Multidisciplinary Evaluation of Interstitial Lung Diseases: New Opportunities Linked to Rheumatologist Involvement
Abstract
1. Introduction
2. Methods
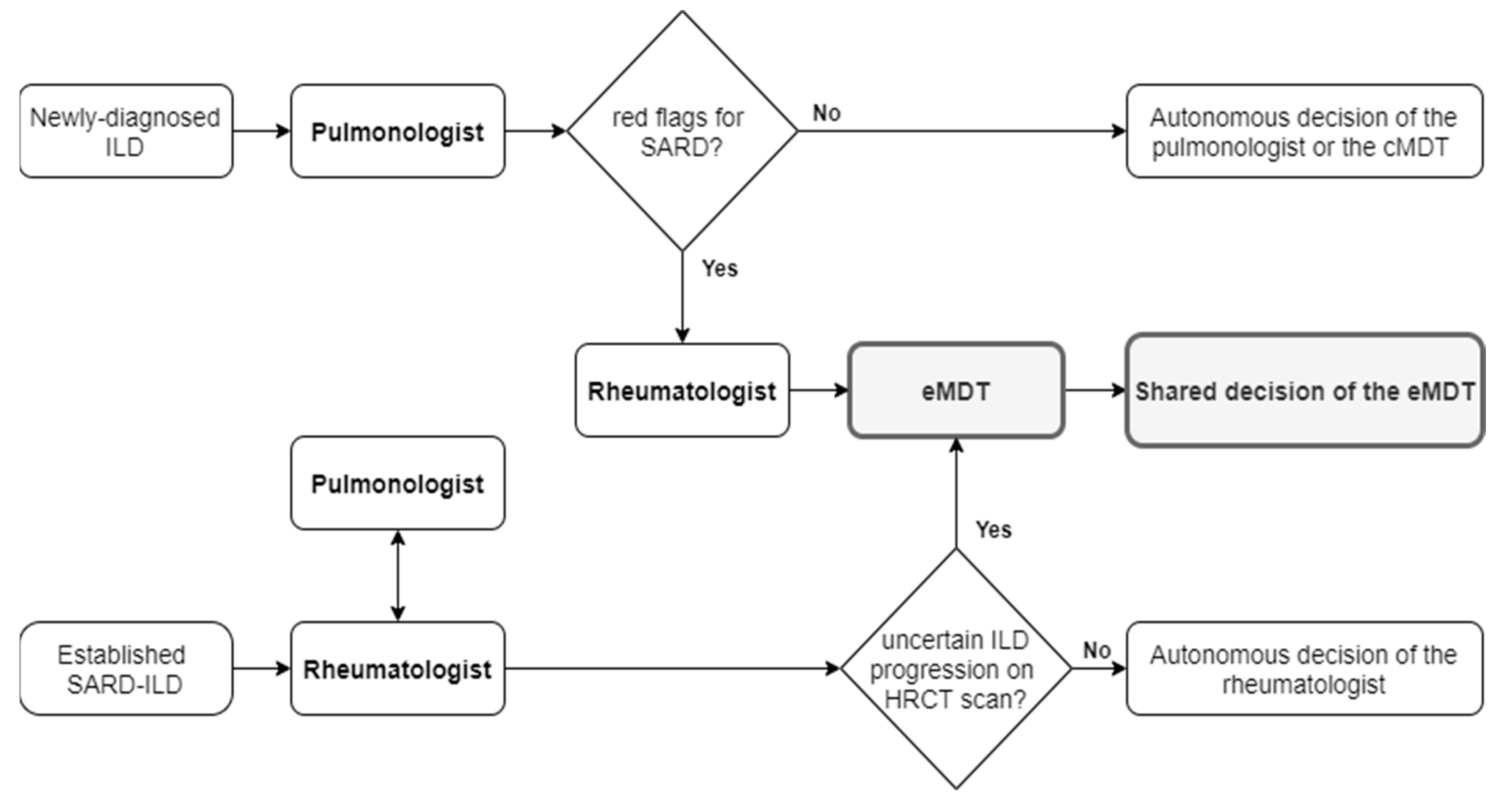
2.1. Multidisciplinary Team Composition and Activity
2.2. Selection of Cases to Be Discussed at eMDT
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Patients
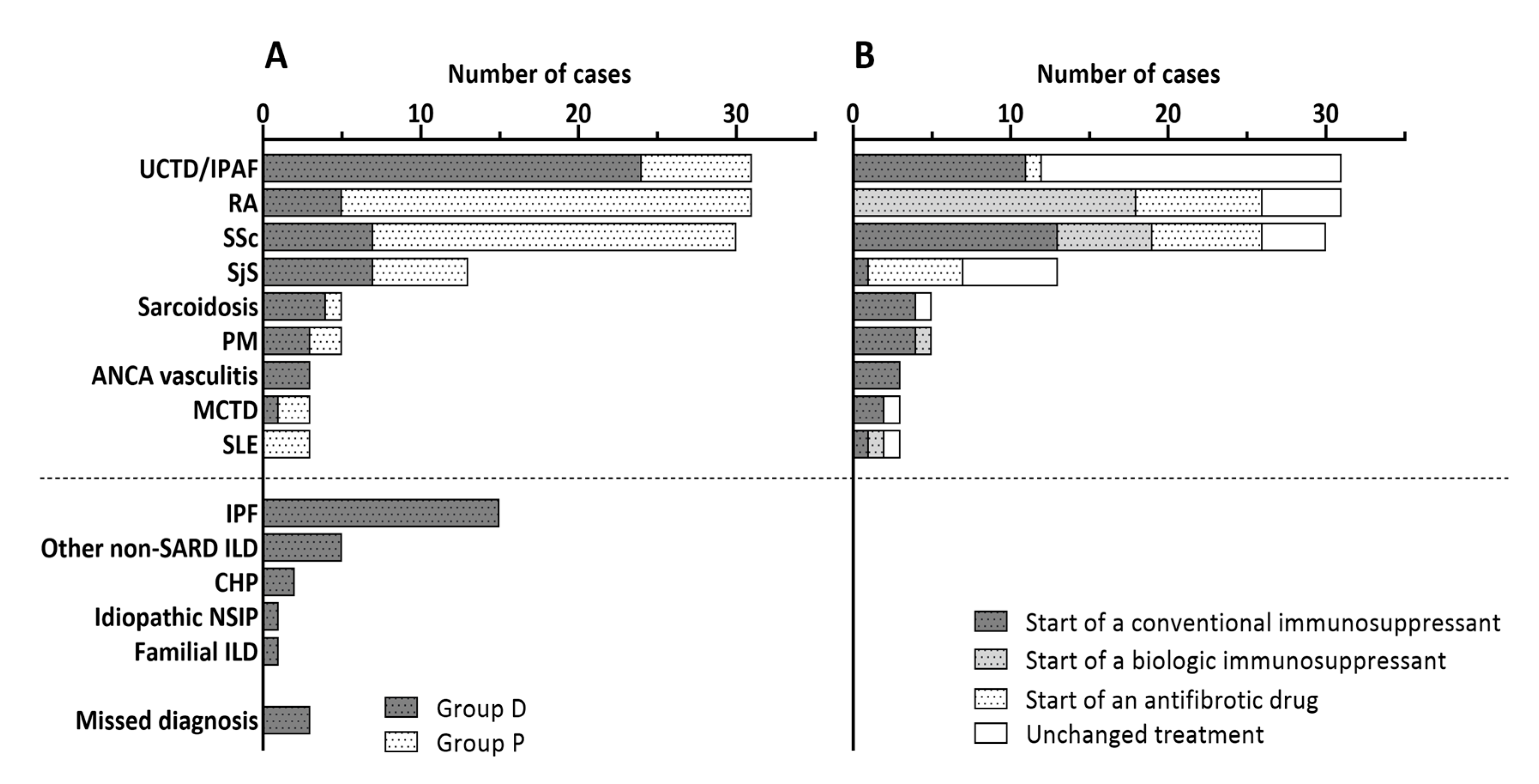
3.2. Evaluation of Patients with Suspected SARD-ILD (Group D)
3.3. Evaluation of Patients with an Uncertain Progression of SARD-ILD (Group P)
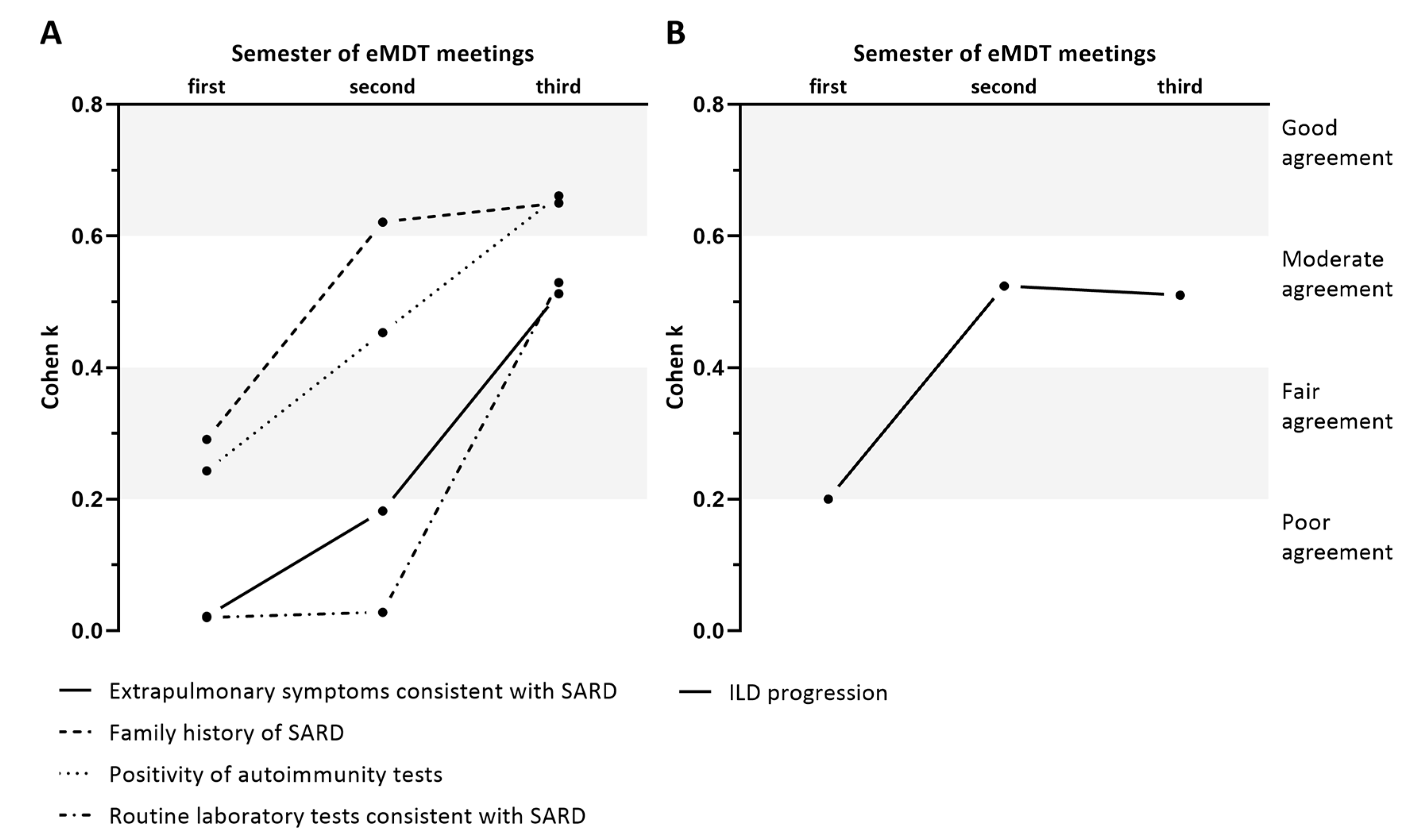
3.4. Modification in the Agreement in Group D and P over Time
3.5. Comparison of Patients with SARD-ILD Observed or Treated (Therapy Initiation or Change)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Fischer, A.; du Bois, R. Interstitial lung disease in connective tissue disorders [published correction appears in Lancet. 2012 Sep 29;380(9848):1148]. Lancet 2012, 380, 689–698. [Google Scholar] [CrossRef]
- Doyle, T.J.; Dellaripa, P.F. Lung Manifestations in the Rheumatic Diseases. Chest 2017, 152, 1283–1295. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Launders, N.; Martinez, F.; Walsh, S.; Myers, J.; Wang, B.; Jones, M.; Chisholm, A.; Flaherty, K.R. The characterisation of interstitial lung disease multidisciplinary team meetings: A global study. ERJ Open Res. 2019, 5, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Levi, Y.; Israeli-Shani, L.; Kuchuk, M.; Epstein Shochet, G.; Koslow, M.; Shitrit, D. Rheumatological Assessment Is Important for Interstitial Lung Disease Diagnosis. J. Rheumatol. 2018, 45, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Murohashi, K.; Hara, Y.; Saigusa, Y.; Kobayashi, N.; Sato, T.; Yamamoto, M.; Kudo, M.; Kaneko, T. Clinical significance of Charlson comorbidity index as a prognostic parameter for patients with acute or subacute idiopathic interstitial pneumonias and acute exacerbation of collagen vascular diseases-related interstitial pneumonia. J. Thorac. Dis. 2019, 11, 2448–2457. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Antoniou, K.M.; Brown, K.K.; Cadranel, J.; Corte, T.J.; du Bois, R.M.; Lee, J.S.; Leslie, K.O.; Lynch, D.A.; Matteson, E.L.; et al. “ERS/ATS Task Force on Undifferentiated Forms of CTD-ILD”. An official European Respiratory Society/American Thoracic Society research statement: Interstitial pneumonia with autoimmune features. Eur. Respir. J. 2015, 46, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Meroni, P.L.; Biggioggero, M.; Pierangeli, S.S.; Sheldon, J.; Zegers, I.; Borghi, M.O. Standardization of autoantibody testing: A paradigm for serology in rheumatic diseases. Nat. Rev. Rheumatol. 2014, 10, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Widell, J.; Lidén, M. Interobserver Variability in High-Resolution CT of the Lungs. Eur. J. Radiol. Open. 2020, 31, 7. [Google Scholar] [CrossRef] [PubMed]
- Al-Khawari, H.; Athyal, R.P.; Al-Saeed, O.; Sada, P.N.; Al-Muthairi, S.; Al-Awadhi, A. Inter- and intraobserver variation between radiologists in the detection of abnormal parenchymal lung changes on high-resolution computed tomography. Ann. Saudi Med. 2010, 30, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Antunes, V.B.; Meirelles, G.S.; Jasinowodolinski, D.; Pereira, C.A.; Verrastro, C.G.; Torlai, F.G.; D’Ippolito, G. Observer agreement in the diagnosis of interstitial lung diseases based on HRCT scans. J. Bras. Pneumol. 2010, 36, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.L.; Calandriello, L.; Sverzellati, N.; Wells, A.U.; Hansell, D.M.; UIP Observer Consort. Interobserver agreement for the ATS/ERS/JRS/ALAT criteria for a UIP pattern on CT. Thorax 2016, 71, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, H.Y.; Zhang, X.X.; Wu, L.L.; Wang, L.L.; Jiang, Y.; Deng, K.M.; Mao, M.M.; Chen, R.C.; Kolb, M.; et al. The Role of Follow-up Evaluation in the Diagnostic Algorithm of Idiopathic Interstitial Pneumonia: A Retrospective Study. Sci. Rep. 2019, 9, 6452. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Archer, J. Impact of workplace based assessment on doctors’ education and performance: A systematic review. BMJ 2010, 341, c5064. [Google Scholar] [CrossRef] [PubMed]
- Sverzellati, N.; Devaraj, A.; Desai, S.R.; Quigley, M.; Wells, A.U.; Hansell, D.M. Method for minimizing observer variation for the quantitation of high-resolution computed tomographic signs of lung disease. J. Comput. Assist. Tomogr. 2011, 35, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.U.; Brown, K.K.; Flaherty, K.R.; Kolb, M.; Thannickal, V.J.; IPF Consensus Working Group. What’s in a name? That which we call IPF, by any other name would act the same. Eur. Respir. J. 2018, 51, 1800692. [Google Scholar] [CrossRef] [PubMed]
| All Patients | Group D | Group P | p * | |
|---|---|---|---|---|
| N | 151 | 81 | 70 | - |
| Age (years), mean ± SD | 60.3 ± 14.1 | 62.1 ± 13.0 | 59.6 ± 13.7 | 0.198 |
| Female, n (%) | 105 (69.5) | 56 (69.1) | 49 (70.0) | 0.908 |
| Current or former smokers, n (%) | 65 (43.0) | 38 (46.9) | 26 (37.1) | 0.281 |
| CCI, mean ± SD | 2.9 ± 1.6 | 2.9 ± 1.6 | 3.1 ± 1.6 | 0.785 |
| ILD duration, years, median (IQR) | 1.0 (0.3–4.3) | 0.6 (0.3–2.5) | 2.0 (0.0–7.9) | 0.036 |
| FVC, %, mean ± SD | 87.2 ± 25.4 | 87.6 ± 26.0 | 86.8 ± 25.0 | 0.333 |
| FVC ≤ 80%, n (%) | 84 (55.6) | 44 (54.3) | 40 (57.1) | 0.728 |
| DLCO, %, mean ± SD | 58.1 ± 21.0 | 60.5 ± 20.7 | 55.5 ± 21.3 | 0.219 |
| DLCO ≤ 50%, n (%) | 87 (57.6) | 45 (56.8) | 41 (58.6) | 0.825 |
| NSIP pattern on HRCT scan, n (%) | 63 (41.7) | 32 (39.5) | 31 (44.3) | 0.553 |
| UIP pattern on HRCT scan, n (%) | 58 (38.4) | 28 (34.6) | 30 (42.9) | 0.296 |
| OP pattern on HRCT scan, n (%) | 6 (4.0) | 4 (4.9) | 2 (2.9) | 0.514 |
| Indeterminate pattern on HRCT, n (%) | 24 (15.9) | 17 (21.0) | 7 (10.0) | 0.066 |
| Oxygen therapy, n (%) | 30 (19.9) | 10 (12.3) | 20 (28.6) | 0.049 |
| Red Flags | Pulmonologist | Rheumatologist | Cohen’s k | Agreement * | CI 95% | p | PPV ** | NPV § |
|---|---|---|---|---|---|---|---|---|
| Positivity of autoimmunity tests, n (%) | 59 (72.8) | 62 (76.5) | 0.475 | Moderate | 0.248–0.723 | <0.001 | 88.1 | 54.5 |
| Family history of SARD, n (%) | 5 (6.2) | 10 (12.3) | 0.491 | Moderate | 0.173–0.806 | <0.001 | 80.0 | 92.1 |
| Extrapulmonary symptoms consistent with SARD, n (%) | 48 (59.3) | 64 (79.0) | 0.225 | Fair | 0.025–0.425 | 0.064 | 87.5 | 33.3 |
| Routine laboratory tests consistent with SARD, n (%) | 7 (8.6) | 44 (54.3) | 0.101 | Poor | −0.007–0.209 | 0.081 | 85.7 | 48.6 |
| All Patients with SARD-ILD | Modification of Treatment | No Modification of Treatment | p * | |
|---|---|---|---|---|
| N | 124 | 72 | 52 | - |
| Group D/Group P, n (%) | 54 (43.5)/70 (56.5) | 42(58.3)/30(41.7) | 31(59.6)/21(40.4) | 0.826 |
| Age (years), mean ± SD | 59.6 ± 13.8 | 59.3 ± 14.5 | 60.1 ± 12.9 | 0.732 |
| Female, n (%) | 87 (70.2) | 46 (63.9) | 41 (78.8) | 0.072 |
| Current or former smokers, n (%) | 51 (41.1) | 30 (41.7) | 21 (40.4) | 0.826 |
| CCI, mean ± SD | 2.9 ± 1.6 | 2.9 ± 1.6 | 2.9 ± 1.5 | 0.940 |
| ILD duration, years, median (IQR) | 1.0 (0.1–4.7) | 0.5 (0.4–4.6) | 0.8 (0.3–2.1) | 0.788 |
| FVC, %, mean ± SD | 84.9 ± 24.2 | 81.0 ± 21.4 | 90.0 ± 27.1 | 0.050 |
| FVC ≤ 80%, n (%) | 68 (54.8) | 43 (59.7) | 25 (48.1) | 0.199 |
| DLCO, %, mean ± SD | 58.8 ± 21.7 | 58.2 ± 23.8 | 60.0 ± 17.6 | 0.692 |
| DLCO ≤ 50%, n (%) | 72 (58.1) | 41 (56.9) | 31 (59.6) | 0.766 |
| NSIP pattern on HRCT scan, n (%) | 59 (47.6) | 37 (51.4) | 22 (42.3) | 0.318 |
| UIP pattern on HRCT scan, n (%) | 45 (36.3) | 24 (33.3) | 21 (40.4) | 0.420 |
| OP pattern on HRCT scan, n (%) | 5 (4.0) | 4 (5.6) | 1 (1.9) | 0.310 |
| Indeterminate pattern on HRCT, n (%) | 15 (12.1) | 7 (9.7) | 8 (15.4) | 0.340 |
| Oxygen therapy, n (%) | 27 (21.8) | 18 (25.0) | 9 (17.3) | 0.332 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lorenzis, E.; Bosello, S.L.; Varone, F.; Sgalla, G.; Calandriello, L.; Natalello, G.; Iovene, B.; Cicchetti, G.; Gigante, L.; Verardi, L.; et al. Multidisciplinary Evaluation of Interstitial Lung Diseases: New Opportunities Linked to Rheumatologist Involvement. Diagnostics 2020, 10, 664. https://doi.org/10.3390/diagnostics10090664
De Lorenzis E, Bosello SL, Varone F, Sgalla G, Calandriello L, Natalello G, Iovene B, Cicchetti G, Gigante L, Verardi L, et al. Multidisciplinary Evaluation of Interstitial Lung Diseases: New Opportunities Linked to Rheumatologist Involvement. Diagnostics. 2020; 10(9):664. https://doi.org/10.3390/diagnostics10090664
Chicago/Turabian StyleDe Lorenzis, Enrico, Silvia Laura Bosello, Francesco Varone, Giacomo Sgalla, Lucio Calandriello, Gerlando Natalello, Bruno Iovene, Giuseppe Cicchetti, Laura Gigante, Lucrezia Verardi, and et al. 2020. "Multidisciplinary Evaluation of Interstitial Lung Diseases: New Opportunities Linked to Rheumatologist Involvement" Diagnostics 10, no. 9: 664. https://doi.org/10.3390/diagnostics10090664
APA StyleDe Lorenzis, E., Bosello, S. L., Varone, F., Sgalla, G., Calandriello, L., Natalello, G., Iovene, B., Cicchetti, G., Gigante, L., Verardi, L., Gremese, E., Richeldi, L., & Larici, A. R. (2020). Multidisciplinary Evaluation of Interstitial Lung Diseases: New Opportunities Linked to Rheumatologist Involvement. Diagnostics, 10(9), 664. https://doi.org/10.3390/diagnostics10090664






