Optimization of the Maximum Skin Dose Measurement Technique Using Digital Imaging and Communication in Medicine—Radiation Dose Structured Report Data for Patients Undergoing Cerebral Angiography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sets
2.2. X-ray Equipment
2.3. Dosimetry of Skin Dose for Patients Who Undergo NIR Procedures
2.4. RPLGD X-Ray Energy Calibration
2.5. Direct Estimation Method: Estimation of Dskin,max,RPLGD from RPLGD Measurements
2.6. Indirect Estimation Method: Estimation of Dskin,max from Total Ka,r by Applying an Arbitrary Constant as a Conversion Factor (CFKa,const) (Estimation Model 1)
2.7. Indirect Estimation Method: Estimation of Dskin,max from Total Ka,r by Applying an Arbitrary Function as a Conversion Factor (CFKa,function) (Estimation Model 2)
2.8. Comparison of the Accuracy of the Estimation of Dskin,max,Ka under Estimation Models 1 and 2
2.9. Validation of the Accuracy of Estimation Models 1 and 2 Using the Validation Data Set
2.10. Statistical Analysis
2.11. Ethical Approval
3. Results
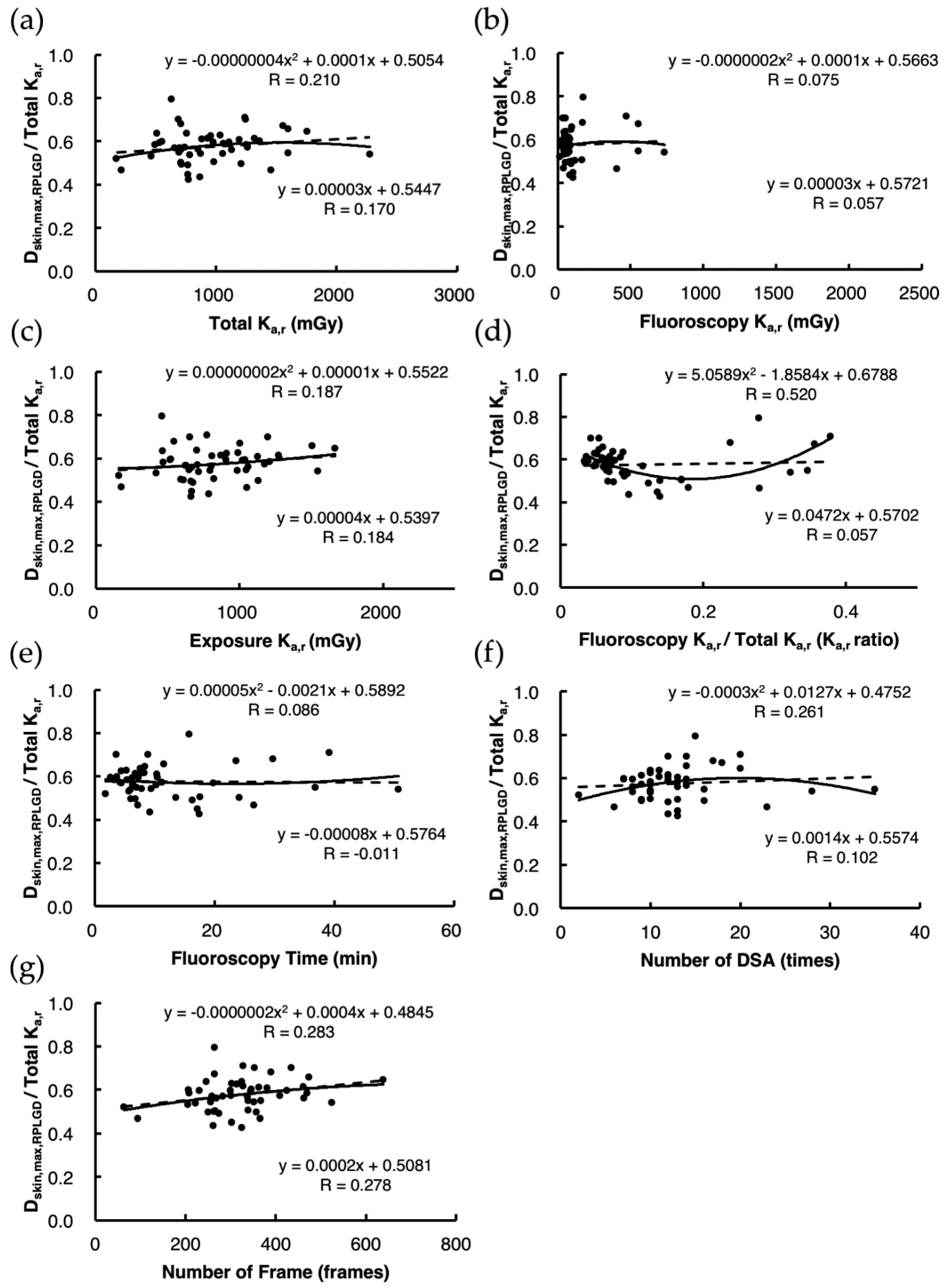
3.1. Direct Estimation of Dskin,max,RPLGD
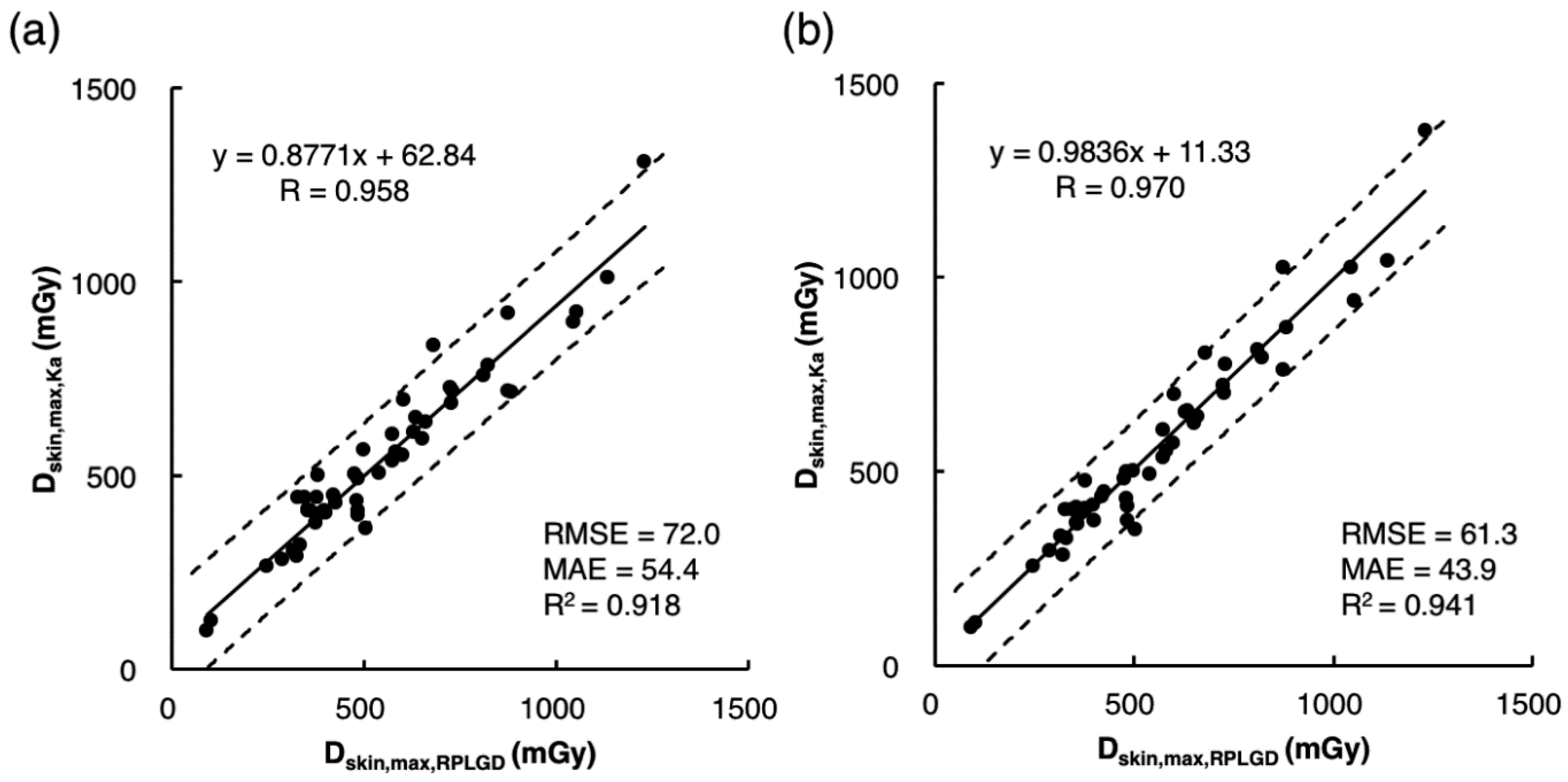
3.2. Indirect Estimation of Dskin,max,Ka Using Estimation Model 1
3.3. Indirect Estimation of Dskin,max,Ka Using Estimation Model 2
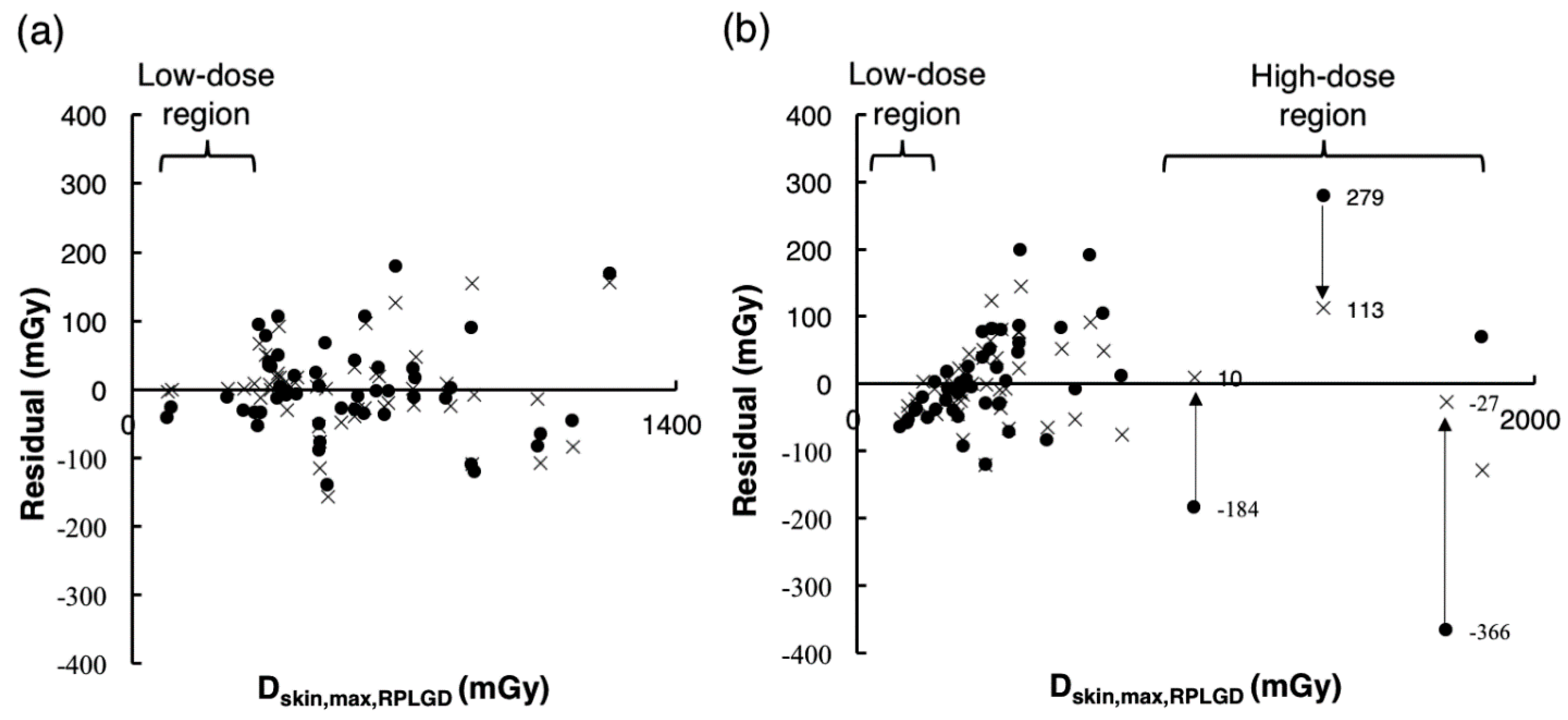
3.4. Comparison of the Accuracy of Dskin,max,Ka Estimated Using Estimation Models 1 and 2
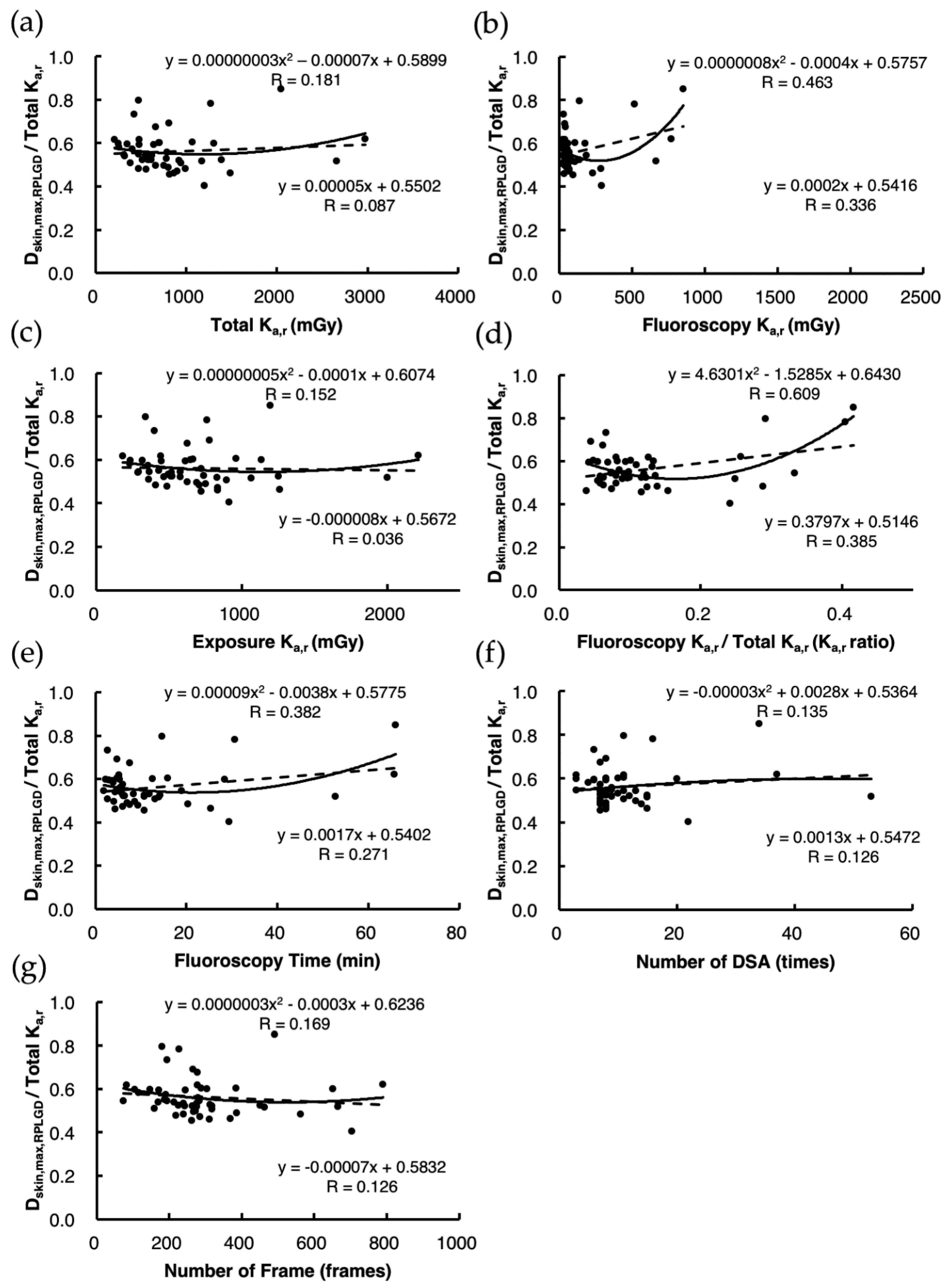
3.5. Validation of the Accuracy of Estimates Using Estimation Models 1 and 2 under the Validation Data Set
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wagner, L.K.; Eifel, P.J.; Geise, R.A. Potential Biological Effects Following High X-ray Dose Interventional Procedures. J. Vasc. Interv. Radiol. 1994, 5, 71–84. [Google Scholar] [CrossRef]
- Mooney, R.B.; McKinstry, C.S.; A Kamel, H. Absorbed dose and deterministic effects to patients from interventional neuroradiology. Br. J. Radiol. 2000, 73, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, M.; Moritake, T.; Kataoka, F.; Takigawa, T.; Koguchi, Y.; Miyamoto, Y.; Akahane, K.; Matsumaru, Y. Direct measurement of patient’s entrance skin dose during neurointerventional procedure to avoid further radiation-induced skin injuries. Clin. Neurol. Neurosurg. 2010, 112, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Magrassi, L.; Bongetta, D.; D’Ercole, L.; Lisciandro, F.; Arienta, C.; Thyrion, F.Z. Neuroembolization may expose patients to radiation doses previously linked to tumor induction. Acta Neurochir. 2012, 154, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Corrigall, R.S.; Martin, C.J.; Scott, I. Observations of tissue reactions following neuroradiology interventional procedures. J. Radiol. Prot. 2020, 40, N9–N15. [Google Scholar] [CrossRef]
- ICRP. Avoidance of radiation injuries from medical interventional procedures. ICRP Publ. 2000, 85, 1–53. [Google Scholar]
- Miller, N.L.; Balter, S.; Noonan, P.T.; Georgia, J.D. Minimizing Radiation-induced Skin Injury in Interventional Radiology Procedures. Radiology 2002, 2002, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Safari, M.J.; Wong, J.H.; Kadir, K.A.; Thorpe, N.K.; Cutajar, D.L.; Petasecca, M.; Lerch, M.L.; Rosenfeld, A.B.; Ng, K.H. Real-time eye lens dose monitoring during cerebral angiography procedures. Eur. Radiol. 2016, 26, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.; Chida, K.; Nakamura, M.; Toyoshima, H.; Terata, K.; Abe, Y. New real-time patient radiation dosimeter for use in radiofrequency catheter ablation. J. Radiat. Res. 2019, 60, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Inaba, Y.; Chida, K.; Murabayashi, Y.; Endo, M.; Otomo, K.; Zuguchi, M. An initial investigation of a wireless patient radiation dosimeter for use in interventional radiology. Radiol. Phys. Technol. 2020, 13, 321–326. [Google Scholar] [CrossRef]
- Sato, F.; Honda, T.; Haga, Y.; Inaba, Y.; Kaga, Y.; Chida, K. Basic characteristic evaluation of the real-time model MOSFET dosimeter. Bull. Sch. Health Sci. Tohoku Univ. 2017, 26, 57–65. [Google Scholar]
- Rana, V.K.; Rudin, S.; Bednarek, D.R. A tracking system to calculate patient skin dose in real-time during neurointerventional procedures using a biplane X-ray imaging system. Med. Phys. 2016, 43, 5131. [Google Scholar] [CrossRef]
- Takata, T.; Kotoku, J.; Maejima, H.; Kumagai, S.; Arai, N.; Kobayashi, T.; Shiraishi, K.; Yamamoto, M.; Kondo, H.; Furui, S. Fast skin dose estimation system for interventional radiology. J. Radiat. Res. 2018, 59, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bor, D.; Sancak, T.; Olgar, T.; Elcim, Y.; Adanali, A.; Sanlıdilek, U.; Akyar, S. Comparison of effective doses obtained from dose–area product and air kerma measurements in interventional radiology. Br. J. Radiol. 2004, 77, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Farah, J.; Trianni, A.; Ciraj-Bjelac, O.; Clairand, I.; De Angelis, C.; Delle Canne, S.; Hadid, L.; Huet, C.; Jarvinen, H.; Negri, A. Characterization of XR-RV3 GafChromic® films in standard laboratory and in clinical conditions and means to evaluate uncertainties and reduce errors. Med. Phys. 2015, 42, 4211–4226. [Google Scholar] [CrossRef]
- Habib Geryes, B.; Hadid-Beurrier, L.; Waryn, M.J.; Jean-Pierre, A.; Farah, J. Benchmarking the DACS-integrated Radiation Dose Monitor(R) skin dose mapping software using XR-RV3 Gafchromic(R) films. Med. Phys. 2018, 45, 4683–4692. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.K.; Ensor, J.E.; Pasciak, A.S. How accurately can the peak skin dose in fluoroscopy be determined using indirect dose metrics? Med. Phys. 2014, 41, 071913. [Google Scholar] [CrossRef] [Green Version]
- Khoury, H.; Garzon, W.; Andrade, G.; Lunelli, N.; Kramer, R.; de Barros, V.; Huda, A. Radiation exposure to patients and medical staff in hepatic chemoembolisation interventional procedures in Recife, Brazil. Radiat. Prot. Dosim. 2015, 165, 263–267. [Google Scholar] [CrossRef]
- Krajinović, M.; Dobrić, M.; Ciraj-Bjelac, O. Skin dose mapping in interventional cardiology: A practical solution. Radiat. Prot. Dosim. 2020, 188, 508–515. [Google Scholar] [CrossRef]
- Bor, D.; Olgar, T.; Toklu, T.; Caglan, A.; Onal, E.; Padovani, R. Patient doses and dosimetric evaluations in interventional cardiology. Phys. Med. 2009, 25, 31–42. [Google Scholar] [CrossRef]
- Nishizawa, K.; Moritake, T.; Matsumaru, Y.; Tsuboi, K.; Iwai, K. Dose measurement for patients and physicians using a glass dosemeter during endovascular treatment for brain disease. Radiat. Prot. Dosim. 2003, 107, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Moritake, T.; Matsumaru, Y.; Takigawa, T.; Nishizawa, K.; Matsumura, A.; Tsuboi, K. Dose measurement on both patients and operators during neurointerventional procedures using photoluminescence glass dosimeters. Am. J. Neuroradiol. 2008, 29, 1910–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moritake, T.; Hayakawa, M.; Matsumaru, Y.; Takigawa, T.; Koguchi, Y.; Miyamoto, Y.; Mizuno, Y.; Chida, K.; Akahane, K.; Tsuboi, K.; et al. Precise mapping system of entrance skin dose during endovascular embolization for cerebral aneurysm. Radiat. Meas. 2011, 46, 2103–2106. [Google Scholar] [CrossRef]
- Kawauchi, S.; Chida, K.; Moritake, T.; Matsumaru, Y.; Hamada, Y.; Sakuma, H.; Yoda, S.; Sun, L.; Sato, M.; Tsuruta, W. Estimation of Patient Lens Dose Associated with C-Arm Cone-Beam Computed Tomography Usage during Interventional Neuroradiology. Radiat. Prot. Dosim. 2018, 184, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, S.; Chida, K.; Moritake, T.; Hamada, Y.; Matsumaru, Y.; Tsuruta, W.; Sato, M.; Hosoo, H.; Sun, L. Treatment of Internal Carotid Aneurysms Using Pipeline Embolization Devices: Measuring the Radiation Dose of the Patient and Determining the Factors Affecting It. Radiat. Prot. Dosim. 2020. [Google Scholar] [CrossRef] [PubMed]
- IEC 60601-2-43, e. Medical Electrical Equipment Part 2-43, 2nd ed.; Particular Requirements for the Basic Safety and Essential Performance of X-Ray Equipment for Interventional Procedures; International Electrotechnical Commission: Geneva, Switzerland, 2010. [Google Scholar]
- Hubbell, J.H.; Seltzer, S.M. Tables of X-Ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients from 1 keV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest. Physical Reference Data (NISTIR 5632); National Institute of Standards and Technology: Gaithersburg, MD, USA, 1995.
- ICRP. Safety in Medicine; International Commission for Radiological Protection (ICRP) Publication 73; Pergamon Press: Oxford, UK; New York, NY, USA, 1996. [Google Scholar]
- ICRP. Diagnostic Reference Levels in Medical Imaging. Ann. Icrp 2017, 1–144. [Google Scholar]
- ICRP. ICRP Publication 105. Radiation protection in medicine. Ann. Icrp 2007, 37, 1–63. [Google Scholar] [CrossRef]
- Directive, C. 97/43/Euratom of 30 June 1997 on health protection of individuals against the dangers of ionizing radiation in relation to medical exposure, and repealing Directive 84/466/Euratom. Off. J. Eur. Communities L 1997, 180, 7. [Google Scholar]
- ACR. ACR–AAPM–SPR Practice Parameter for Diagnostic Reference Levels and Achievable Doses In medical X-Ray Imaging; ACR: Hong Kong, China, 2018. [Google Scholar]
- J-RIME. Diagnostic Reference Levels Based on Latest Surveys in Japan–Japan DRLs 2015. Available online: http://www.radher.jp/J-RIME/report/DRLhoukokusyoEng.pdf (accessed on 22 December 2020).
- J-RIME. Diagnostic Reference Levels Based on Latest Surveys in Japan–Japan DRLs 2020. Available online: http://www.radher.jp/J-RIME/report/JapanDRL2020_jp.pdf (accessed on 22 December 2020).
- Vano, E.; Fernandez Soto, J. Patient dose management in digital radiography. Biomed. Imaging Interv. J. 2007, 3, e26. [Google Scholar] [CrossRef]
- Vano, E.; Ten, J.I.; Fernandez, J.M.; Prieto, C.; Ordiales, J.M.; Martinez, D. Quality control and patient dosimetry in digital radiology. On line system: New features and transportability. Radiat. Prot. Dosim. 2008, 129, 144–146. [Google Scholar] [CrossRef]
- Ten, J.I.; Fernandez, J.M.; Vano, E. Automatic management system for dose parameters in interventional radiology and cardiology. Radiat. Prot. Dosim. 2011, 147, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Khodadadegan, Y.; Zhang, M.; Pavlicek, W.; Paden, R.G.; Chong, B.; Schueler, B.A.; Fetterly, K.A.; Langer, S.G.; Wu, T. Automatic monitoring of localized skin dose with fluoroscopic and interventional procedures. J. Digit. Imaging 2011, 24, 626–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, L.; Dixon, M.; Rowles, N.; Stevens, G. A practical method for skin dose estimation in interventional cardiology based on fluorographic DICOM information. Radiat. Prot. Dosim. 2015, 168, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.; Bujila, R.; Fransson, A.; Andreo, P.; Poludniowski, G. A framework for organ dose estimation in X-ray angiography and interventional radiology based on dose-related data in DICOM structured reports. Phys. Med. Biol. 2016, 61, 3063–3083. [Google Scholar] [CrossRef] [PubMed]
- Vano, E.; Ten, J.I.; Fernandez-Soto, J.M.; Sanchez-Casanueva, R.M. Experience with patient dosimetry and quality control online for diagnostic and interventional radiology using DICOM services. Am. J. Roentgenol. 2013, 200, 783–790. [Google Scholar] [CrossRef]



| Acquisition No. | No. of Frames | Fluoroscopy /Exposure | LAO /RAO (Degree) | CAUD /CRAN (Degree) | Tube Voltage (kV) | Tube Current (mA) | Pulse Width (ms) | SID (mm) | Ka,r (mGy) | Collimated Field Size (m2) | Pulse Rate (Pulse/s) | Fluoroscopy Time (s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 819 | Fluoroscopy | LAO 0 | CRAN 0 | 76 | 6.9 | 66.7 | 976 | 7.71 | 0.023 | 15 | 54.6 |
| 2 | 28 | Fluoroscopy | RAO 30 | CRAN 0 | 82 | 7.9 | 66.7 | 1041 | 0.34 | 0.020 | 15 | 1.9 |
| 3 | 11 | Exposure | RAO 30 | CRAN 0 | 71 | 654.0 | 41.4 | 1005 | 37.39 | 0.021 | NA | − |
| 4 | 195 | Fluoroscopy | RAO 30 | CRAN 0 | 82 | 7.9 | 66.7 | 1005 | 2.63 | 0.021 | 15 | 13.0 |
| 5 | 1938 | Fluoroscopy | RAO 30 | CRAN 0 | 82 | 7.9 | 66.7 | 1005 | 26.04 | 0.021 | 15 | 129.2 |
| 6 | 43 | Fluoroscopy | LAO 0 | CRAN 20 | 84 | 8.2 | 66.7 | 950 | 0.68 | 0.018 | 15 | 2.9 |
| 7 | 104 | Fluoroscopy | LAO 0 | CRAN 20 | 83 | 8.1 | 66.7 | 919 | 1.54 | 0.019 | 15 | 6.9 |
| 8 | 14 | Fluoroscopy | LAO 0 | CRAN 25 | 83 | 8.1 | 66.7 | 904 | 0.19 | 0.020 | 15 | 1.0 |
| 9 | 28 | Exposure | LAO 0 | CRAN 25 | 70 | 642.0 | 39.0 | 904 | 86.06 | 0.020 | NA | − |
| 10 | 329 | Fluoroscopy | LAO 0 | CRAN 0 | 78 | 7.2 | 66.7 | 957 | 3.56 | 0.018 | 15 | 21.9 |
| ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ |
| ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ |
| ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ |
| 61 | 30 | Fluoroscopy | LAO 0 | CRAN 0 | 76 | 6.7 | 66.7 | 1016 | 3.02 | 0.021 | 15 | 2.0 |
| 62 | 767 | Fluoroscopy | LAO 0 | CRAN 0 | 81 | 7.8 | 66.7 | 1016 | 9.89 | 0.021 | 15 | 51.1 |
| Test Data Set | Validation Data Set | p-Value * | |
|---|---|---|---|
| Period of investigation | October 2015–July 2016 | August 2016–September 2017 | |
| Number of cases (NIR) | 50 (7) | 50 (7) | |
| Men | 20 (40%) | 24 (48%) | |
| Women | 30 (60%) | 26 (52%) | |
| Age, y [range] | 57.5 ± 13.9 [30–78] | 62.8 ± 14.4 [33–88] | N.S. |
| BMI, kg·m−2 [range] | 22.5 ± 3.1 [16.8–32.4] | −23.5 ± 3.8 [16.9–33.4] | N.S. |
| Dskin,max,RPLGD, mGy | 552.4 ± 250.3 | 457.9 ± 353.6 | N.S. |
| Total Ka,r, mGy | 951.2 ± 398.2 | 807.8 ± 548.5 | N.S. |
| Fluoroscopy Ka,r, mGy | 117.3 ± 151.8 | 124.5 ± 185.5 | N.S. |
| Exposure Ka,r, mGy | 833.9 ± 322.7 | 683.3 ± 397.5 | p = 0.04 |
| Fluoroscopy Time, min | 11.9 ± 10.1 | 12.7 ± 14.4 | N.S. |
| Number of DSA | 12.7 ± 5.4 | 11.3 ± 8.8 | N.S. |
| Number of Frames | 322.8 ± 100.5 | 297.8 ± 155.9 | N.S. |
| Case No. | Fluoroscopy | Exposure | Ka,r Ratio | CFRPLGD,weighted | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tube Voltage * (kV) | Effective Energy ** (keV) | CFRPLGD *** (a) | Tube Voltage * (kV) | Effective Energy ** (keV) | CFRPLGD *** (b) | Fluoroscopy Ka,r/Total Ka,r (c) | Exposure Ka,r/Total Ka,r (d) | ||
| 1 | 79.3 | 51.8 | 0.302 | 69.4 | 29.1 | 0.269 | 0.074 | 0.926 | 0.271 |
| 2 | 74.9 | 50.5 | 0.295 | 68.6 | 28.9 | 0.269 | 0.074 | 0.926 | 0.271 |
| 3 | 83.8 | 53.1 | 0.311 | 71.0 | 29.4 | 0.268 | 0.278 | 0.722 | 0.280 |
| ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ |
| ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ |
| ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ |
| 48 | 73.2 | 50.0 | 0.292 | 68.0 | 28.8 | 0.270 | 0.087 | 0.913 | 0.272 |
| 49 | 74.8 | 50.5 | 0.295 | 68.3 | 28.8 | 0.270 | 0.139 | 0.861 | 0.273 |
| 50 | 74.3 | 50.3 | 0.294 | 68.4 | 28.8 | 0.269 | 0.046 | 0.954 | 0.271 |
| Mean | 76.8 | 51.1 | 0.298 | 70.0 | 29.2 | 0.268 | 0.112 | 0.888 | 0.272 |
| S.D. | 3.4 | 1.0 | 0.006 | 2.0 | 0.4 | 0.001 | 0.091 | 0.091 | 0.004 |
| Range | 69.7—87.3 | 49.0—54.2 | 0.287—0.318 | 67.5—76.2 | 28.7—30.5 | 0.265—0.270 | 0.034—0.378 | 0.622—0.966 | 0.267—0.284 |
| Case No. | Fluoroscopy | Exposure | Ka,r Ratio | CFRPLGD,weighted | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tube Voltage * (kV) | Effective Energy ** (keV) | CFRPLGD *** (a) | Tube Voltage * (kV) | Effective Energy ** (keV) | CFRPLGD *** (b) | Fluoroscopy Ka,r/Total Ka,r (c) | Exposure Ka,r/Total Ka,r (d) | ||
| 51 | 76.7 | 51.0 | 0.298 | 69.3 | 29.0 | 0.269 | 0.055 | 0.945 | 0.270 |
| 52 | 80.0 | 52.0 | 0.303 | 70.0 | 29.2 | 0.268 | 0.075 | 0.925 | 0.271 |
| 53 | 77.6 | 51.3 | 0.299 | 68.8 | 28.9 | 0.269 | 0.136 | 0.864 | 0.273 |
| ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ |
| ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ |
| ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ | ∙ |
| 98 | 73.1 | 50.0 | 0.292 | 67.9 | 28.7 | 0.270 | 0.120 | 0.880 | 0.272 |
| 99 | 74.8 | 50.5 | 0.295 | 70.1 | 29.2 | 0.268 | 0.097 | 0.903 | 0.271 |
| 100 | 75.8 | 50.7 | 0.296 | 69.2 | 29.0 | 0.269 | 0.292 | 0.708 | 0.277 |
| Mean | 77.3 | 51.2 | 0.299 | 69.3 | 29.0 | 0.269 | 0.124 | 0.876 | 0.273 |
| S.D. | 2.9 | 0.8 | 0.005 | 1.5 | 0.3 | 0.001 | 0.090 | 0.090 | 0.004 |
| Range | 73.1—85.9 | 50.0—53.7 | 0.292—0.315 | 67.7—78.4 | 28.7—31.0 | 0.264—0.270 | 0.039—0.416 | 0.584—0.961 | 0.270—0.287 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morota, K.; Moritake, T.; Nagamoto, K.; Matsuzaki, S.; Nakagami, K.; Sun, L.; Kunugita, N. Optimization of the Maximum Skin Dose Measurement Technique Using Digital Imaging and Communication in Medicine—Radiation Dose Structured Report Data for Patients Undergoing Cerebral Angiography. Diagnostics 2021, 11, 14. https://doi.org/10.3390/diagnostics11010014
Morota K, Moritake T, Nagamoto K, Matsuzaki S, Nakagami K, Sun L, Kunugita N. Optimization of the Maximum Skin Dose Measurement Technique Using Digital Imaging and Communication in Medicine—Radiation Dose Structured Report Data for Patients Undergoing Cerebral Angiography. Diagnostics. 2021; 11(1):14. https://doi.org/10.3390/diagnostics11010014
Chicago/Turabian StyleMorota, Koichi, Takashi Moritake, Keisuke Nagamoto, Satoru Matsuzaki, Koichi Nakagami, Lue Sun, and Naoki Kunugita. 2021. "Optimization of the Maximum Skin Dose Measurement Technique Using Digital Imaging and Communication in Medicine—Radiation Dose Structured Report Data for Patients Undergoing Cerebral Angiography" Diagnostics 11, no. 1: 14. https://doi.org/10.3390/diagnostics11010014
APA StyleMorota, K., Moritake, T., Nagamoto, K., Matsuzaki, S., Nakagami, K., Sun, L., & Kunugita, N. (2021). Optimization of the Maximum Skin Dose Measurement Technique Using Digital Imaging and Communication in Medicine—Radiation Dose Structured Report Data for Patients Undergoing Cerebral Angiography. Diagnostics, 11(1), 14. https://doi.org/10.3390/diagnostics11010014





