A Paper-Based Analytical Device for Analysis of Paraquat in Urine and Its Validation with Optical-Based Approaches
Abstract
:1. Introduction
2. Methods
2.1. Chemicals
2.2. Paper-Based Analytical Device
2.3. Colorimetric Assay
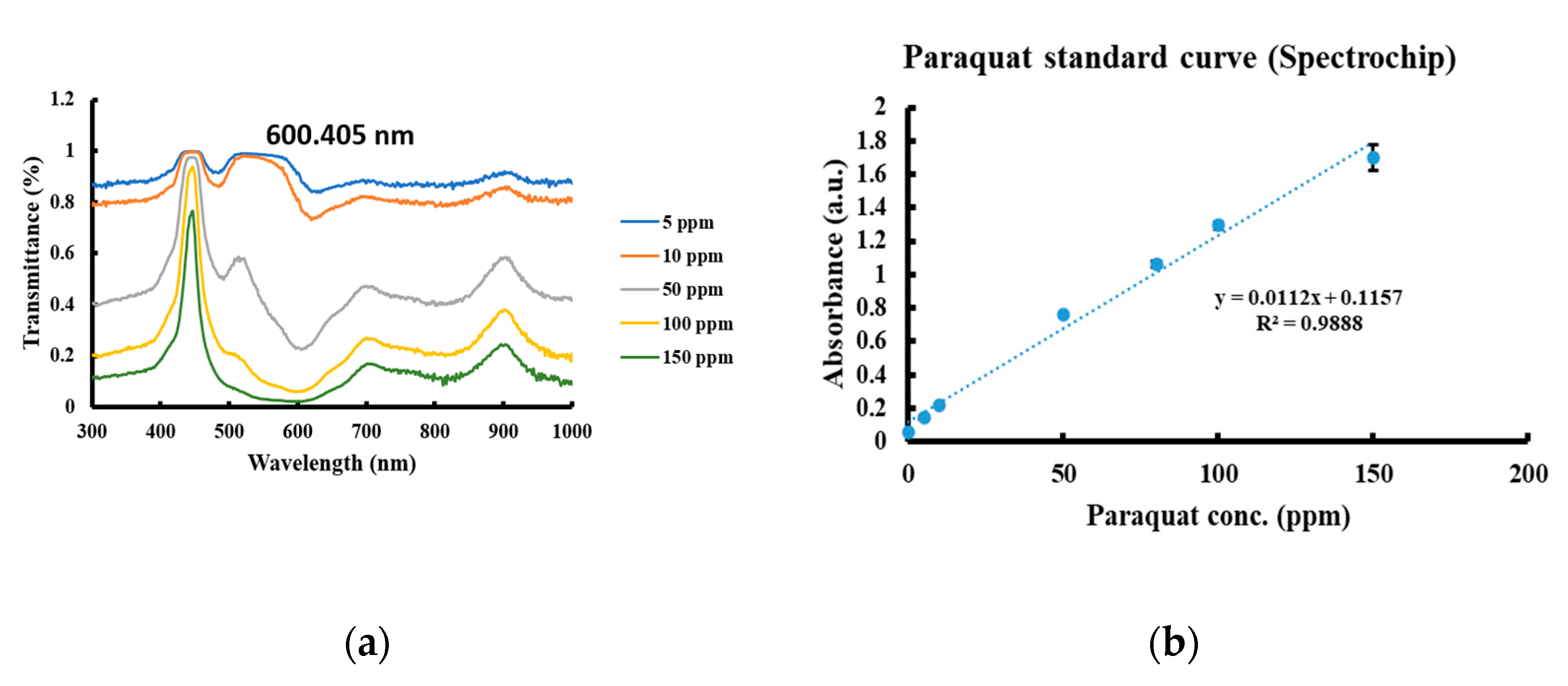
2.4. Spectrochip and NanoDrop
2.5. Statistical Analysis
2.6. Clinical Validation and Collection of Patient URINE Samples
3. Results
3.1. Paraquat Detection with the Paper-Based Analytical Device
3.2. Paraquat Detection with Spectrochip and NanoDrop
3.3. Comparison of Paraquat Detection via Different Methods Using Bland–Altman Analysis
3.4. Clinical Validation Using Six Patient Urine Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gil, H.W.; Hong, J.R.; Jang, S.H.; Hong, S.Y. Diagnostic and therapeutic approach for acute paraquat intoxication. J. Korean Med. Sci. 2014, 29, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.H.; Eddleston, M.; Senarathna, L.; Mohamed, F.; Gawarammana, I.; Bowe, S.J.; Manuweera, G.; Buckley, N.A. Acute human lethal toxicity of agricultural pesticides: A prospective cohort study. PLoS Med. 2010, 7, e1000357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.S.; Lu, T.H.; Eddleston, M.; Konradsen, F.; Sterne, J.A.; Lin, J.J.; Gunnell, D. Factors associated with the decline in suicide by pesticide poisoning in Taiwan: A time trend analysis, 1987–2010. Clin. Toxicol. (Phila) 2012, 50, 471–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.L. Mechanism of paraquat toxicity in lung and its relevance to treatment. Hum. Toxicol. 1987, 6, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Oliveira, R.J.; Duarte, J.A.; Sanchez-Navarro, A.; Remiao, F.; Bastos, M.L.; Carvalho, F. Paraquat poisonings: Mechanisms of lung toxicity, clinical features, and treatment. Crit. Rev. Toxicol. 2008, 38, 13–71. [Google Scholar] [CrossRef]
- Sawada, Y.; Yamamoto, I.; Hirokane, T.; Nagai, Y.; Satoh, Y.; Ueyama, M. Severity index of paraquat poisoning. Lancet 1988, 1, 1333. [Google Scholar] [CrossRef]
- Proudfoot, A.T.; Stewart, M.S.; Levitt, T.; Widdop, B. Paraquat poisoning: Significance of plasma-paraquat concentrations. Lancet 1979, 2, 330–332. [Google Scholar] [CrossRef]
- Scherrmann, J.M.; Houze, P.; Bismuth, C.; Bourdon, R. Prognostic value of plasma and urine paraquat concentration. Hum. Toxicol. 1987, 6, 91–93. [Google Scholar] [CrossRef]
- Liu, X.W.; Ma, T.; Li, L.L.; Qu, B.; Liu, Z. Predictive values of urine paraquat concentration, dose of poison, arterial blood lactate and APACHE II score in the prognosis of patients with acute paraquat poisoning. Exp. Med. 2017, 14, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Koo, J.R.; Yoon, J.W.; Han, S.J.; Choi, M.J.; Park, I.I.; Lee, Y.K.; Kim, S.G.; Oh, J.E.; Seo, J.W.; Kim, H.J.; et al. Rapid analysis of plasma paraquat using sodium dithionite as a predictor of outcome in acute paraquat poisoning. Am. J. Med. Sci. 2009, 338, 373–377. [Google Scholar] [CrossRef]
- Li, C.B.; Li, X.H.; Wang, Z.; Jiang, C.H.; Peng, A. Serum paraquat concentration detected by spectrophotometry in patients with paraquat poisoning. World J. Emerg. Med. 2011, 2, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. Engl. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Si, J.; Li, Z. Fabrication techniques for microfluidic paper-based analytical devices and their applications for biological testing: A review. Biosens. Bioelectron. 2016, 77, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.B.; Fernandes, S.C.; Rajaratnam, A.; DeChiara, N.S.; Mace, C.R. Measurement of the hematocrit using paper-based microfluidic devices. Lab Chip 2016, 16, 3689–3694. [Google Scholar] [CrossRef]
- Chinnadayyala, S.R.; Park, J.; Le, H.T.N.; Santhosh, M.; Kadam, A.N.; Cho, S. Recent advances in microfluidic paper-based electrochemiluminescence analytical devices for point-of-care testing applications. Biosens. Bioelectron. 2019, 126, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.M.; Lin, S.T.; Yen, T.H.; Wang, Y.L.; Cheng, C.M. Paper-based diagnostic devices for clinical paraquat poisoning diagnosis. Biomicrofluidics 2016, 10, 034118. [Google Scholar] [CrossRef]
- Chang, T.H.; Tung, K.H.; Gu, P.W.; Yen, T.H.; Cheng, C.M. Rapid simultaneous determination of paraquat and creatinine in human serum using a piece of paper. Micromachines 2018, 9, 586. [Google Scholar] [CrossRef] [Green Version]
- Kita, D.M.; Miranda, B.; Favela, D.; Bono, D.; Michon, J.; Lin, H.; Gu, T.; Hu, J. High-performance and scalable on-chip digital Fourier transform spectroscopy. Nat. Commun. 2018, 9, 4405. [Google Scholar] [CrossRef]
- Ko, C.-H.; Hong, C.; Chiu, W.-H. MEMS Based SpectroChip for Healthcare, Food Safety and Blockchain Applications; SPIE: Bellingham, DC, USA, 2019; Volume 10983. [Google Scholar]
- Hsu, C.W.; Lin, J.L.; Lin-Tan, D.T.; Chen, K.H.; Yen, T.H.; Wu, M.S.; Lin, S.C. Early hemoperfusion may improve survival of severely paraquat-poisoned patients. PLoS ONE 2012, 7, e48397. [Google Scholar] [CrossRef]
- Nasr Isfahani, S.; Farajzadegan, Z.; Sabzghabaee, A.M.; Rahimi, A.; Samasamshariat, S.; Eizadi-Mood, N. Does hemoperfusion in combination with other treatments reduce the mortality of patients with paraquat poisoning more than hemoperfusion alone: A systematic review with meta-analysis. J. Res. Med. Sci. 2019, 24, 2. [Google Scholar] [CrossRef]
- Hadland, S.E.; Levy, S. Objective Testing: Urine and Other Drug Tests. Child Adolesc. Psychiatr. Clin. N. Am. 2016, 25, 549–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Delta RGB * | 0 ppm | 5 ppm | 10 ppm | 25 ppm | 50 ppm | 100 ppm |
|---|---|---|---|---|---|---|
| Test 1 | 3.26457 | 6.07037 | 8.28258 | 42.7104 | 66.5518 | 111.381 |
| Test 2 | 5.25096 | 6.65480 | 20.7853 | 36.3824 | 69.7230 | 118.404 |
| Test 3 | 2.23013 | 9.92608 | 21.4377 | 42.8390 | 80.4217 | 114.011 |
| Average | 3.58189 | 7.55042 | 16.8352 | 40.6439 | 72.2322 | 114.599 |
| Standard deviation | 1.53521 | 2.07803 | 7.41396 | 3.69117 | 7.26746 | 3.54797 |
| Limit of detection: 3.01 ppm | ||||||
| Limit of quantification: 10.02 ppm | ||||||
| Patient Number | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Age | 24 | 26 | 49 | 63 | 94 | 76 |
| Sex | Male | Male | Male | Male | Male | Male |
| Time elapsed between paraquat ingestion and hospital arrival, (h) | 1.5 | 2.0 | 4.0 | 5.0 | 3.0 | 3.0 |
| Blood paraquat level, (ppm) | 26.4 | 142.4 | >8.0 | 4.9 | >10.0 | >10.0 |
| SIPP, (ppm h) | 39.6 | 284.8 | >32.0 | 24.5 | >30.0 | >30.0 |
| Treatment | Charcoal hemoperfusion, glucocorticoid/cyclophosphamide pulse therapies | Charcoal hemoperfusion | Death before treatment | Charcoal hemoperfusion, glucocorticoid/cyclophosphamide pulse therapies | Death before treatment | Death before treatment |
| Duration of hospitalization, (day) | 2 | 0.5 | 0.1 | 2 | 0.2 | 0.1 |
| Outcome | Dead | Dead | Dead | Dead | Dead | Dead |
| Patient Number | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Quick urine sodium dithionite test (ppm) | >50 | >50 | >50 | >50 | >50 | >50 |
| Paper-based (ppm) | 2145.6 | 804.0 | 1267.5 | 372.8 | 4275.0 | 51.5 |
| Spectrochip (ppm) | 3613.6 | 930.0 | 1380.6 | 354.2 | 5939.0 | 47.2 |
| NanoDrop (ppm) | 3375.0 | 1112.6 | 1430.7 | 397.4 | 5148.2 | 39.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-Y.; Lee, Y.-T.; Chen, H.-Y.; Ko, C.-H.; Hong, C.-T.; Wen, J.-W.; Yen, T.-H.; Cheng, C.-M. A Paper-Based Analytical Device for Analysis of Paraquat in Urine and Its Validation with Optical-Based Approaches. Diagnostics 2021, 11, 6. https://doi.org/10.3390/diagnostics11010006
Wang T-Y, Lee Y-T, Chen H-Y, Ko C-H, Hong C-T, Wen J-W, Yen T-H, Cheng C-M. A Paper-Based Analytical Device for Analysis of Paraquat in Urine and Its Validation with Optical-Based Approaches. Diagnostics. 2021; 11(1):6. https://doi.org/10.3390/diagnostics11010006
Chicago/Turabian StyleWang, Tse-Yao, Yi-Tzu Lee, Hsien-Yi Chen, Cheng-Hao Ko, Chi-Tsung Hong, Jyun-Wei Wen, Tzung-Hai Yen, and Chao-Min Cheng. 2021. "A Paper-Based Analytical Device for Analysis of Paraquat in Urine and Its Validation with Optical-Based Approaches" Diagnostics 11, no. 1: 6. https://doi.org/10.3390/diagnostics11010006






