In Vivo Measurement of Neurochemical Abnormalities in the Hippocampus in a Rat Model of Cuprizone-Induced Demyelination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Cuprizone Intoxication
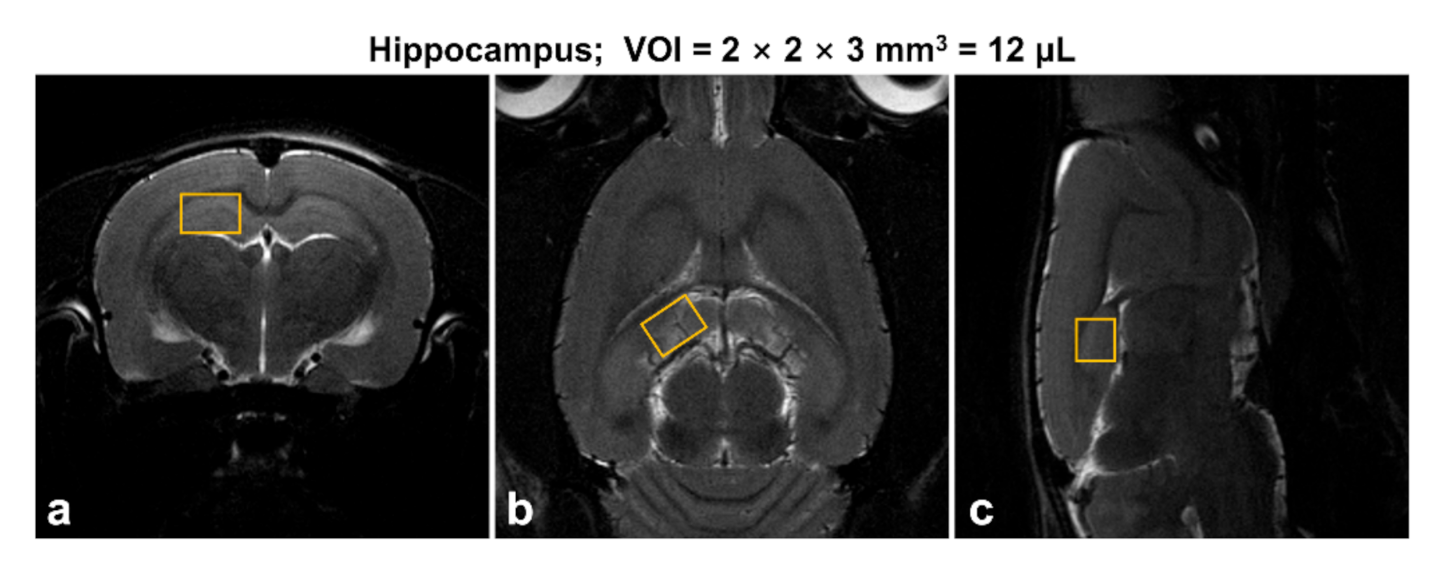
2.2. In Vivo 1H MR Spectroscopy
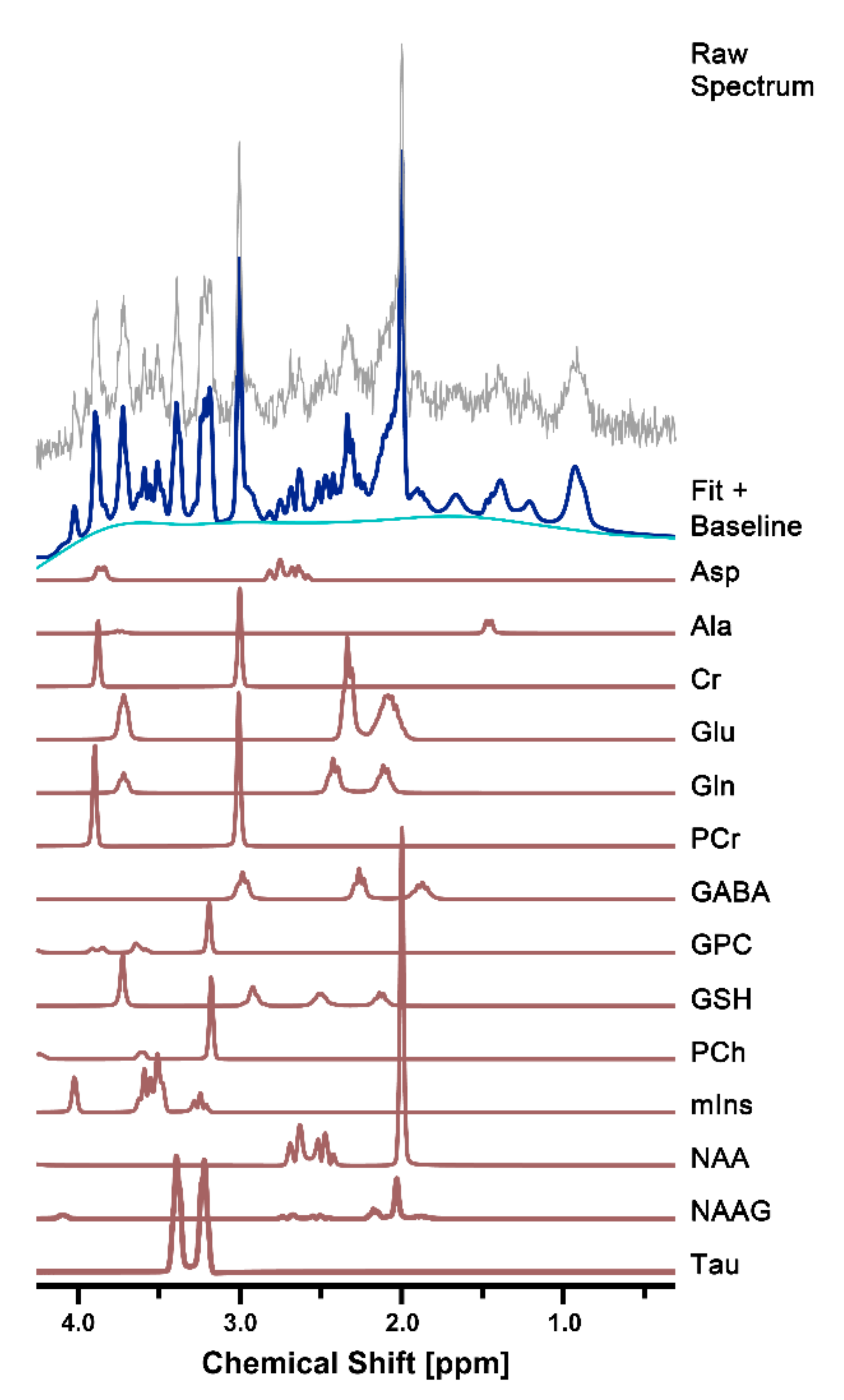
2.3. Spectral Quantification
2.4. Statistical Analysis
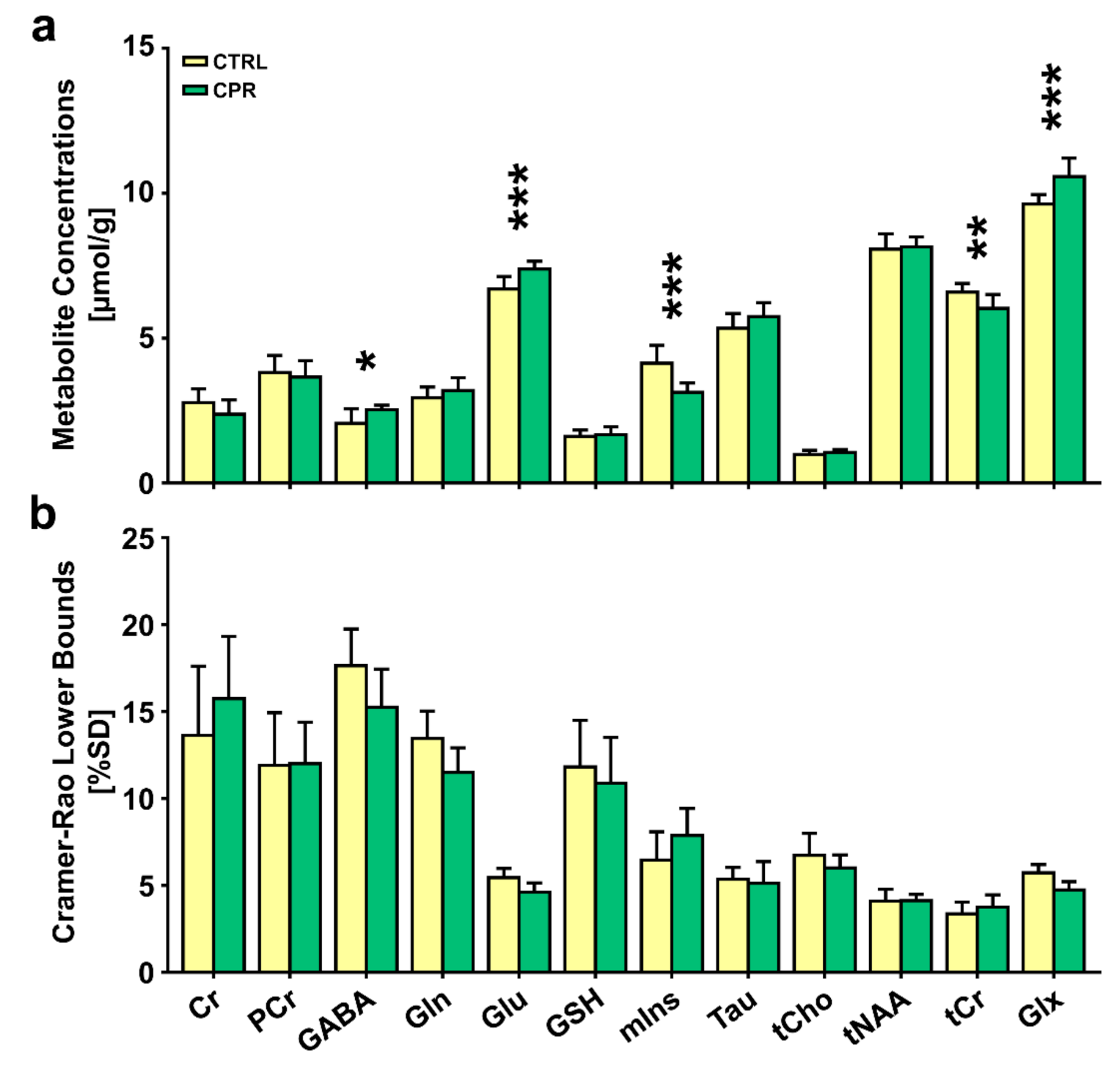
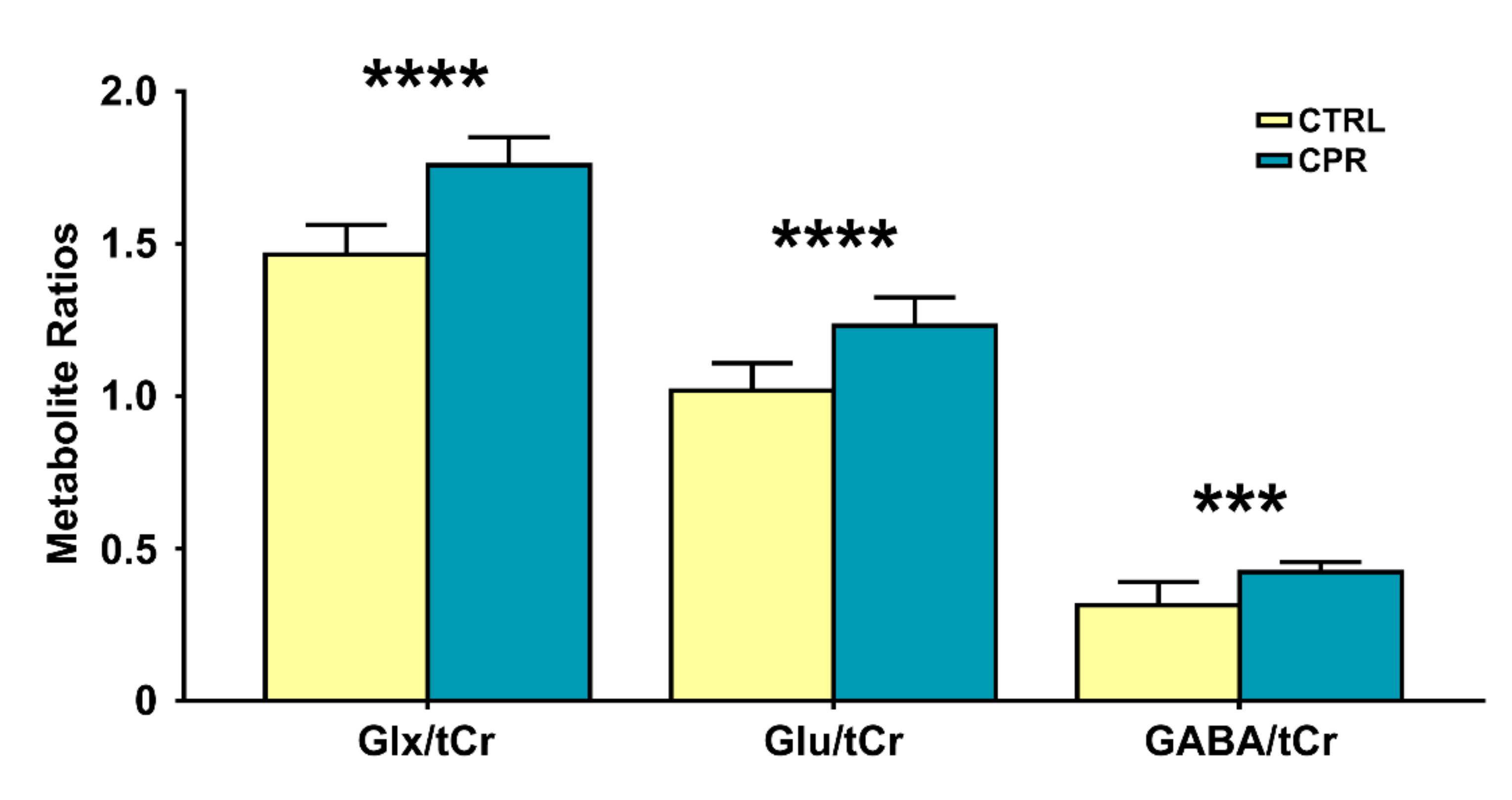
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Koutsoudaki, P.N.; Skripuletz, T.; Gudi, V.; Moharregh-Khiabani, D.; Hildebrandt, H.; Trebst, C.; Stangel, M. Demyelination of the hippocampus is prominent in the cuprizone model. Neurosci. Lett. 2009, 451, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, D.; Dukes, S.; Patel, R.; Nicholas, R.; Vora, A.; Reynolds, R. Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathol. 2009, 19, 238–253. [Google Scholar] [CrossRef]
- Varga, E.; Pandur, E.; Abrahám, H.; Horváth, A.; Ács, P.; Komoly, S.; Miseta, A.; Sipos, K. Cuprizone Administration Alters the Iron Metabolism in the Mouse Model of Multiple Sclerosis. Cell. Mol. Neurobiol. 2018, 38, 1081–1097. [Google Scholar] [CrossRef] [PubMed]
- Mahad, D.H.; Trapp, B.D.; Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015, 14, 183–193. [Google Scholar] [CrossRef]
- Chiaravalloti, N.D.; DeLuca, J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008, 7, 1139–1151. [Google Scholar] [CrossRef]
- Ohgomori, T.; Jinno, S. Cuprizone-induced demyelination in the mouse hippocampus is alleviated by phytoestrogen genistein. Toxicol. Appl. Pharmacol. 2019, 363, 98–110. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, N.; Zhang, R.; Jin, L.; Petridis, A.K.; Loers, G.; Zheng, X.; Wang, Z.; Siebert, H.C. Cuprizone-Induced Demyelination in Mouse Hippocampus Is Alleviated by Ketogenic Diet. J. Agric. Food Chem. 2020, 68, 11215–11228. [Google Scholar] [CrossRef]
- Kim, W.; Hahn, K.R.; Jung, H.Y.; Kwon, H.J.; Nam, S.M.; Kim, J.W.; Park, J.H.; Yoo, D.Y.; Kim, D.W.; Won, M.H.; et al. Melatonin ameliorates cuprizone-induced reduction of hippocampal neurogenesis, brain-derived neurotrophic factor, and phosphorylation of cyclic AMP response element-binding protein in the mouse dentate gyrus. Brain Behav. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Klein, B.; Mrowetz, H.; Barker, C.M.; Lange, S.; Rivera, F.J.; Aigner, L. Age Influences Microglial Activation After Cuprizone-Induced Demyelination. Front. Aging Neurosci. 2018, 10, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Denic, A.; Johnson, A.J.; Bieber, A.J.; Warrington, A.E.; Rodriguez, M.; Pirko, I. The relevance of animal models in multiple sclerosis research. Pathophysiology 2011, 18, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Bénardais, K.; Kotsiari, A.; Škuljec, J.; Koutsoudaki, P.N.; Gudi, V.; Singh, V.; Vulinović, F.; Skripuletz, T.; Stangel, M. Cuprizone [bis(cyclohexylidenehydrazide)] is selectively toxic for mature oligodendrocytes. Neurotox. Res. 2013, 24, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, G.K.; Morell, P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001, 11, 107–116. [Google Scholar] [CrossRef]
- Orije, J.; Kara, F.; Guglielmetti, C.; Praet, J.; Van der Linden, A.; Ponsaerts, P.; Verhoye, M. Longitudinal monitoring of metabolic alterations in cuprizone mouse model of multiple sclerosis using 1H-magnetic resonance spectroscopy. Neuroimage 2015, 114, 128–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laule, C.; Vavasour, I.M.; Kolind, S.H.; Li, D.K.B.; Traboulsee, T.L.; Moore, G.R.W.; MacKay, A.L. Magnetic resonance imaging of myelin. Neurotherapeutics 2007, 4, 460–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tkáč, I.; Gruetter, R. Methodology of 1H NMR spectroscopy of the human brain at very high magnetic fields. Appl. Magn. Reson. 2005, 29, 139–157. [Google Scholar] [CrossRef]
- Tkáč, I.; Henry, P.G.; Andersen, P.; Keene, C.D.; Low, W.C.; Gruetter, R. Highly resolved in vivo 1H NMR spectroscopy of the mouse brain at 9.4 T. Magn. Reson. Med. 2004, 52, 478–484. [Google Scholar] [CrossRef]
- Lee, D.W.; Chung, S.; Yoo, H.J.; Kim, S.J.; Woo, C.W.; Kim, S.T.; Lee, D.H.; Kim, K.W.; Kim, J.K.; Lee, J.S.; et al. Neurochemical changes associated with stress-induced sleep disturbance in rats: In vivo and in vitro measurements. PLoS ONE 2016, 11, 1–17. [Google Scholar] [CrossRef]
- Wang, A.M.; Pradhan, S.; Coughlin, J.M.; Trivedi, A.; Dubois, S.L.; Crawford, J.L.; Sedlak, T.W.; Nucifora, F.C.; Nestadt, G.; Nucifora, L.G.; et al. Assessing Brain Metabolism with 7 T Proton Magnetic Resonance Spectroscopy in Patients with First-Episode Psychosis. JAMA Psychiatry 2019, 76, 314–323. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, D.W.; Kwon, J.I.; Kim, S.T.; Woo, C.W.; Kon Kim, J.; Won Kim, K.; Seong Lee, J.; Gon Choi, C.; Suh, J.Y.; et al. Changes to gamma-aminobutyric acid levels during short-term epileptiform activity in a kainic acid-induced rat model of status epilepticus: A chemical exchange saturation transfer imaging study. Brain Res. 2019, 1717, 176–181. [Google Scholar] [CrossRef]
- Jansen, J.F.A.; Backes, W.H.; Nicolay, K.; Kooi, M.E. 1H MR spectroscopy of the brain: Absolute quantification of metabolites. Radiology 2006, 240, 318–332. [Google Scholar] [CrossRef]
- Pfeuffer, J.; Tkáč, I.; Provencher, S.W.; Gruetter, R. Toward an in vivo Neurochemical Profile: Quantification of 18 Metabolites in Short-Echo-Time 1H NMR Spectra of the Rat Brain. J. Magn. Reson. 1999, 141, 104–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.H.; Woo, C.W.; Kwon, J.I.; Chae, Y.J.; Ham, S.J.; Suh, J.Y.; Kim, S.T.; Kim, J.K.; Kim, K.W.; Woo, D.C.; et al. Cerebral mapping of glutamate using chemical exchange saturation transfer imaging in a rat model of stress-induced sleep disturbance at 7.0T. J. Magn. Reson. Imaging 2019, 50, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Geurts, J.J.G.; Bö, L.; Roosendaal, S.D.; Hazes, T.; Daniëls, R.; Barkhof, F.; Witter, M.P.; Huitinga, I.; Van Der Valk, P. Extensive hippocampal demyelination in multiple sclerosis. J. Neuropathol. Exp. Neurol. 2007, 66, 819–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norkute, A.; Hieble, A.; Braun, A.; Johann, S.; Clarner, T.; Baumgartner, W.; Beyer, C.; Kipp, M. Cuprizone treatment induces demyelination and astrocytosis in the mouse hippocampus. J. Neurosci. Res. 2009, 87, 1343–1355. [Google Scholar] [CrossRef]
- De Stefano, N.; Bartolozzi, M.L.; Guidi, L.; Stromillo, M.L.; Federico, A. Magnetic resonance spectroscopy as a measure of brain damage in multiple sclerosis. J. Neurol. Sci. 2005, 233, 203–208. [Google Scholar] [CrossRef]
- De Stefano, N.; Filippi, M. MR spectroscopy in multiple sclerosis. J. Neuroimaging 2007, 17, 31–35. [Google Scholar] [CrossRef]
- Srinivasan, R.; Sailasuta, N.; Hurd, R.; Nelson, S.; Pelletier, D. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain 2005, 128, 1016–1025. [Google Scholar] [CrossRef] [Green Version]
- Taraboletti, A.; Walker, T.; Avila, R.; Huang, H.; Caporoso, J.; Manandhar, E.; Leeper, T.C.; Modarelli, D.A.; Medicetty, S.; Shriver, L.P. Cuprizone Intoxication Induces Cell Intrinsic Alterations in Oligodendrocyte Metabolism Independent of Copper Chelation. Biochemistry 2017, 56, 1518–1528. [Google Scholar] [CrossRef]
- Messori, L.; Casini, A.; Gabbiani, C.; Sorace, L.; Muniz-Miranda, M.; Zatta, P. Unravelling the chemical nature of copper cuprizone. Dalt. Trans. 2007, 2112–2114. [Google Scholar] [CrossRef]
- Buschmann, J.P.; Berger, K.; Awad, H.; Clarner, T.; Beyer, C.; Kipp, M. Inflammatory response and chemokine expression in the white matter corpus callosum and gray matter cortex region during cuprizone-induced demyelination. J. Mol. Neurosci. 2012, 48, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Piani, D.; Frei, K.; Do, K.Q.; Cuénod, M.; Fontana, A. Murine brain macrophages induce NMDA receptor mediated neurotoxicity in vitro by secreting glutamate. Neurosci. Lett. 1991, 133, 159–162. [Google Scholar] [CrossRef]
- Swanberg, K.M.; Landheer, K.; Pitt, D.; Juchem, C. Quantifying the Metabolic Signature of Multiple Sclerosis by in vivo Proton Magnetic Resonance Spectroscopy: Current Challenges and Future Outlook in the Translation from Proton Signal to Diagnostic Biomarker. Front. Neurol. 2019, 10, 1173. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Edden, R.A.E.; Gao, F.; Li, H.; Gong, T.; Chen, W.; Liu, X.; Wang, G.; Zhao, B. Reduced GABA levels correlate with cognitive impairment in patients with relapsing-remitting multiple sclerosis. Eur. Radiol. 2018, 28, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Meknatkhah, S.; Dashti, P.S.; Raminfard, S.; Rad, H.S.; Mousavi, M.-S.; Riazi, G.H. The Changes in 1H-MRS Metabolites in Cuprizone-Induced Model of Multiple Sclerosis: Effects of Prior Psychological Stress. J. Mol. Neurosci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Praet, J.; Orije, J.; Kara, F.; Guglielmetti, C.; Santermans, E.; Daans, J.; Hens, N.; Verhoye, M.; Berneman, Z.; Ponsaerts, P.; et al. Cuprizone-induced demyelination and demyelination-associated inflammation result in different proton magnetic resonance metabolite spectra. NMR Biomed. 2015, 28, 505–513. [Google Scholar] [CrossRef]
- Cawley, N.; Solanky, B.S.; Muhlert, N.; Tur, C.; Edden, R.A.E.; Wheeler-Kingshott, C.A.M.; Miller, D.H.; Thompson, A.J.; Ciccarelli, O. Reduced gamma-aminobutyric acid concentration is associated with physical disability in progressive multiple sclerosis. Brain 2015, 138, 2584–2595. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Yin, X.; Edden, R.A.E.; Evans, A.C.; Xu, J.; Cao, G.; Li, H.; Li, M.; Zhao, B.; Wang, J.; et al. Altered hippocampal GABA and glutamate levels and uncoupling from functional connectivity in multiple sclerosis. Hippocampus 2018, 28, 813–823. [Google Scholar] [CrossRef]
- Nantes, J.C.; Proulx, S.; Zhong, J.; Holmes, S.A.; Narayanan, S.; Brown, R.A.; Hoge, R.D.; Koski, L. GABA and glutamate levels correlate with MTR and clinical disability: Insights from multiple sclerosis. Neuroimage 2017, 157, 705–715. [Google Scholar] [CrossRef]
- Bhattacharyya, P.K.; Phillips, M.D.; Stone, L.A.; Bermel, R.A.; Lowe, M.J. Sensorimotor cortex gamma-aminobutyric acid concentration correlates with impaired performance in patients with MS. Am. J. Neuroradiol. 2013, 34, 1733–1739. [Google Scholar] [CrossRef] [Green Version]
- Bhat, R.; Axtell, R.; Mitra, A.; Miranda, M.; Lock, C.; Tsien, R.W.; Steinman, L. Inhibitory role for GABA in autoimmune inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 2580–2585. [Google Scholar] [CrossRef] [Green Version]
- Vélez-Fort, M.; Audinat, E.; Angulo, M.C. Central role of GABA in neuron-glia interactions. Neuroscientist 2012, 18, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Crowley, T.; Fitzpatrick, J.M.; Kuijper, T.; Cryan, J.F.; O’Toole, O.; O’Leary, O.F.; Downer, E.J. Modulation of TLR3/TLR4 inflammatory signaling by the GABAB receptor agonist baclofen in glia and immune cells: Relevance to therapeutic effects in multiple sclerosis. Front. Cell. Neurosci. 2015, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.; Mendu, S.K.; Birnir, B. GABA is an effective immunomodulatory molecule. Amino Acids 2013, 45, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Erecinska, M.; Silver, I.A. ATP and Brain Function. J. Cereb. Blood Flow Metab. 1989, 9, 2–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, C.G.; Bueno, A.R.F.; Schuck, P.F.; Leipnitz, G.; Ribeiro, C.A.J.; Rosa, R.B.; Dutra Filho, C.S.; Wyse, A.T.S.; Wannmacher, C.M.D.; Wajner, M. Inhibition of creatine kinase activity from rat cerebral cortex by D-2-hydroxyglutaric acid in vitro. Neurochem. Int. 2004, 44, 45–52. [Google Scholar] [CrossRef]
- Bessman, S. The Creatine-Creatine Phosphate Energy Shuttle. Annu. Rev. Biochem. 1985, 54, 831–862. [Google Scholar] [CrossRef] [PubMed]
- Dolder, M.; Walzel, B.; Speer, O.; Schlattner, U.; Wallimann, T. Inhibition of the mitochondrial permeability transition by creatine kinase substrates. Requirement for microcompartmentation. J. Biol. Chem. 2003, 278, 17760–17766. [Google Scholar] [CrossRef] [Green Version]
- Meyer, L.E.; Machado, L.B.; Santiago, A.P.S.A.; Da-Silva, W.S.; De Felice, F.G.; Holub, O.; Oliveira, M.F.; Galina, A. Mitochondrial creatine kinase activity prevents reactive oxygen species generation: Antioxidant role of mitochondrial kinase-dependent ADP re-cycling activity. J. Biol. Chem. 2006, 281, 37361–37371. [Google Scholar] [CrossRef] [Green Version]
- Xuan, Y.; Yan, G.; Wu, R.; Huang, Q.; Li, X.; Xu, H. The cuprizone-induced changes in 1H-MRS metabolites and oxidative parameters in C57BL/6 mouse brain: Effects of quetiapine. Neurochem. Int. 2015, 90, 185–192. [Google Scholar] [CrossRef]
- Ciccarelli, O.; Barkhof, F.; Bodini, B.; De Stefano, N.; Golay, X.; Nicolay, K.; Pelletier, D.; Pouwels, P.J.W.; Smith, S.A.; Wheeler-Kingshott, C.A.M.; et al. Pathogenesis of multiple sclerosis: Insights from molecular and metabolic imaging. Lancet Neurol. 2014, 13, 807–822. [Google Scholar] [CrossRef]
- Ciccarelli, O.; Thomas, D.L.; De Vita, E.; Wheeler-Kingshott, C.A.M.; Kachramanoglou, C.; Kapoor, R.; Leary, S.; Matthews, L.; Palace, J.; Chard, D.; et al. Low Myo-inositol indicating astrocytic damage in a case series of neuromyelitis optica. Ann. Neurol. 2013, 74, 301–305. [Google Scholar] [CrossRef]
- Llufriu, S.; Kornak, J.; Ratiney, H.; Oh, J.; Brenneman, D.; Cree, B.A.; Sampat, M.; Hauser, S.L.; Nelson, S.J.; Pelletier, D. Magnetic resonance spectroscopy markers of disease progression in multiple sclerosis. JAMA Neurol. 2014, 71, 840–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häussinger, D.; Laubenberger, J.; Vom Dahl, S.; Ernst, T.; Bayer, S.; Langer, M.; Gerok, W.; Hennig, J. Proton magnetic resonance spectroscopy studies on human brain Myo-inositol in hypo-osmolarity and hepatic encephalopathy. Gastroenterology 1994, 107, 1475–1480. [Google Scholar] [CrossRef] [Green Version]
- Fernando, K.T.M.; McLean, M.A.; Chard, D.T.; MacManus, D.G.; Dalton, C.M.; Miszkiel, K.A.; Gordon, R.M.; Plant, G.T.; Thompson, A.J.; Miller, D.H. Elevated white matter myo-inositol in clinically isolated syndromes suggestive of multiple sclerosis. Brain 2004, 127, 1361–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, K.A.; Nanga, R.P.R.; Das, S.; Chen, S.H.; Hadar, P.N.; Pollard, J.R.; Lucas, T.H.; Shinohara, R.T.; Litt, B.; Hariharan, H.; et al. Glutamate imaging (GluCEST) lateralizes epileptic foci in nonlesional temporal lobe epilepsy. Sci. Transl. Med. 2015, 7, 309ra161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haris, M.; Cai, K.; Singh, A.; Hariharan, H.; Reddy, R. In vivo mapping of brain myo-inositol. Neuroimage 2011, 54, 2079–2085. [Google Scholar] [CrossRef] [Green Version]
- Kogan, F.; Haris, M.; Debrosse, C.; Singh, A.; Nanga, R.P.; Cai, K.; Hariharan, H.; Reddy, R. In vivo chemical exchange saturation transfer imaging of creatine (CrCEST) in skeletal muscle at 3T. J. Magn. Reson. Imaging 2014, 40, 596–602. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.W.; Woo, C.W.; Woo, D.C.; Kim, J.K.; Kim, K.W.; Lee, D.H. Regional mapping of brain glutamate distributions using glutamate-weighted chemical exchange saturation transfer imaging. Diagnostics 2020, 10, 571. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-W.; Kwon, J.-I.; Woo, C.-W.; Heo, H.; Kim, K.W.; Woo, D.-C.; Kim, J.K.; Lee, D.-H. In Vivo Measurement of Neurochemical Abnormalities in the Hippocampus in a Rat Model of Cuprizone-Induced Demyelination. Diagnostics 2021, 11, 45. https://doi.org/10.3390/diagnostics11010045
Lee D-W, Kwon J-I, Woo C-W, Heo H, Kim KW, Woo D-C, Kim JK, Lee D-H. In Vivo Measurement of Neurochemical Abnormalities in the Hippocampus in a Rat Model of Cuprizone-Induced Demyelination. Diagnostics. 2021; 11(1):45. https://doi.org/10.3390/diagnostics11010045
Chicago/Turabian StyleLee, Do-Wan, Jae-Im Kwon, Chul-Woong Woo, Hwon Heo, Kyung Won Kim, Dong-Cheol Woo, Jeong Kon Kim, and Dong-Hoon Lee. 2021. "In Vivo Measurement of Neurochemical Abnormalities in the Hippocampus in a Rat Model of Cuprizone-Induced Demyelination" Diagnostics 11, no. 1: 45. https://doi.org/10.3390/diagnostics11010045




