Postembolization Syndrome after Prostatic Artery Embolization: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Information Sources and Search Strategy
2.3. Eligibility Criteria and Study Selection
2.4. Outcome Measures
2.5. Risk of Bias
2.6. Statistical Considerations
3. Results
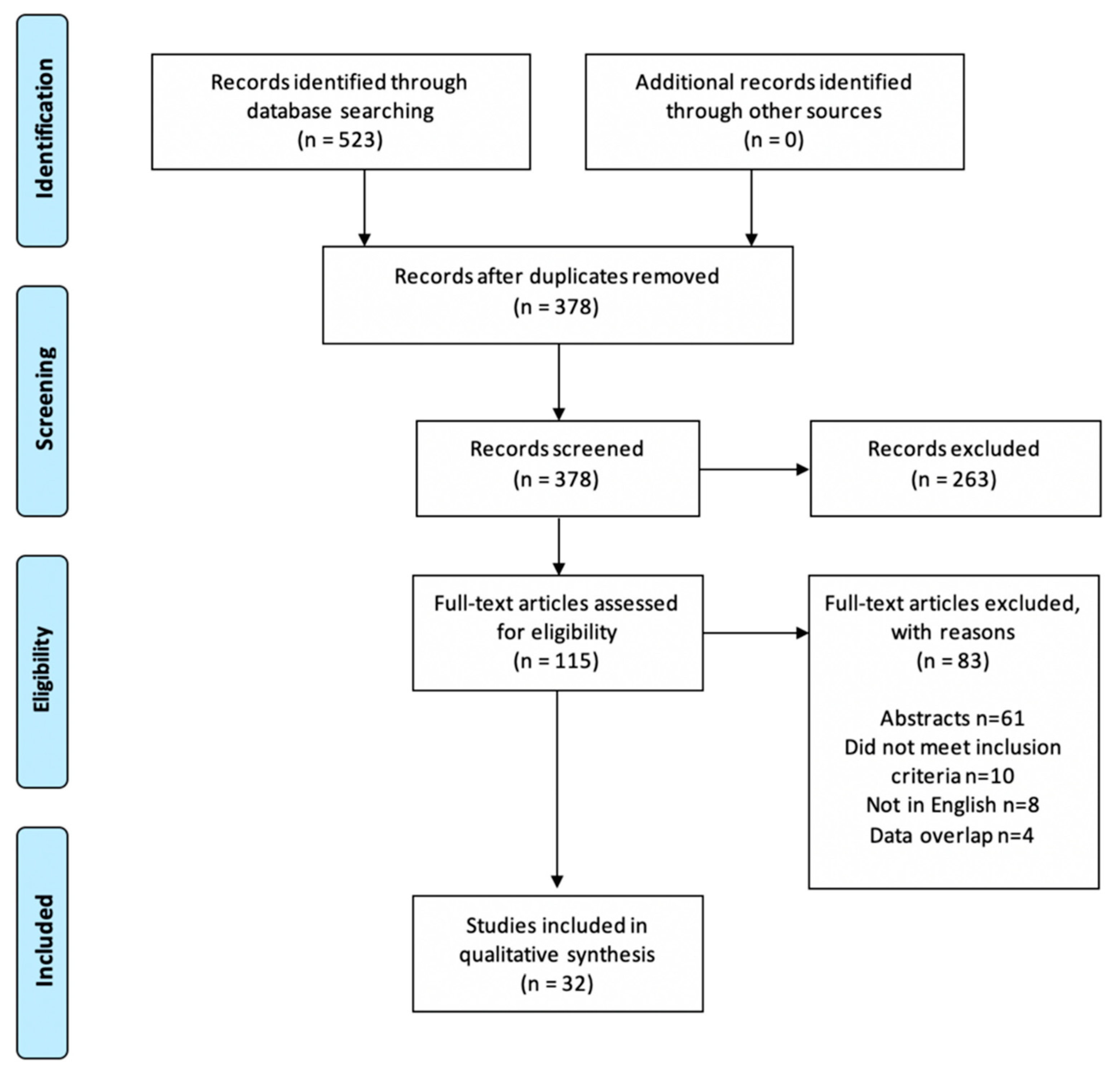
3.1. Study Selection and Overview
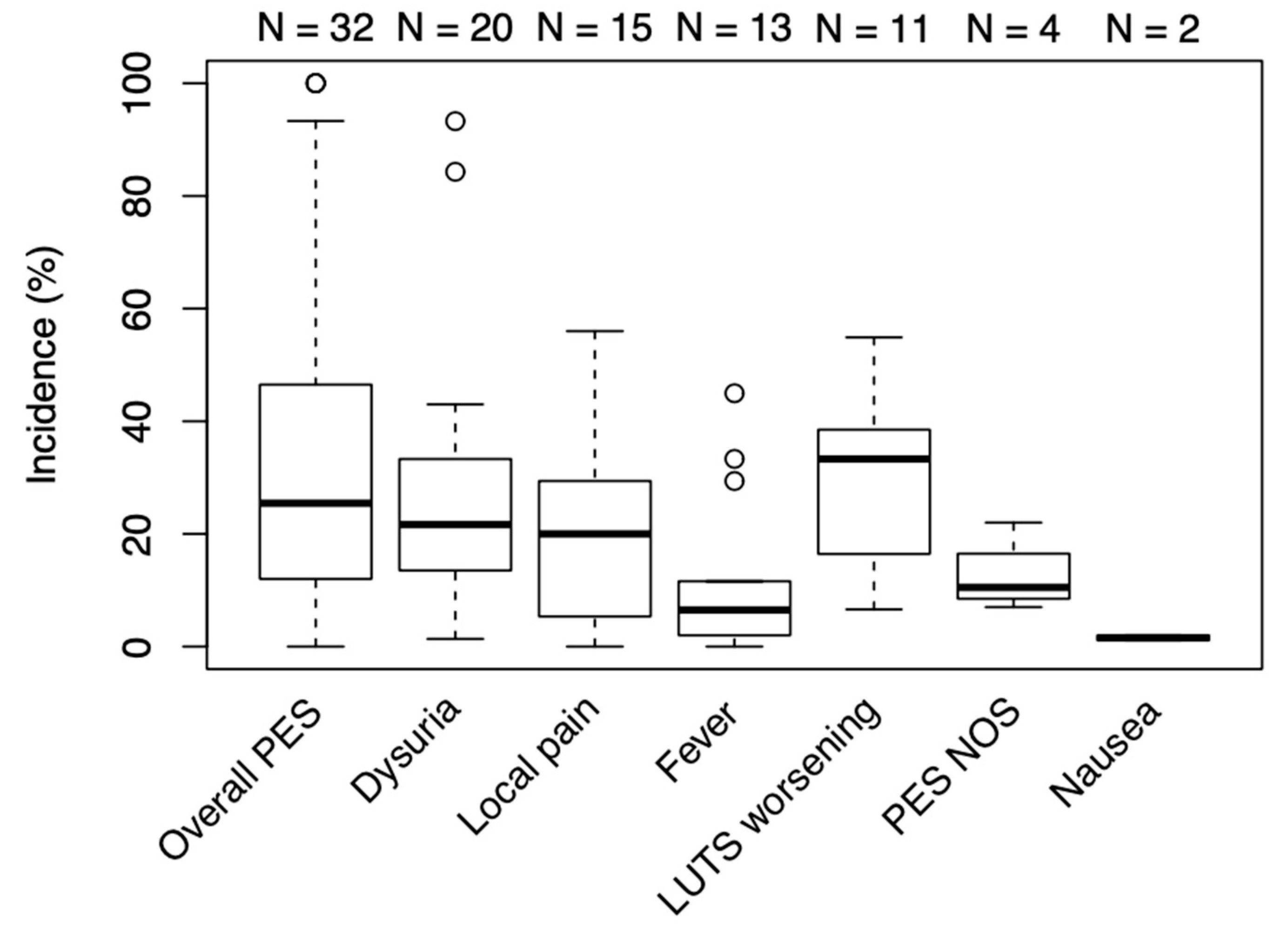
3.2. Outcome Measures
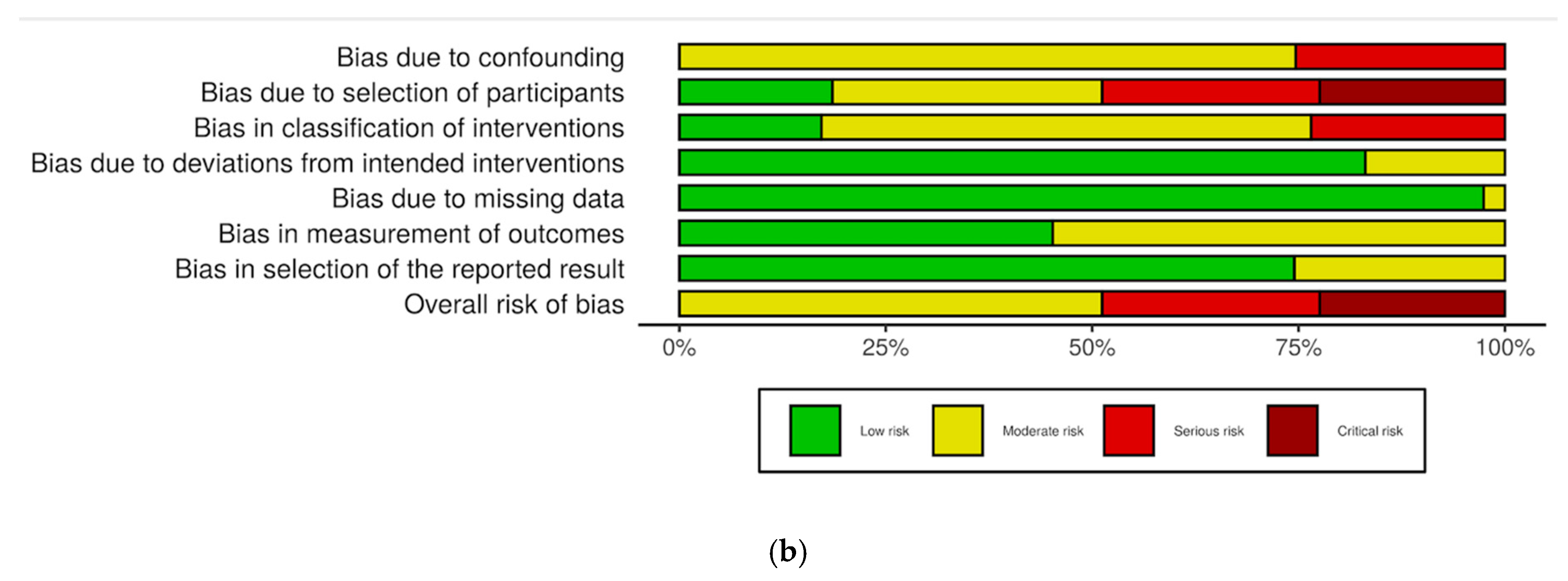
3.3. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Roehrborn, C.G. Benign prostatic hyperplasia: An overview. Rev. Urol. 2005, 7 (Suppl. S9), S3–S14. [Google Scholar] [PubMed]
- Thorpe, A.; Neal, D. Benign prostatic hyperplasia. Lancet 2003, 361, 1359–1367. [Google Scholar] [CrossRef]
- Irwin, D.E.; Kopp, Z.S.; Agatep, B.; Milsom, I.; Abrams, P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction: Worldwide prevalence of luts. BJU Int. 2011, 108, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Malling, B.; Røder, M.A.; Brasso, K.; Forman, J.; Taudorf, M.; Lönn, L. Prostate artery embolisation for benign prostatic hyperplasia: A systematic review and meta-analysis. Eur. Radiol. 2019, 29, 287–298. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, Y.; Zhang, R.; Yang, Y.; Zhang, Q.; Hou, M.; Wang, Y. Benign Prostatic Hyperplasia: Prostatic Arterial Embolization versus Transurethral Resection of the Prostate—A Prospective, Randomized, and Controlled Clinical Trial. Radiology 2014, 270, 920–928. [Google Scholar] [CrossRef]
- Carnevale, F.C.; Iscaife, A.; Yoshinaga, E.M.; Moreira, A.M.; Antunes, A.A.; Srougi, M. Transurethral Resection of the Prostate (TURP) Versus Original and PErFecTED Prostate Artery Embolization (PAE) Due to Benign Prostatic Hyperplasia (BPH): Preliminary Results of a Single Center, Prospective, Urodynamic-Controlled Analysis. Cardiovasc. Interv. Radiol. 2016, 39, 44–52. [Google Scholar] [CrossRef]
- Abt, D.; Hechelhammer, L.; Müllhaupt, G.; Markart, S.; Güsewell, S.; Kessler, T.M.; Schmid, H.-P.; Engeler, D.S.; Mordasini, L. Comparison of prostatic artery embolisation (PAE) versus transurethral resection of the prostate (TURP) for benign prostatic hyperplasia: Randomised, open label, non-inferiority trial. BMJ 2018, 361, k2338. [Google Scholar] [CrossRef]
- Moreira, A.M.; de Assis, A.M.; Carnevale, F.C.; Antunes, A.A.; Srougi, M.; Cerri, G.G. A Review of Adverse Events Related to Prostatic Artery Embolization for Treatment of Bladder Outlet Obstruction Due to BPH. Cardiovasc. Interv. Radiol. 2017, 40, 1490–1500. [Google Scholar] [CrossRef]
- Leung, D.A.; Goin, J.E.; Sickles, C.; Raskay, B.J.; Soulen, M.C. Determinants of postembolization syndrome after hepatic chemoembolization. J. Vasc. Interv. Radiol. 2001, 12, 321–326. [Google Scholar] [CrossRef]
- Bissler, J.J.; Racadio, J.; Donnelly, L.F.; Johnson, N.D. Reduction of postembolization syndrome after ablation of renal angiomyolipoma. Am. J. Kidney Dis. 2002, 39, 966–971. [Google Scholar] [CrossRef]
- Ganguli, S.; Faintuch, S.; Salazar, G.M.; Rabkin, D.J. Postembolization Syndrome: Changes in White Blood Cell Counts Immediately after Uterine Artery Embolization. J. Vasc. Interv. Radiol. 2008, 19, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Svarc Petra Components and Incidence of the Postembolization Syndrome after Prostatic Artery Embolization for Benign Prostatic Hyperplasia: A Systematic Review. Available online: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=164472 (accessed on 1 August 2020).
- Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia. Available online: https://www.covidence.org (accessed on 20 January 2020).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualisation (robvis): An R package and Shiny web app for visualising risk-of-bias assessments. Res. Synth. Methods 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Kurbatov, D.; Russo, G.I.; Lepetukhin, A.; Dubsky, S.; Sitkin, I.; Morgia, G.; Rozhivanov, R.; Cimino, S.; Sansalone, S. Prostatic Artery Embolization for Prostate Volume Greater Than 80 cm3: Results From a Single-center Prospective Study. Urology 2014, 84, 400–404. [Google Scholar] [CrossRef]
- Rampoldi, A.; Barbosa, F.; Secco, S.; Migliorisi, C.; Galfano, A.; Prestini, G.; Harward, S.H.; Di Trapani, D.; Brambillasca, P.M.; Ruggero, V.; et al. Prostatic Artery Embolization as an Alternative to Indwelling Bladder Catheterization to Manage Benign Prostatic Hyperplasia in Poor Surgical Candidates. Cardiovasc. Interv. Radiol. 2017, 40, 530–536. [Google Scholar] [CrossRef]
- Amouyal, G.; Thiounn, N.; Pellerin, O.; Yen-Ting, L.; Del Giudice, C.; Dean, C.; Pereira, H.; Chatellier, G.; Sapoval, M. Clinical Results After Prostatic Artery Embolization Using the PErFecTED Technique: A Single-Center Study. Cardiovasc. Interv. Radiol. 2016, 39, 367–375. [Google Scholar] [CrossRef]
- Malling; Lönn; Jensen; Lindh; Frevert; Brasso; Røder Prostate Artery Embolization for Lower Urinary Tract Symptoms in Men Unfit for Surgery. Diagnostics 2019, 9, 46. [CrossRef]
- Bilhim, T.; Pisco, J.; Campos Pinheiro, L.; Rio Tinto, H.; Fernandes, L.; Pereira, J.A.; Duarte, M.; Oliveira, A.G. Does Polyvinyl Alcohol Particle Size Change the Outcome of Prostatic Arterial Embolization for Benign Prostatic Hyperplasia? Results from a Single-Center Randomized Prospective Study. J. Vasc. Interv. Radiol. 2013, 24, 1595–1602. [Google Scholar] [CrossRef]
- Bilhim, T.; Costa, N.V.; Torres, D.; Pisco, J.; Carmo, S.; Oliveira, A.G. Randomized Clinical Trial of Balloon Occlusion versus Conventional Microcatheter Prostatic Artery Embolization for Benign Prostatic Hyperplasia. J. Vasc. Interv. Radiol. 2019, 30, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.; Costa, N.V.; Pisco, J.; Pinheiro, L.C.; Oliveira, A.G.; Bilhim, T. Prostatic Artery Embolization for Benign Prostatic Hyperplasia: Prospective Randomized Trial of 100–300 μm versus 300–500 μm versus 100- to 300-μm + 300- to 500-μm Embospheres. J. Vasc. Interv. Radiol. 2019, 30, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Q.; Zhang, J.L.; Xin, H.N.; Yuan, K.; Yan, J.; Wang, Y.; Zhang, G.D.; Fu, J.X. Comparison of Clinical Outcomes of Prostatic Artery Embolization with 50-μm Plus 100-μm Polyvinyl Alcohol (PVA) Particles versus 100-μm PVA Particles Alone: A Prospective Randomized Trial. J. Vasc. Interv. Radiol. 2018, 29, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Ayyagari, R.; Powell, T.; Staib, L.; Chapiro, J.; Schoenberger, S.; Devito, R.; Pollak, J. Case-Control Comparison of Conventional End-Hole versus Balloon-Occlusion Microcatheter Prostatic Artery Embolization for Treatment of Symptomatic Benign Prostatic Hyperplasia. J. Vasc. Interv. Radiol. 2019, 30, 1459–1470. [Google Scholar] [CrossRef]
- Bagla, S.; Martin, C.P.; van Breda, A.; Sheridan, M.J.; Sterling, K.M.; Papadouris, D.; Rholl, K.S.; Smirniotopoulos, J.B.; van Breda, A. Early Results from a United States Trial of Prostatic Artery Embolization in the Treatment of Benign Prostatic Hyperplasia. J. Vasc. Interv. Radiol. 2014, 25, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Sinha, V.K.; Harward, S.; Gomez, C.; Kava, B.R.; Parekh, D.J. Prostate Artery Embolization in Patients with Prostate Volumes of 80 mL or More: A Single-Institution Retrospective Experience of 93 Patients. J. Vasc. Interv. Radiol. 2018, 29, 1392–1398. [Google Scholar] [CrossRef]
- Bilhim, T.; Pisco, J.; Rio Tinto, H.; Fernandes, L.; Campos Pinheiro, L.; Duarte, M.; Pereira, J.A.; Oliveira, A.G.; O’Neill, J. Unilateral Versus Bilateral Prostatic Arterial Embolization for Lower Urinary Tract Symptoms in Patients with Prostate Enlargement. Cardiovasc. Interv. Radiol. 2013, 36, 403–411. [Google Scholar] [CrossRef]
- Brown, N.; Walker, D.; McBean, R.; Pokorny, M.; Kua, B.; Gianduzzo, T.; Dunglison, N.; Esler, R.; Yaxley, J. Prostate artery Embolisation Assessment of Safety and feasibilitY (P-EASY): A potential alternative to long-term medical therapy for benign prostate hyperplasia. BJU Int. 2018, 122, 27–34. [Google Scholar] [CrossRef]
- Carnevale, F.C.; da Motta-Leal-Filho, J.M.; Antunes, A.A.; Baroni, R.H.; Marcelino, A.S.Z.; Cerri, L.M.O.; Yoshinaga, E.M.; Cerri, G.G.; Srougi, M. Quality of Life and Clinical Symptom Improvement Support Prostatic Artery Embolization for Patients with Acute Urinary Retention Caused by Benign Prostatic Hyperplasia. J. Vasc. Interv. Radiol. 2013, 24, 535–542. [Google Scholar] [CrossRef]
- Franiel, T.; Aschenbach, R.; Trupp, S.; Lehmann, T.; von Rundstedt, F.-C.; Grimm, M.-O.; Teichgräber, U. Prostatic Artery Embolization with 250-μm Spherical Polyzene-Coated Hydrogel Microspheres for Lower Urinary Tract Symptoms with Follow-up MR Imaging. J. Vasc. Interv. Radiol. 2018, 29, 1127–1137. [Google Scholar] [CrossRef]
- Gonçalves, O.M.; Carnevale, F.C.; Moreira, A.M.; Antunes, A.A.; Rodrigues, V.C.; Srougi, M. Comparative Study Using 100–300 Versus 300–500 μm Microspheres for Symptomatic Patients Due to Enlarged-BPH Prostates. Cardiovasc. Interv. Radiol. 2016, 39, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Kenny, A.G.; Pellerin, O.; Amouyal, G.; Desgranchamps, F.; Méria, P.; De Gouvello, A.; Dariane, C.; Déan, C.; Pereira, H.; Thiounn, N.; et al. Prostate Artery Embolization in Patients With Acute Urinary Retention. Am. J. Med. 2019, 132, e786–e790. [Google Scholar] [CrossRef] [PubMed]
- Kløw, N.E.; Grøtta, O.J.; Bay, D.; Sandbæk, G.; Johansen, T.E.B.; Hagen, T.; Baco, E. Outcome after prostatic artery embolization in patients with symptomatic benign prostatic hyperplasia. Acta Radiol. 2019, 60, 1175–1180. [Google Scholar] [CrossRef]
- Lindgren, H.; Bläckberg, M. Introduction of prostate artery embolization (PAE) in Sweden. Scand. J. Urol. 2019, 53, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Pisco, J.; Bilhim, T.; Pinheiro, L.C.; Fernandes, L.; Pereira, J.; Costa, N.V.; Duarte, M.; Oliveira, A.G. Prostate Embolization as an Alternative to Open Surgery in Patients with Large Prostate and Moderate to Severe Lower Urinary Tract Symptoms. J. Vasc. Interv. Radiol. 2016, 27, 700–708. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhang, C.; Wang, X.; Cheng, K.; Liang, X.; Wang, D.; Hou, S. Clinical evaluation of embolization of the superior vesical prostatic artery for treatment of benign prostatic hyperplasia: A single-center retrospective study. Videosurgery Other Miniinvasive Tech. 2017, 4, 409–416. [Google Scholar] [CrossRef]
- Ray, A.F.; Powell, J.; Speakman, M.J.; Longford, N.T.; DasGupta, R.; Bryant, T.; Modi, S.; Dyer, J.; Harris, M.; Carolan-Rees, G.; et al. Efficacy and safety of prostate artery embolization for benign prostatic hyperplasia: An observational study and propensity-matched comparison with transurethral resection of the prostate (the UK-ROPE study). BJU Int. 2018, 122, 270–282. [Google Scholar] [CrossRef]
- Russo, G.I.; Kurbatov, D.; Sansalone, S.; Lepetukhin, A.; Dubsky, S.; Sitkin, I.; Salamone, C.; Fiorino, L.; Rozhivanov, R.; Cimino, S.; et al. Prostatic Arterial Embolization vs Open Prostatectomy: A 1-Year Matched-pair Analysis of Functional Outcomes and Morbidities. Urology 2015, 86, 343–348. [Google Scholar] [CrossRef]
- Salem, R.; Hairston, J.; Hohlastos, E.; Riaz, A.; Kallini, J.; Gabr, A.; Ali, R.; Jenkins, K.; Karp, J.; Desai, K.; et al. Prostate Artery Embolization for Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: Results From a Prospective FDA-Approved Investigational Device Exemption Study. Urology 2018, 120, 205–210. [Google Scholar] [CrossRef]
- Tian, W.; Zhou, C.; Leng, B.; Shi, H.; Liu, S. Prostatic Artery Embolization for Control of Gross Hematuria in Patients with Benign Prostatic Hyperplasia: A Single-Center Retrospective Study in 20 Patients. J. Vasc. Interv. Radiol. 2019, 30, 661–667. [Google Scholar] [CrossRef]
- Wang, M.; Guo, L.; Duan, F.; Yuan, K.; Zhang, G.; Li, K.; Yan, J.; Wang, Y.; Kang, H. Prostatic arterial embolization for the treatment of lower urinary tract symptoms caused by benign prostatic hyperplasia: A comparative study of medium- and large-volume prostates. BJU Int. 2016, 117, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Q.; Wang, Y.; Yan, J.Y.; Yuan, K.; Zhang, G.D.; Duan, F.; Li, K. Prostatic artery embolization for the treatment of symptomatic benign prostatic hyperplasia in men ≥75 years: A prospective single-center study. World J. Urol. 2016, 34, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.C.H.; Cho, C.C.M.; Hung, E.H.Y.; Chiu, P.K.F.; Yee, C.H.; Ng, C.F. Prostate Artery Embolization for Complete Urinary Outflow Obstruction Due to Benign Prostatic Hypertrophy. Cardiovasc. Interv. Radiol. 2017, 40, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.C.H.; Cho, C.C.M.; Hung, E.H.Y.; Zou, J.; Yuen, B.T.Y.; Shi, L.; Chiu, P.K.F.; Yee, S.C.H.; Ng, A.C.F. Thickness-to-Height Ratio of Intravesical Prostatic Protrusion Predicts the Clinical Outcome and Morbidity of Prostatic Artery Embolization for Benign Prostatic Hyperplasia. J. Vasc. Interv. Radiol. 2019, 30, 1807–1816. [Google Scholar] [CrossRef]
- Marik, P.E.; Taeb, A.M. SIRS, qSOFA and new sepsis definition. J. Thorac. Dis. 2017, 9, 943–945. [Google Scholar] [CrossRef]
- de Assis, A.M.; Moreira, A.M.; de Paula Rodrigues, V.C.; Yoshinaga, E.M.; Antunes, A.A.; Harward, S.H.; Srougi, M.; Carnevale, F.C. Prostatic Artery Embolization for Treatment of Benign Prostatic Hyperplasia in Patients with Prostates > 90 g: A Prospective Single-Center Study. J. Vasc. Interv. Radiol. 2015, 26, 87–93. [Google Scholar] [CrossRef]
- Cochran, S.T.; Barbaric, Z.L.; Lee, J.J.; Kashfian, P. Percutaneous nephrostomy tube placement: An outpatient procedure? Radiology 1991, 179, 843–847. [Google Scholar] [CrossRef] [PubMed]
- de la Motte, L.; Kehlet, H.; Vogt, K.; Nielsen, C.H.; Groenvall, J.B.; Nielsen, H.B.; Andersen, A.; Schroeder, T.V.; Lönn, L. Preoperative Methylprednisolone Enhances Recovery After Endovascular Aortic Repair: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Ann. Surg. 2014, 260, 540–549. [Google Scholar] [CrossRef]
- Ogasawara, S.; Chiba, T.; Ooka, Y.; Kanogawa, N.; Motoyama, T.; Suzuki, E.; Tawada, A.; Nagai, K.; Nakagawa, T.; Sugawara, T.; et al. A randomized placebo-controlled trial of prophylactic dexamethasone for transcatheter arterial chemoembolization. Hepatology 2018, 67, 575–585. [Google Scholar] [CrossRef]
- De Oliveira, G.S.; Almeida, M.D.; Benzon, H.T.; McCarthy, R.J. Perioperative Single Dose Systemic Dexamethasone for Postoperative Pain: A Meta-analysis of Randomized Controlled Trials. Anesthesiology 2011, 115, 575–588. [Google Scholar] [CrossRef]

| Author and (Year) | Study Design | Data Collection Period | Study Location | Patients Included in Intervention Group(s) (n) | Mean Age | Intervention | Control/Comparator |
|---|---|---|---|---|---|---|---|
| Abt (2018) [7] | open-label RCT | Feb 2014–May 2017 | Switzerland | 48 | 65.7 | PAE with 250–400 µm Embozene® | TURP |
| Bilhim (2013) [22] | single-blind RCT | May 2011–Dec 2011 | Portugal | 80 | 63.9 | PAE with 80–180 µm or 180–300 µm particles | |
| Bilhim (2019) [23] | single-blind RCT | Nov 2017–Nov 2018 | Portugal | 84 | 67.3 cPAE; 65.8 bPAE | cPAE, bPAE (both with with 300–500 µm Embosphere®) | |
| Carnevale (2016) [6] | open-label RCT | Nov 2010–Dec 2012 | Brazil | 15 | 60.4 | PAE PErFecTED with 300–500 µm Embosphere® | original PAE and TURP |
| Gao (2014) [5] | open-label RCT | Jan 2007–Jan 2012 | China | 54 | 67.7 | PAE with 355–500 µm Ivalon® | TURP |
| Torres (2019) [24] | open-label RCT | Jul 2015–Dec 2016 | Portugal | 137 | 66.1 | PAE (3 groups: 100–300 µm, 300–500 µm, and 100–300 followed by 300–500 µm microspheres) | |
| Wang (2018) [25] | double-blind RCT | Jan 2010–Oct 2015 | China | 110 | 69.5 | PAE (2 groups: 50 µm followed by 100 µm and 100 µm spheres alone) |
| Author and (Year) | Study Design | Data Collection Period | Study Location | Patients Included in Intervention Group(s) (n) | Mean Age | Intervention | Control/Comparator |
|---|---|---|---|---|---|---|---|
| Bagla (2014) [27] | prospective | Jan 2012–Mar 2013 | United States | 19 | 66.5 | PAE with 100–400 µm Embozene® | |
| Bilhim (2013) [29] | prospective | Mar 2009–Dec 2011 | Portugal | 122 | 65.8 bilateral PAE; 71.3 unilateral PAE | PAE with 100- and 200 µm particle sizes, unilateral vs. bilateral | |
| Brown (2018) [30] | prospective | Nov 2015–Feb 2017 | Australia | 51 | 67 | PAE with 250 µm Embozene® | |
| Carnevale (2013) [31] | prospective | Jun 2008–Nov 2011 | Brazil | 11 | 68.5 | PAE with 300–500 µm Embosphere® | |
| Franiel (2018) [32] | prospective | Jul 2014–Dec 2015 | Germany | 27 | 66 | PAE with 250 µm Embozene® | |
| Goncalves (2016) [33] | prospective | Aug 2011–Jun 2013 | Brazil | 30 | not mentioned | PAE with 100–300 or 300–500 µm Embosphere® | |
| Kenny (2019) [34] | prospective | Not mentioned | France | 20 | 75.3 | PAE with 300–500 µm Bead Block® in patients with indwelling catheters | |
| Kløw (2018) [35] | prospective | Dec 2015–Mar 2017 | Norway | 29 | 69 | PAE with 300–500 µm Embosphere® | |
| Kurbatov (2014) [18] | prospective | Jan 2009–Jan 2012 | Russia and Italy | 88 | 66.4 | PAE with 300–500 µm Embosphere® in prostates >80 cm3 | |
| Lindgren (2019) [36] | prospective | Jan 2015–Jun 2018 | Sweden | 37 | 73 | PAE with 300–500 µm Embosphere® | |
| Malling (2019) [21] | prospective | Jul 2017–Jul 2018 | Denmark | 11 | 75.2 | PAE PErFecTED with 300–500 µm Embosphere® | |
| Rampoldi (2017) [19] | prospective | Not mentioned | Italy | 41 | 77.9 | PAE PErFecTED with 300–500 µm Embosphere® in patients with indwelling catheters | Indwelling urinary catheter |
| Ray (2018) [39] | prospective | Jul 2014–Jan 2016 | United Kingdom | 199 | 66 | PAE | TURP |
| Russo (2015) [40] | prospective matched pair | Jan 2006–Jan 2014 | Italy | 80 | 67 | PAE with 300–500 µm Embosphere® | open prostatectomy |
| Salem (2018) [41] | prospective | Dec 2014–Jun 2017 | United States | 45 | 67 | PAE with 300–500 µm Embosphere® | |
| Wang (2016) [43] | prospective | Apr 2010–Dec 2013 | China | 115 | 72.5 (>80 cm3); 66 (50–80 cm3) | PAE with 100 µm particles in prostates >80 cm3 and 50–80 cm3 | |
| Wang (2016) [44] | prospective | Feb 2009–Apr 2014 | China | 158 | 82.5 (>75 yrs), 67.5 (<75 yrs) | PAE with 100 µm particles in men >75 years and <75 years | |
| Yu (2016) [45] | prospective | Jun 2015–Mar 2016 | Hong Kong SAR | 16 | 66 | PAE with 100–300 µm Embosphere® in patients with BPH and acute urinary retention | PAE with 100–300 µm Embosphere® n patients with BPH without urinary retention |
| Yu (2019) [46] | prospective | Jun 2015–Dec 2018 | Hong Kong SAR | 82 | 66 | PAE with 100–300 µm Embosphere® |
| Author and (Year) | Study Design | Data Collection Period | Study Location | Patients Included in Intervention group(s) (n) | Mean Age | Intervention | Control/Comparator |
|---|---|---|---|---|---|---|---|
| Amouyal (2016) [20] | retrospective | Dec 2013–Jan 2015 | France | 32 | 65 | PAE PErFecTED with 300–500 µm Embosphere® | |
| Ayyagari (2019) [26] | retrospective | Apr 2013–Aug 2018 | United States | 93 | 76.0 end-hole; 72,8 balloon occlusion | end-hole vs. balloon occlusion PAE (both with 100–300 µm Embosphere®) | |
| Bhatia (2018) [28] | retrospective | Apr 2014–Oct 2017 | United States | 93 | 68.5 | PAE with 100–300 or 300–500 µm Embosphere® | |
| Pisco (2016) [37] | retrospective | Mar 2009–Sep 2014 | Portugal | 152 | 67.4 | PAE 100–200 µm PVA spheres, 300–500 µm Bead Block®, 300–500 µm Embosphere® or 400 µm Embozene® | |
| Qiu (2017) [38] | retrospective | Feb 2012–Mar 2015 | China | 17 | 75.53 | PAE with 90–180 µm Embosphere® | TURP |
| Tian (2019) [42] | retrospective | Feb 2014–Dec 2017 | China | 20 | 80.8 | PAE with 90–180 µm or 180–300 µm particles for control of gross haematuria in BPH |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svarc, P.; Taudorf, M.; Nielsen, M.B.; Stroomberg, H.V.; Røder, M.A.; Lönn, L. Postembolization Syndrome after Prostatic Artery Embolization: A Systematic Review. Diagnostics 2020, 10, 659. https://doi.org/10.3390/diagnostics10090659
Svarc P, Taudorf M, Nielsen MB, Stroomberg HV, Røder MA, Lönn L. Postembolization Syndrome after Prostatic Artery Embolization: A Systematic Review. Diagnostics. 2020; 10(9):659. https://doi.org/10.3390/diagnostics10090659
Chicago/Turabian StyleSvarc, Petra, Mikkel Taudorf, Michael Bachmann Nielsen, Hein Vincent Stroomberg, Martin Andreas Røder, and Lars Lönn. 2020. "Postembolization Syndrome after Prostatic Artery Embolization: A Systematic Review" Diagnostics 10, no. 9: 659. https://doi.org/10.3390/diagnostics10090659
APA StyleSvarc, P., Taudorf, M., Nielsen, M. B., Stroomberg, H. V., Røder, M. A., & Lönn, L. (2020). Postembolization Syndrome after Prostatic Artery Embolization: A Systematic Review. Diagnostics, 10(9), 659. https://doi.org/10.3390/diagnostics10090659







