C-C, Li C-J, Sheu JJ-C, Chiang A-J. Detection of Recurrent Cervical Cancer and Prediction of Its Patient Survival with Serum Squamous-Cell Carcinoma-Antigen and 2-[18F] Fluoro-2-Deoxy-d-Glucose-Positron Emission Tomography/Computed Tomography. Diagnostics. 2020; 10(9):657. https://doi.org/10.3390/diagnostics10090657
Chicago/Turabian StylePeng, Nan-Jing, Chin Hu, Yu-Li Chiu, Chang-Ching Yu, Chia-Jung Li, Jim Jinn-Chyuan Sheu, and An-Jen Chiang. 2020. "Detection of Recurrent Cervical Cancer and Prediction of Its Patient Survival with Serum Squamous-Cell Carcinoma-Antigen and 2-[18F] Fluoro-2-Deoxy-d-Glucose-Positron Emission Tomography/Computed Tomography" Diagnostics 10, no. 9: 657. https://doi.org/10.3390/diagnostics10090657
APA StylePeng, N.-J., Hu, C., Chiu, Y.-L., Yu, C.-C., Li, C.-J., Sheu, J. J.-C., & Chiang, A.-J. (2020). Detection of Recurrent Cervical Cancer and Prediction of Its Patient Survival with Serum Squamous-Cell Carcinoma-Antigen and 2-[18F] Fluoro-2-Deoxy-d-Glucose-Positron Emission Tomography/Computed Tomography. Diagnostics, 10(9), 657. https://doi.org/10.3390/diagnostics10090657







