Nuclear Imaging for Bone Metastases in Prostate Cancer: The Emergence of Modern Techniques Using Novel Radiotracers
Abstract
:1. Introduction
2. Conventional Imaging in the Detection and Management of Prostate Cancer
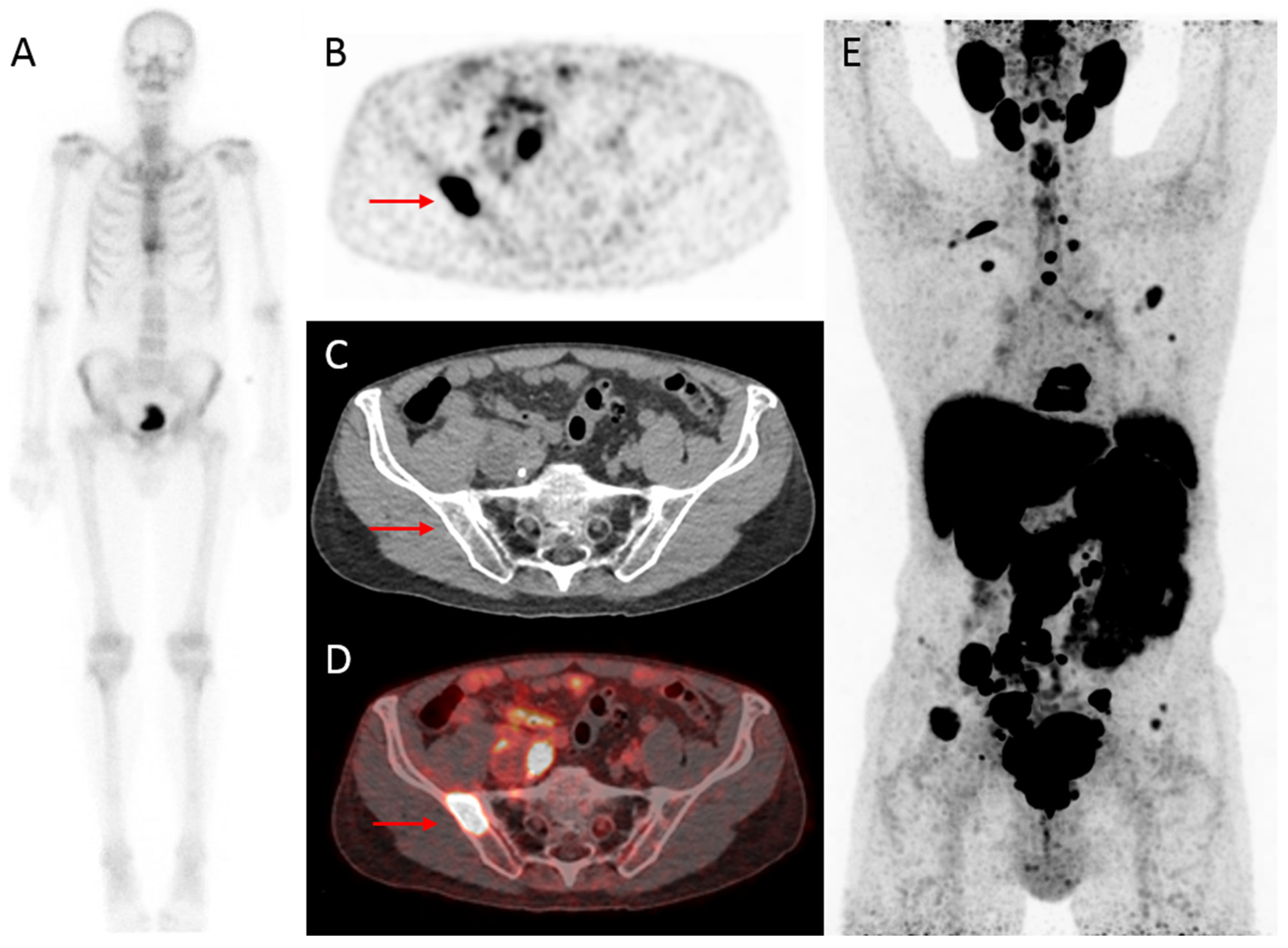
2.1. Bone Scintigraphy: The Historical Standard for Nuclear Imaging in Prostate Cancer
2.2. Computed Tomography in the Workup of Prostate Cancer
3. Initial Treatment of Metastatic Prostate Cancer and Importance of High vs. Low Burden of Disease: Rationale for Improved Imaging and Metastasis Detection
4. Modern Nuclear Imaging in Prostate Cancer: New PET/CT Radiotracers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 68Ga | 68Gallium |
| 18F | 18Fluorine |
| 18F-FCH | 18F-fluorocholine |
| 18F-FDHT | 18F-fluordihydrotestosterone |
| 18F-NaF | 18F-sodium fluoride |
| ADT | androgen-deprivation therapy |
| AR | androgen receptor |
| BCR | biochemical recurrence |
| BS | bone scintigraphy |
| CRPC | castration-resistant prostate cancer |
| CT | computed tomography |
| DRE | digital rectal examination |
| EAU | European Association of Urology |
| ECOG | Eastern Cooperative Oncology Group |
| GG | Grade Group |
| GS | Gleason Score |
| HR | hazard ratio |
| ISUP | International Society of Urological Pathology |
| mHSPCa | metastatic hormone-sensitive prostate cancer |
| MRI | magnetic resonance imaging |
| OS | overall survival |
| PCa | prostate cancer |
| PET | positron emission tomography |
| PS | performance status |
| PSA | prostate-specific antigen |
| SAE | serious adverse events |
| TRUS | transrectal ultrasound |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2020. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading, C. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.J.; et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef]
- Corfield, J.; Perera, M.; Bolton, D.; Lawrentschuk, N. (68)Ga-prostate specific membrane antigen (PSMA) positron emission tomography (PET) for primary staging of high-risk prostate cancer: A systematic review. World J. Urol. 2018, 36, 519–527. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 77, 403–417. [Google Scholar] [CrossRef]
- Zhou, J.; Gou, Z.; Wu, R.; Yuan, Y.; Yu, G.; Zhao, Y. Comparison of PSMA-PET/CT, choline-PET/CT, NaF-PET/CT, MRI, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: A systematic review and meta-analysis. Skeletal. Radiol. 2019, 48, 1915–1924. [Google Scholar] [CrossRef]
- Sheikhbahaei, S.; Jones, K.M.; Werner, R.A.; Salas-Fragomeni, R.A.; Marcus, C.V.; Higuchi, T.; Rowe, S.P.; Solnes, L.B.; Javadi, M.S. (18)F-NaF-PET/CT for the detection of bone metastasis in prostate cancer: A meta-analysis of diagnostic accuracy studies. Ann. Nucl. Med. 2019, 33, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Deng, H.; Hu, S.; Jia, Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: A meta-analysis. Skeletal. Radiol. 2014, 43, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Briganti, A.; Passoni, N.; Ferrari, M.; Capitanio, U.; Suardi, N.; Gallina, A.; Da Pozzo, L.F.; Picchio, M.; Di Girolamo, V.; Salonia, A.; et al. When to perform bone scan in patients with newly diagnosed prostate cancer: External validation of the currently available guidelines and proposal of a novel risk stratification tool. Eur. Urol. 2010, 57, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Abuzallouf, S.; Dayes, I.; Lukka, H. Baseline staging of newly diagnosed prostate cancer: A summary of the literature. J. Urol. 2004, 171, 2122–2127. [Google Scholar] [CrossRef]
- Lebastchi, A.H.; Gupta, N.; DiBianco, J.M.; Piert, M.; Davenport, M.S.; Ahdoot, M.A.; Gurram, S.; Bloom, J.B.; Gomella, P.T.; Mehralivand, S.; et al. Comparison of cross-sectional imaging techniques for the detection of prostate cancer lymph node metastasis: A critical review. Transl. Androl. Urol. 2020, 9, 1415–1427. [Google Scholar] [CrossRef]
- McMahon, C.J.; Rofsky, N.M.; Pedrosa, I. Lymphatic Metastases from Pelvic Tumors: Anatomic Classification, Characterization, and Staging. Radiology 2010, 254, 31–46. [Google Scholar] [CrossRef]
- Kiss, B.; Thoeny, H.C.; Studer, U.E. Current Status of Lymph Node Imaging in Bladder and Prostate Cancer. Urology 2016, 96, 1–7. [Google Scholar] [CrossRef]
- Larson, S.M.; Morris, M.; Gunther, I.; Beattie, B.; Humm, J.L.; Akhurst, T.A.; Finn, R.D.; Erdi, Y.; Pentlow, K.; Dyke, J.; et al. Tumor Localization of 16β-18F-Fluoro-5α-Dihydrotestosterone Versus 18F-FDG in Patients with Progressive, Metastatic Prostate Cancer. J. Nucl. Med. 2004, 45, 366–373. [Google Scholar]
- Taneja, S.S. Imaging in the diagnosis and management of prostate cancer. Rev. Urol. 2004, 6, 101–113. [Google Scholar]
- Carroll, P.; Coley, C.; McLeod, D.; Schellhammer, P.; Sweat, G.; Wasson, J.; Zietman, A.; Thompson, I. Prostate-specific antigen best practice policy—Part II: Prostate cancer staging and post-treatment follow-up. Urology 2001, 57, 225–229. [Google Scholar] [CrossRef]
- Flanigan, R.C.; McKay, T.C.; Olson, M.; Shankey, T.V.; Pyle, J.; Waters, W.B. Limited efficacy of preoperative computed tomographic scanning for the evaluation of lymph node metastasis in patients before radical prostatectomy. Urology 1996, 48, 428–432. [Google Scholar] [CrossRef]
- Hövels, A.M.; Heesakkers, R.A.; Adang, E.M.; Jager, G.J.; Strum, S.; Hoogeveen, Y.L.; Severens, J.L.; Barentsz, J.O. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin. Radiol. 2008, 63, 387–395. [Google Scholar] [CrossRef]
- Gabriele, D.; Collura, D.; Oderda, M.; Stura, I.; Fiorito, C.; Porpiglia, F.; Terrone, C.; Zacchero, M.; Guiot, C.; Gabriele, P. Is there still a role for computed tomography and bone scintigraphy in prostate cancer staging? An analysis from the EUREKA-1 database. World J. Urol. 2016, 34, 517–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagliarulo, V.; Bracarda, S.; Eisenberger, M.A.; Mottet, N.; Schroder, F.H.; Sternberg, C.N.; Studer, U.E. Contemporary role of androgen deprivation therapy for prostate cancer. Eur. Urol. 2012, 61, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Gravis, G.; Fizazi, K.; Joly, F.; Oudard, S.; Priou, F.; Esterni, B.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 149–158. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Gravis, G.; Boher, J.M.; Joly, F.; Soulie, M.; Albiges, L.; Priou, F.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Androgen Deprivation Therapy (ADT) Plus Docetaxel Versus ADT Alone in Metastatic Non castrate Prostate Cancer: Impact of Metastatic Burden and Long-term Survival Analysis of the Randomized Phase 3 GETUG-AFU15 Trial. Eur. Urol. 2016, 70, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, C.E.; Chen, Y.H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J. Clin. Oncol. 2018, 36, 1080–1087. [Google Scholar] [CrossRef] [Green Version]
- Clarke, N.W.; Ali, A.; Ingleby, F.C.; Hoyle, A.; Amos, C.L.; Attard, G.; Brawley, C.D.; Calvert, J.; Chowdhury, S.; Cook, A.; et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: Long-term survival results from the STAMPEDE trial. Ann. Oncol. 2019, 30, 1992–2003. [Google Scholar] [CrossRef] [Green Version]
- Beheshti, M.; Rezaee, A.; Geinitz, H.; Loidl, W.; Pirich, C.; Langsteger, W. Evaluation of Prostate Cancer Bone Metastases with 18F-NaF and 18F-Fluorocholine PET/CT. J. Nucl. Med. 2016, 57, 55S–60S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zacho, H.D.; Jochumsen, M.R.; Langkilde, N.C.; Mortensen, J.C.; Haarmark, C.; Hendel, H.W.; Jensen, J.B.; Petersen, L.J. No Added Value of (18)F-Sodium Fluoride PET/CT for the Detection of Bone Metastases in Patients with Newly Diagnosed Prostate Cancer with Normal Bone Scintigraphy. J. Nucl. Med. 2019, 60, 1713–1716. [Google Scholar] [CrossRef] [PubMed]
- Cysouw, M.C.F.; Kramer, G.M.; Heijtel, D.; Schuit, R.C.; Morris, M.J.; van den Eertwegh, A.J.M.; Voortman, J.; Hoekstra, O.S.; Oprea-Lager, D.E.; Boellaard, R. Sensitivity of (18)F-fluorodihydrotestosterone PET-CT to count statistics and reconstruction protocol in metastatic castration-resistant prostate cancer. EJNMMI Res. 2019, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Kramer, G.M.; Yaqub, M.; Vargas, H.A.; Schuit, R.C.; Windhorst, A.D.; van den Eertwegh, A.J.M.; van der Veldt, A.A.M.; Bergman, A.M.; Burnazi, E.M.; Lewis, J.S.; et al. Assessment of Simplified Methods for Quantification of (18)F-FDHT Uptake in Patients with Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2019, 60, 1221–1227. [Google Scholar] [CrossRef] [Green Version]
- Rathkopf, D.E.; Morris, M.J.; Fox, J.J.; Danila, D.C.; Slovin, S.F.; Hager, J.H.; Rix, P.J.; Chow Maneval, E.; Chen, I.; Gonen, M.; et al. Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J. Clin. Oncol. 2013, 31, 3525–3530. [Google Scholar] [CrossRef]
- Scher, H.I.; Beer, T.M.; Higano, C.S.; Anand, A.; Taplin, M.E.; Efstathiou, E.; Rathkopf, D.; Shelkey, J.; Yu, E.Y.; Alumkal, J.; et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: A phase 1–2 study. Lancet 2010, 375, 1437–1446. [Google Scholar] [CrossRef] [Green Version]
- Dehdashti, F.; Picus, J.; Michalski, J.M.; Dence, C.S.; Siegel, B.A.; Katzenellenbogen, J.A.; Welch, M.J. Positron tomographic assessment of androgen receptors in prostatic carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 344–350. [Google Scholar] [CrossRef]
- Oprea-Lager, D.E.; van Kanten, M.P.; van Moorselaar, R.J.; van den Eertwegh, A.J.; van de Ven, P.M.; Bijnsdorp, I.V.; Hoekstra, O.S.; Geldof, A.A. [18F]fluoromethylcholine as a chemotherapy response read-out in prostate cancer cells. Mol. Imaging Biol. 2015, 17, 319–327. [Google Scholar] [CrossRef]
- Evangelista, L.; Zattoni, F.; Guttilla, A.; Saladini, G.; Zattoni, F.; Colletti, P.M.; Rubello, D. Choline PET or PET/CT and biochemical relapse of prostate cancer: A systematic review and meta-analysis. Clin. Nucl. Med. 2013, 38, 305–314. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Zechmann, C.M.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Holland-Letz, T.; Hadaschik, B.A.; Giesel, F.L.; Debus, J.; et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Schwenck, J.; Rempp, H.; Reischl, G.; Kruck, S.; Stenzl, A.; Nikolaou, K.; Pfannenberg, C.; la Fougere, C. Comparison of (68)Ga-labelled PSMA-11 and (11)C-choline in the detection of prostate cancer metastases by PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Perner, S.; Hofer, M.D.; Kim, R.; Shah, R.B.; Li, H.; Moller, P.; Hautmann, R.E.; Gschwend, J.E.; Kuefer, R.; Rubin, M.A. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum. Pathol. 2007, 38, 696–701. [Google Scholar] [CrossRef]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar] [PubMed]
- Uprimny, C.; Kroiss, A.S.; Decristoforo, C.; Fritz, J.; von Guggenberg, E.; Kendler, D.; Scarpa, L.; di Santo, G.; Roig, L.G.; Maffey-Steffan, J.; et al. (68)Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.P.; Gorin, M.A.; Allaf, M.E.; Pienta, K.J.; Tran, P.T.; Pomper, M.G.; Ross, A.E.; Cho, S.Y. PET imaging of prostate-specific membrane antigen in prostate cancer: Current state of the art and future challenges. Prostate Cancer Prostatic Dis. 2016, 19, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Bodar, Y.J.L.; Jansen, B.H.E.; van der Voorn, J.P.; Zwezerijnen, G.J.C.; Meijer, D.; Nieuwenhuijzen, J.A.; Boellaard, R.; Hendrikse, N.H.; Hoekstra, O.S.; van Moorselaar, R.J.A.; et al. Detection of prostate cancer with 18F-DCFPyL PET/CT compared to final histopathology of radical prostatectomy specimens: Is PSMA-targeted biopsy feasible? The DeTeCT trial. World J. Urol. 2020. [Google Scholar] [CrossRef]
- van Leeuwen, P.J.; Donswijk, M.; Nandurkar, R.; Stricker, P.; Ho, B.; Heijmink, S.; Wit, E.M.K.; Tillier, C.; van Muilenkom, E.; Nguyen, Q.; et al. Gallium-68-prostate-specific membrane antigen ((68) Ga-PSMA) positron emission tomography (PET)/computed tomography (CT) predicts complete biochemical response from radical prostatectomy and lymph node dissection in intermediate- and high-risk prostate cancer. BJU Int. 2019, 124, 62–68. [Google Scholar] [CrossRef]
- Wondergem, M.; van der Zant, F.M.; Roeleveld, T.A.; Srbljin, S.; Kartachova, M.S.; van Dongen, A.; Franken, V.; Knol, R.J.J. 18F-DCFPyL PET/CT in primary staging of prostate cancer. Eur. J. Hybrid Imaging 2018, 2, 26. [Google Scholar] [CrossRef]
- Szabo, Z.; Mena, E.; Rowe, S.P.; Plyku, D.; Nidal, R.; Eisenberger, M.A.; Antonarakis, E.S.; Fan, H.; Dannals, R.F.; Chen, Y.; et al. Initial Evaluation of [(18)F]DCFPyL for Prostate-Specific Membrane Antigen (PSMA)-Targeted PET Imaging of Prostate Cancer. Mol. Imaging Biol. 2015, 17, 565–574. [Google Scholar] [CrossRef]
- Giesel, F.L.; Hadaschik, B.; Cardinale, J.; Radtke, J.; Vinsensia, M.; Lehnert, W.; Kesch, C.; Tolstov, Y.; Singer, S.; Grabe, N.; et al. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 678–688. [Google Scholar] [CrossRef] [Green Version]
- Dietlein, M.; Kobe, C.; Kuhnert, G.; Stockter, S.; Fischer, T.; Schomacker, K.; Schmidt, M.; Dietlein, F.; Zlatopolskiy, B.D.; Krapf, P.; et al. Comparison of [(18)F]DCFPyL and [ (68)Ga]Ga-PSMA-HBED-CC for PSMA-PET Imaging in Patients with Relapsed Prostate Cancer. Mol. Imaging Biol. 2015, 17, 575–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wondergem, M.; van der Zant, F.M.; Broos, W.A.M.; Roeleveld, T.A.; Donker, R.; Ten Oever, D.; Geenen, R.W.F.; Knol, R.J.J. (18)F-DCFPyL PET/CT for primary staging in 160 high-risk prostate cancer patients; metastasis detection rate, influence on clinical management and preliminary results of treatment efficacy. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef]
- Jansen, B.H.E.; Bodar, Y.J.L.; Zwezerijnen, G.J.C.; Meijer, D.; van der Voorn, J.P.; Nieuwenhuijzen, J.A.; Wondergem, M.; Roeleveld, T.A.; Boellaard, R.; Hoekstra, O.S.; et al. Pelvic lymph-node staging with (18)F-DCFPyL PET/CT prior to extended pelvic lymph-node dissection in primary prostate cancer—The SALT trial. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef] [PubMed]
- Weichselbaum, R.R.; Hellman, S. Oligometastases revisited. Nat. Rev. Clin. Oncol. 2011, 8, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Ost, P.; Bossi, A.; Decaestecker, K.; De Meerleer, G.; Giannarini, G.; Karnes, R.J.; Roach, M., 3rd; Briganti, A. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: A systematic review of the literature. Eur. Urol. 2015, 67, 852–863. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; Bruycker, A.D.; Lambert, B.; Delrue, L.; Bultijnck, R.; Goetghebeur, E.; et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): Five-year results of a randomized phase II trial. J. Clin. Oncol. 2020, 38, 10. [Google Scholar] [CrossRef]

| Sensitivity (%) | Specificity (%) | Reference | Type of Article | |
|---|---|---|---|---|
| Bone scintigraphy | 79 | 82 | Shen [12] | Meta-analysis |
| CT | 8.8 | 98 | Gabriele [23] | Retrospective cohort |
| 18F-NaF PET/CT | 98 | 90 | Sheikhbahaei [11] | Meta-analysis |
| 18F-FDHT PET/CT | 63 | - | Dehdashti [37] | Prospective cohort |
| 18F-FCH PET/CT | 87 | 97 | Shen [12] | Meta-analysis |
| 68Ga-PSMA PET/CT | 77 | 97 | Perera [9] | Systematic review and meta-analysis |
| 18F-DCFPyL PET/CT | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luining, W.I.; Meijer, D.; Dahele, M.R.; Vis, A.N.; Oprea-Lager, D.E. Nuclear Imaging for Bone Metastases in Prostate Cancer: The Emergence of Modern Techniques Using Novel Radiotracers. Diagnostics 2021, 11, 117. https://doi.org/10.3390/diagnostics11010117
Luining WI, Meijer D, Dahele MR, Vis AN, Oprea-Lager DE. Nuclear Imaging for Bone Metastases in Prostate Cancer: The Emergence of Modern Techniques Using Novel Radiotracers. Diagnostics. 2021; 11(1):117. https://doi.org/10.3390/diagnostics11010117
Chicago/Turabian StyleLuining, Wietske I., Dennie Meijer, Max R. Dahele, André N. Vis, and Daniela E. Oprea-Lager. 2021. "Nuclear Imaging for Bone Metastases in Prostate Cancer: The Emergence of Modern Techniques Using Novel Radiotracers" Diagnostics 11, no. 1: 117. https://doi.org/10.3390/diagnostics11010117
APA StyleLuining, W. I., Meijer, D., Dahele, M. R., Vis, A. N., & Oprea-Lager, D. E. (2021). Nuclear Imaging for Bone Metastases in Prostate Cancer: The Emergence of Modern Techniques Using Novel Radiotracers. Diagnostics, 11(1), 117. https://doi.org/10.3390/diagnostics11010117





