Pelvic Lymphadenectomy in Gynecologic Oncology—Significance of Anatomical Variations
Abstract
1. Introduction
2. Pelvic Lymph Nodes and Regions
3. Selective PLND—Anatomical Landmarks and Techniques
4. Systematic Open PLND—Surgical Technique and Steps
- (1)
- Peritoneal incision. After transecting (not a necessary step) the round ligament, the peritoneum is incised in a posterior (lateral and parallel to the infundibulopelvic ligament) and an anterior (ventrally and laterally to the obliterated umbilical artery) direction. The iliac vessels are exposed from the bifurcation of the aorta to the inguinal ligament.
- (2)
- Identification of the ureter.
- (3)
- Lateral paravesical and lateral (Latzko’s space) pararectal space dissection. The lateral paravesical space is dissected between the obliterated umbilical artery and the external iliac vessels. Lateral pararectal space is dissected between the internal iliac artery and the ureter.
- (4)
- Genitofemoral nerve identification. Lateral incision to the fascia of the psoas muscle is preferable in order to avoid genitofemoral nerve injury. The nerve is located lateral to the external iliac vessels and sometimes overlying them.
- (5)
- External iliac region dissection: lateral and medial external iliac vessels dissection. The dissection begins at the origin of the external iliac vessels and finishes down to the point where the deep circumflex iliac vein crosses over the external iliac artery.
- (6)
- Obturator region dissection. The obturator space is approached by retracting the external iliac vessels medially and the psoas muscle laterally, and by dissection of the areolar tissue that lies directly between these vessels and the lateral pelvic wall. The obturator nerve is identified. The procedure is followed by lateral retraction of the external iliac vessels to expose the obturator space. Superficial obturator lymph nodes are dissected after obturator nerve visualization (obturator nerve stripping). For locally advanced cervical cancer cases, PLND continues with dissection of the deep obturator lymph nodes and the gluteal nodes.
- (7)
- Internal iliac region dissection. Lymph nodes are removed medially and anteriorly to the internal iliac vessels.
- (8)
- Common iliac region dissection. Lymph nodes are removed ventrally and laterally from both common iliac vessels to the aortic bifurcation. Middle common iliac lymph nodes are located in the lumbosacral fossa. It is approached by medial retraction of the common iliac vessels and lateral retraction of the psoas muscle. The obturator nerve, entering the obturator fossa through the body of the psoas muscle, the iliolumbar artery/vein, and the lumbosacral plexus are exposed.
- (9)
- Sacral (presacral) region dissection. After medial mobilization of the sigma-rectum, the peritoneum and the presacral fascia are incised medially to the right common iliac artery. Sacral lymph nodes, localized below the bifurcation of the abdominal aorta and inferior vena cava, in the triangle between the left and right common iliac vessels, are dissected [1,5,7,16,17,18,19].
5. Anatomical Landmarks and Anatomical Variations, Related to Systematic PLND
6. Anatomical Variations of the Ureter, Related to PLND in Gynecologic Oncology (PLNDGO)
- (A)
- Multiplication of ureter;
- (B)
- Ureteral diverticulum;
- (C)
- Unusual ureteral position—retro-iliac ureter.
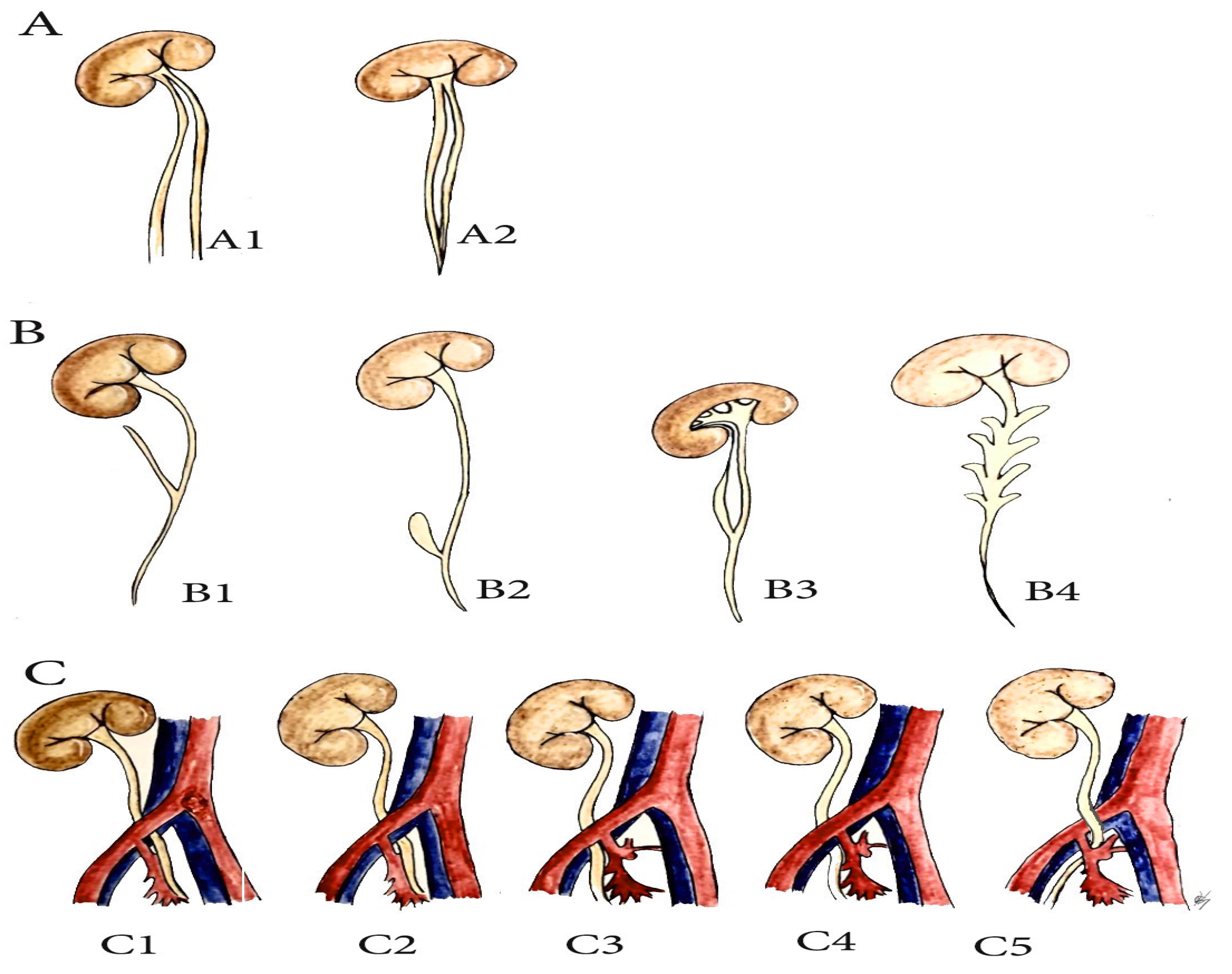
7. Iliac Vessel Variations
7.1. Iliac Arteries
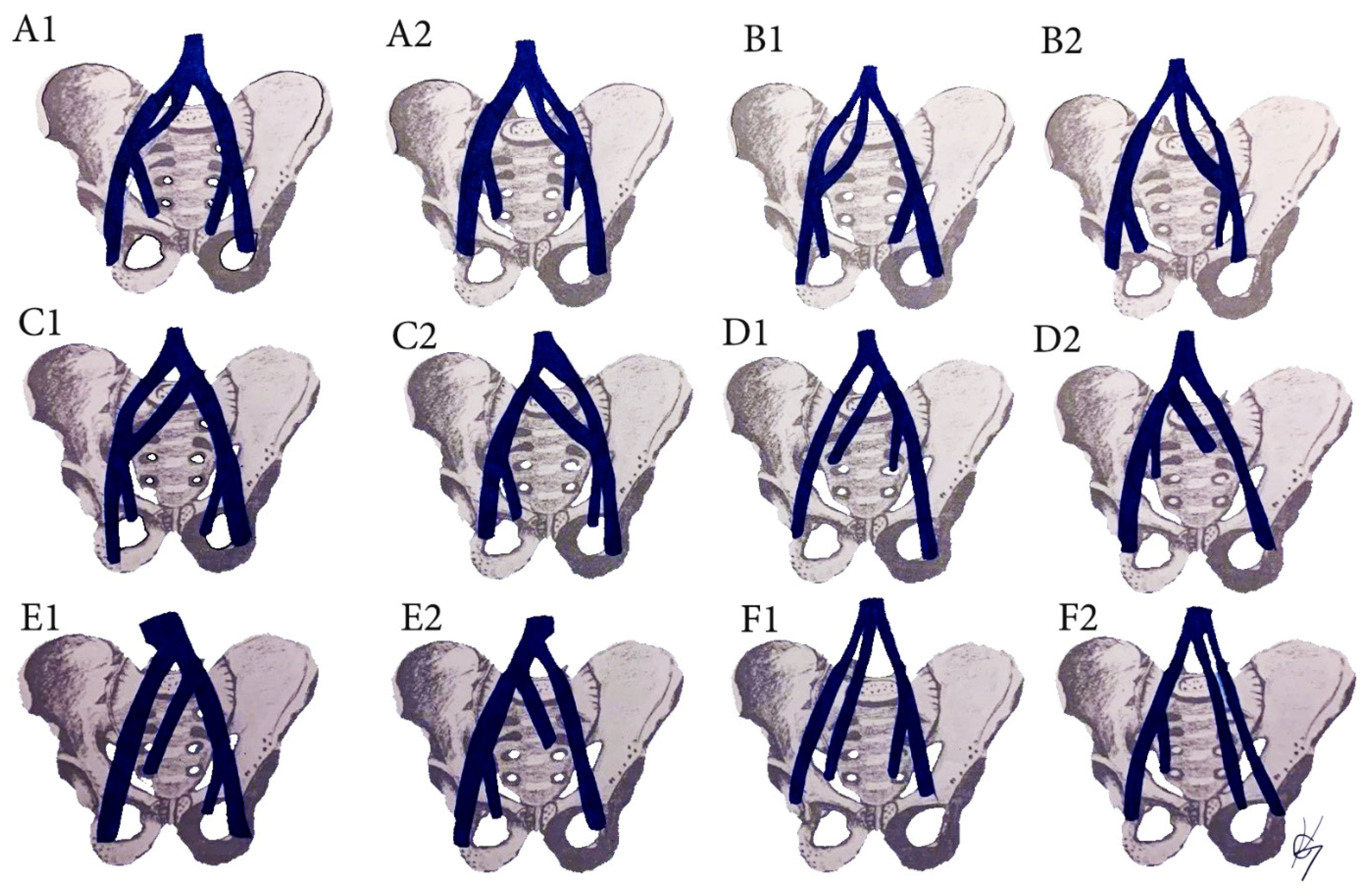
7.2. Common Iliac Artery (CIA) Anatomy
7.3. CIA Variations Related to PLNDGO
8. External/Internal Iliac Artery Anatomy
9. EIA Variations Related to PLNDGO
- (A)
- Hypoplasia, agenesis of the EIA;
- (B)
- Anomalous origin and position of the EIA;
- (C)
- Morphological variations (variations in the shape of the EIA);
- (D)
- Variations in the branching pattern of the EIA.
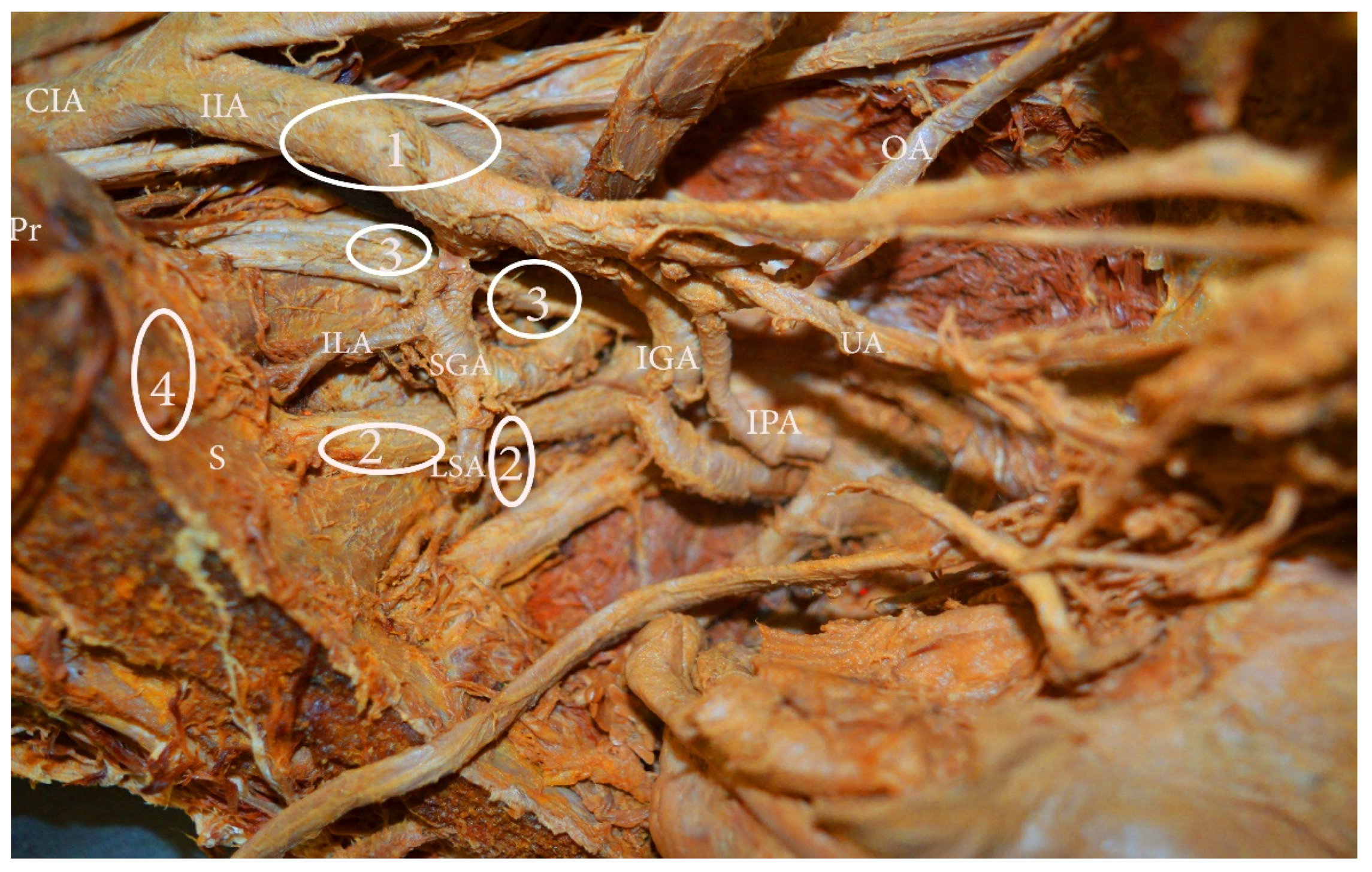
10. IIA Variations, Related to PLNDGO
11. Uterine and Obturator Artery Variations Related to PLNDGO
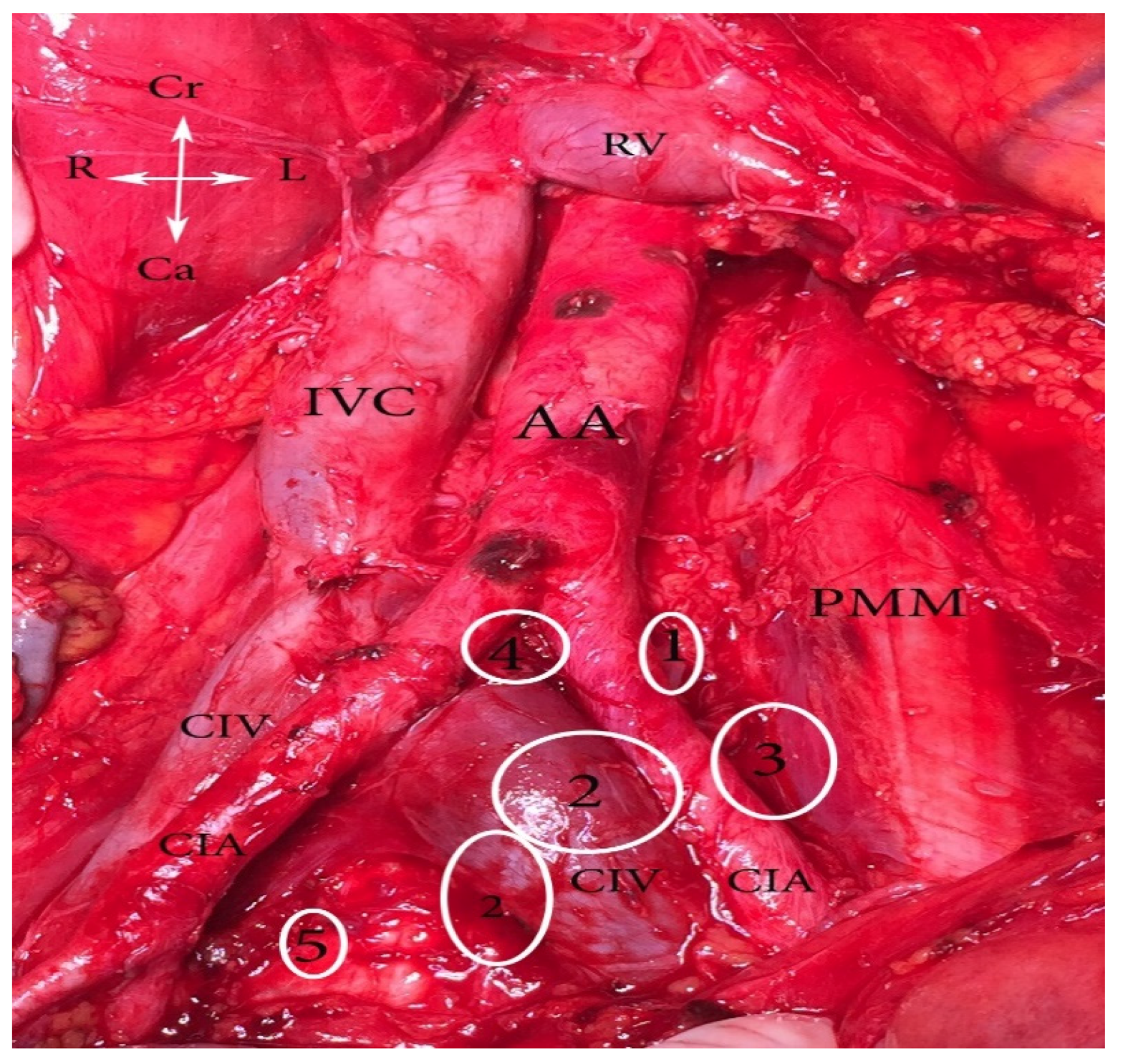
12. Iliac Veins
12.1. Common Iliac Vein Anatomy (CIV)
12.2. CIV Variations Related to the PLNDGO
12.3. CIV Tributaries Variations Related to PLNDGO
13. Median Sacral Vein (MSV) and Lateral Sacral Veins (LSVs) Anatomy and Variations Related to PLNDGO
14. EIV Variations Related to PLNDGO
15. EIV Tributaries Variations Related to PLNDOG
16. Internal Iliac Vein (IIV) Anatomy
17. IIV Variations Related to PLNDGO
18. Corona Mortis, Aberrant and Accessory Obturator Veins Related to PLNDGO
19. Nerves Anatomy
19.1. Obturator Nerve (ON) Anatomy
19.2. ON Variations Related to PLNDGO
19.3. Genitofemoral Nerve (GFN) Anatomy
19.4. The GFN Variations Related to PLNDGO
20. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability
Acknowledgments
Conflicts of Interest
References
- Singh, K.; Fares, R. Pelvic and Para-aortic Lymphadenectomy in Gynecologic Cancers. In Gynecologic and Obstetric Surgery: Challenges and Management Options, 1st ed.; Coomarasamy, A., Shafi, M., Davila, G.W., Chan, K.K., Eds.; John Wiley & Sons Inc.: New York, NY, USA, 2016; pp. 408–411. [Google Scholar] [CrossRef]
- Mangan, C.E.; Rubin, S.C.; Rabin, D.S.; Mikuta, J.J. Lymph node nomenclature in gynecologic oncology. Gynecol. Oncol. 1986, 23, 222–226. [Google Scholar] [CrossRef]
- Bakkum Gamez, J.N. Lymphadenectomy in the Management of Gynecologic Cancer. Clin. Obstet. Gynecol. 2019, 62, 749–755. [Google Scholar] [CrossRef]
- Fowler, J.; Backes, F. Pelvic and Paraaortic Lymphadenectomy in Gynecologic Cancers up to Date 2020. Available online: https://www.uptodate.com/contents/pelvic-and-paraaortic-lymphadenectomy-in-gynecologic-cancers (accessed on 8 November 2020).
- Cibula, D.; Abu-Rustum, N.R. Pelvic lymphadenectomy in cervical cancer—surgical anatomy and proposal for a new classification system. Gynecol. Oncol. 2010, 116, 33–37. [Google Scholar] [CrossRef]
- Canessa, C.E.; Miegge, L.M.; Bado, J.; Silveri, C.; Labandera, D. Anatomic Study of Lateral Pelvic Lymph Nodes: Implications in the Treatment of Rectal Cancer. Dis. Colon Rectum 2004, 47, 297–303. [Google Scholar] [CrossRef]
- Selçuk, İ.; Uzuner, B.; Boduç, E.; Baykuş, Y.; Akar, B.; Güngör, T. Pelvic lymphadenectomy: Step-by-step surgical education video. J. Turk. Ger. Gynecol. Assoc. 2020, 21, 66–69. [Google Scholar] [CrossRef]
- McMahon, C.J.; Rofsky, N.M.; Pedrosa, I. Lymphatic Metastases from Pelvic Tumors: Anatomic Classification, Characterization, and Staging. Radiology 2010, 254, 31–46. [Google Scholar] [CrossRef]
- Kolbenstvedt, A.; Kolstad, P. The difficulties of complete pelvic lymph node dissection in radical hysterectomy for carcinoma of the cervix. Gynecol. Oncol. 1976, 4, 244–254. [Google Scholar] [CrossRef]
- Ovcharov, V.; Vankov, V. Human Anatomy, 14th ed.; ARSO Publishing: Sofia, Bulgaria, 2019; pp. 632–633. [Google Scholar]
- Vincent, P. Lymphatic System 9 Quick Study Academic, 1st ed.; BarCharts Publishing: Boca Raton, FL, USA, 2016. [Google Scholar]
- Paño, B.; Sebastià, C.; Ripoll, E.; Paredes, P.; Salvador, R.; Buñesch, L.; Nicolau, C. Pathways of Lymphatic Spread in Gynecologic Malignancies. RadioGraphics 2015, 35, 916–945. [Google Scholar] [CrossRef] [PubMed]
- Sakuragi, N.; Satoh, C.; Takeda, N.; Hareyama, H.; Takeda, M.; Yamamoto, R.; Fujimoto, T.; Oikawa, M.; Fujino, T.; Fujimoto, S. Incidence and distribution pattern of pelvic and paraaortic lymph node metastasis in patients with stages IB, IIA, and IIB cervical carcinoma treated with radical hysterectomy. Cancer 1999, 85, 1547–1554. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, K.; Liu, A.; Shen, J.; Hou, X.; Lian, X.; Sun, S.; Yan, J.; Zhang, F. Patterns of lymph node metastasis in locally advanced cervical cancer. Medicine 2016, 95, e4814. [Google Scholar] [CrossRef] [PubMed]
- Panici, P.B.; Scambia, G.I.; Baiocchi, G.A.; Matonti, G.I.; Capelli, A.R.; Mancuso, S.A. Anatomical study of para-aortic and pelvic lymph nodes in gynecologic malignancies. Obstet. Gynecol. 1992, 79, 498–502. [Google Scholar] [PubMed]
- Centini, G.; Fernandes, R.P.; Afors, K.; Murtada, R.; Puga, M.F.; Wattiez, A. Radical Hysterectomy and Pelvic Lymphadenectomy (French School). Hysterectomy 2017, 589–595. [Google Scholar] [CrossRef]
- Benedetti Panici, P.; Basile, S.; Angioli, R. Pelvic and aortic lymphadenectomy in cervical cancer: The standardization of surgical procedure and its clinical impact. Gynecol. Oncol. 2009, 113, 284–290. [Google Scholar] [CrossRef]
- Jones, H.W.; Rock, J.A. Te Linde’s Operative Gynecology, 10th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Chen, J.J.; Zhu, Z.S.; Zhu, Y.Y.; Shi, H.Q. Applied anatomy of pelvic lymph nodes and its clinical significance for prostate cancer:a single-center cadaveric study. BMC Cancer 2020, 20, 330. [Google Scholar] [CrossRef]
- Frober, R. Surgical anatomy of the ureter. BJU Int. 2007, 100, 949–965. [Google Scholar] [CrossRef]
- Razvan, R.; Geiorgescu, D.; Geavlete, P.; Bogdan, B. Retrograde Ureteroscopy: Handbook of Endourology. In Notions of Histology, Anatomy, and Physiology of the Upper Urinary Tract, 1st ed.; Geavlete, P., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 7–19. [Google Scholar]
- Wedel, T. Topographical Anatomy for Hysterectomy Procedures. In Hysterectomy a Comprehensive Surgical Approach, 1st ed.; Alkatout, I., Mettler, L., Eds.; Springer: Cham, Switzerland, 2018; pp. 37–60. [Google Scholar]
- Selçuk, İ.; Ersak, B.; Tatar, İ.; Güngör, T.; Huri, E. Basic clinical retroperitoneal anatomy for pelvic surgeons. Turk. J. Obstet. Gynecol. 2018, 15, 259–269. [Google Scholar] [CrossRef]
- Amar, A.D.; Hutch, J.A. Anomalies of the Ureter. In Malformations (Handbuch der Urologie/Encyclopedia of Urology/Encyclopédie d’Urologie); Springer: Berlin, Germany, 1968. [Google Scholar] [CrossRef]
- Nation, E.F. Duplication of the Kidney and Ureter: A Statistical Study of 230 New Cases. J. Urol. 1944, 51, 456–465. [Google Scholar] [CrossRef]
- Foley, C.E.; Mansuria, S. Ureteral anomalies in gynecologic surgery. J. Minim. Invasive Gynecol. 2020, 27, 566–567. [Google Scholar] [CrossRef] [PubMed]
- Mayers, M.M. Diverticulum of the ureter. J. Urol. 1949, 61, 344–350. [Google Scholar] [CrossRef]
- McLoughlin, L.C.; Davis, N.F.; Dowling, C.; Eng, M.P.; Power, R.E. Ureteral diverticulum: A review of the current literature. Can. J. Urol. 2013, 20, 6893–6896. [Google Scholar]
- Papin, E.; Eisendrath, D.N. Classification of Renal and Ureteral Anomalies. Ann. Surg. 1927, 85, 735–756. [Google Scholar] [PubMed]
- Tawfik, A.M.; Younis, M.H. Computed Tomography Imaging Appearance of a Unique Variant of Retroiliac Ureter. Urology 2016, 88, e7–e9. [Google Scholar] [CrossRef]
- Prasad, H.L.; Karthikeyan, V.S.; Shivalingaiah, M.; Ratkal, C.S. Retroiliac ureter presenting as right upper ureteric obstruction—Report of a rare case. Int. J. Res. Med. Sci. 2015, 3, 2143–2144. [Google Scholar] [CrossRef]
- Ebiloglu, T.; Kaya, E.; Zorba, O.; Kibar, Y.; Gok, F. Retro-Iliac Ureters: Review of Literature and Two Cases with Two Different Techniques. Turk. Klin. J. Case Rep. 2016, 24, 66–69. [Google Scholar] [CrossRef]
- Parashar, M.; Sharma, R.K.; Kumar, S. Ureteric injury in gynaecological surgery: A rare but serious event. Int. J. Health Sci. Res. 2019, 9, 397–401. [Google Scholar]
- Mylonas, I.; Briese, V.; Vogt-Weber, B.; Friese, K. Complete bilateral crossed ureteral duplication observed during a radical hysterectomy with pelvic lymphadenectomy for ovarian cancer. A case report. Arch. Gynecol. Obstet. 2003, 267, 250–251. [Google Scholar] [CrossRef]
- Ostrzenski, A.; Radolinski, B.; Ostrzenska, K.M. A review of laparoscopic ureteral injury in pelvic surgery. Obstet. Gynecol. Surv. 2003, 58, 794–799. [Google Scholar] [CrossRef]
- Davis, A.A. Transection of Duplex Ureter During Vaginal Hysterectomy. Cureus 2020, 12, e6597. [Google Scholar] [CrossRef]
- Benedetti-Panici, P.; Maneschi, F.; Scambia, G.; Greggi, S.; Mancuso, S. Anatomic abnormalities of the retroperitoneum encountered during aortic and pelvic lymphadenectomy. Am. J. Obstet. Gynecol. 1994, 170 Pt 1, 111–116. [Google Scholar] [CrossRef]
- Dalzell, A.P.B.; Robinson, R.G.; Crooke, K.M. Duplex ureter damaged during laparoscopic hysterectomy. Pelviperineology 2010, 29, 88. [Google Scholar]
- Subbiah, S.; Rajaraman, N.N. Congenital anomalies in surgical oncology practice. Edorium J. Surg. 2015, 2, 29–36. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Chen, C.; Liu, P.; Duan, H.; Chen, L.; Wang, J.; Tan, H.; Li, P.; Zhao, C.; et al. Distribution of iliac veins posterior to the common iliac artery bifurcation related to pelvic lymphadenectomy: A digital in vivo anatomical study of 442 Chinese females. Gynecol. Oncol. 2016, 141, 538–542. [Google Scholar] [CrossRef]
- Gray, H.; Standring, S.; Hrold Ellis, H.; Berkovitz, B. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 39th ed.; Elsevier Churchill Livingstone Edinburgh: New York, NY, USA, 2005; pp. 2171–2175. [Google Scholar]
- Tubbs, R.S.; Shoja, M.M.; Loukas, M. Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 669–673, 884–889. [Google Scholar]
- Shetty, S.; Kantha, L.; Sheshgiri, C. Bilateral absence of common iliac artery—A cadaveric observation. Int. J. Anat. Var. 2013, 6, 7–8. [Google Scholar]
- Dabydeen, D.A.; Shabashov, A.; Shaffer, K. Congenital Absence of the Right Common Iliac Artery. Radiol. Case Rep. 2015, 3, 47. [Google Scholar] [CrossRef]
- Llauger, J.; Sabaté, J.M.; Guardia, E.; Escudero, J. Congenital absence of the right common iliac artery: CT and angiographic demonstration. Eur. J. Radiol. 1995, 21, 128–130. [Google Scholar] [CrossRef]
- Rusu, M.C.; Cergan, R.; Dermengiu, D.; Curcă, G.C.; Folescu, R.; Motoc, A.G.M.; Jianu, A.M. The iliolumbar artery—Anatomic considerations and details on the common iliac artery trifurcation. Clin. Anat. 2009, 23, 93–100. [Google Scholar] [CrossRef]
- Lierse, W. Applied Anatomy of the Pelvis, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1987; pp. 47–58. [Google Scholar] [CrossRef]
- Badagabettu Nayak, S.; Padur Aithal, A.; Kumar, N.; Regunathan, D.; Shetty, P.; Alathady Maloor, P. A cadaveric study of variations of external iliac artery and its implication in trauma and radiology. Morphologie 2019, 103, 24–31. [Google Scholar] [CrossRef]
- Tamisier, D.; Melki, J.-P.; Cormier, J.-M. Congenital Anomalies of the External Iliac Artery: Case Report and Review of the Literature. Ann. Vasc. Surg. 1990, 4, 510–514. [Google Scholar] [CrossRef]
- Kawashima, T.; Sato, K.; Sasaki, H. A human case of hypoplastic external iliac artery and its collateral pathways. Folia Morphol. 2006, 65, 157–160. [Google Scholar]
- Okamoto, K.; Wakebe, T.; Saiki, K.; Nagashima, S. Consideration of the potential courses of the common iliac artery. Anat. Sci. Int. 2005, 80, 116–119. [Google Scholar] [CrossRef]
- Safi, K.C.; Teber, D.; Moazen, M.; Anghel, G.; Maldonado, R.V.; Rassweiler, J.J. Laparoscopic repair of external iliac-artery transection during laparoscopic radical prostatectomy. J. Endourol. 2006, 20, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Boonruangsri, P.; Suwannapong, I.; Rattanasuwan, S.; Iamsaard, S. Aneurysm, tortuosity, and kinking of abdominal aorta and iliac arteries in Thai cadavers. Int. J. Morphol. 2015, 33, 73–76. [Google Scholar] [CrossRef][Green Version]
- Moul, J.W.; Wind, G.G.; Wright, C.R. Tortuous and aberrant external iliac artery precluding radical retropubic prostatectomy for prostate cancer. Urology 1993, 42, 450–452. [Google Scholar] [CrossRef]
- Kostov, S.; Slavchev, S.; Dzhenkov, D.; Stoyanov, G.; Dimitrov, N.; Yordanov, A. Corona mortis, aberrant obturator vessels, accessory obturator vessels: Clinical applications in gynecology. Folia Morphol. 2020. [Google Scholar] [CrossRef]
- Sañudo, J.R.; Roig, M.; Rodriguez, A.; Ferreira, B.; Domenech, J.M. Rare origin of the obturator, inferior epigastric and medial circumflex femoral arteries from a common trunk. J. Anat. 1993, 183, 161–163. [Google Scholar]
- Fătu, C.; Puişoru, M.; Fătu, I.C. Morphometry of the internal iliac artery in different ethnic groups. Ann. Anat. 2006, 188, 541–546. [Google Scholar] [CrossRef]
- Adachi, B. Das Arteriensystem der Japaner, Bd. II; Kyoto. Supp. to Acta Scholae Medicinalis Universitatis Imperalis in Kioto: Tokyo, Japan, 1928; Volume 9, pp. 1926–1927. [Google Scholar]
- Yamaki, K.; Saga, T.; Doi, Y.; Aida, K.; Yoshizuka, M. A statistical study of the branching of the human internal iliac artery. Kurume Med. J. 1998, 45, 333–340. [Google Scholar] [CrossRef]
- Liapis, K.; Tasis, N.; Tsouknidas, I.; Tsakotos, G.; Skandalakis, P.; Vlasis, K.; Filippou, D. Anatomic variations of the Uterine Artery. Review of the literature and their clinical significance. Turk. J. Obstet. Gynecol. 2020, 17, 58–62. [Google Scholar] [CrossRef]
- Kumari, S.; Trinesh Gowda, M.S. A study of variations of origin of obturator artery: Review in south Indian population. J. Anat. Soc. India 2016, 65, S1–S4. [Google Scholar] [CrossRef]
- Granite, G.; Meshida, K.; Wind, G. Frequency and Clinical Review of the Aberrant Obturator Artery: A Cadaveric Study. Diagnostics 2020, 10, 546. [Google Scholar] [CrossRef]
- Sañudo, J.R.; Mirapeix, R.; Rodriguez-Niedenführ, M.; Maranillo, E.; Parkin, I.G.; Vázquez, T. Obturator artery revisited. Int. Urogynecol. J. 2011, 22, 1313–1318. [Google Scholar] [CrossRef]
- Bae, J.W.; Lee, J.H.; Choi, J.S.; Son, C.E.; Jeon, S.W.; Hong, J.H.; Eom, J.M.; Joo, K.J. Laparoscopic lymphadenectomy for gynecologic malignancies: Evaluation of the surgical approach and outcomes over a seven-year experience. Arch Gynecol. Obstet. 2012, 285, 823–829. [Google Scholar] [CrossRef]
- Ricciardi, E.; di Martino, G.; Maniglio, P.; Schimberni, M.; Frega, A.; Jakimovska, M.; Kobal, B.; Moscarini, M. Life-threatening bleeding after pelvic lymphadenectomy for cervical cancer: Endovascular management of ruptured false aneurysm of the external iliac artery. World J. Surg. Onc. 2012, 10, 149. [Google Scholar] [CrossRef]
- Ishikawa, M.; Nakayama, K.; Razia, S.; Yamashita, H.; Ishibashi, T.; Sato, S.; Sasamori, H.; Sawada, K.; Kurose, S.; Ishikawa, N.; et al. External Iliac Artery Injury and Thrombosis during Laparoscopic Gynecologic Surgery. Case Rep. Obstet. Gynecol. 2020. [Google Scholar] [CrossRef]
- Gyimadu, A.; Salman, M.C.; Karcaaltincaba, M.; Yuce, K. Retroperitoneal vascular aberrations increase the risk of vascular injury during lymphadenectomy in gynecologic cancers. Arch Gynecol. Obstet. 2012, 286, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, C.; Neumann, U.; Kraetschell, R.; Lichtenegger, W.; Sehouli, J. External iliac artery ligation due to late postoperative rupture after radical lymphadenectomy for advanced ovarian cancer—Two case reports. Eur. J. Gynaecol. Oncol. 2010, 31, 198–200. [Google Scholar] [PubMed]
- Mehta, K.; Iwanaga, J.; Tubbs, R.S. Absence of the Right Common Iliac Vein with the Right Internal Iliac Vein Arising from the Left Common Iliac Vein: Case Report. Cureus 2019, 11, e4575. [Google Scholar] [CrossRef]
- Morris, H. Human Anatomy: A Complete Systematic Treatise by English and American Authors, 5th ed.; P. Blakiston, Son & Co.: Philadelphia, PA, USA, 1893. [Google Scholar] [CrossRef]
- Cardinot, T.M.; Aragão, A.H.; Babinski, M.A.; Favorito, L.A. Rare variation in course and affluence of internal iliac vein due to its anatomical and surgical significance. Surg. Radiol. Anat. 2006, 28, 422–425. [Google Scholar] [CrossRef]
- Yahyayev, A.; Bulakci, M.; Yilmaz, E.; Ucar, A.; Sayin, O.A.; Yekeler, E. Absence of the right iliac vein and an unusual connection between both common femoral veins. Phlebology 2013, 28, 162–164. [Google Scholar] [CrossRef]
- Panchal, P.; Chaturvedi, H. Agenesis of common iliac vein encroaching development of inferior vena cava. IJAV 2014, 7, 21–23. [Google Scholar]
- Nusrath, S.; Pawar, S.; Goel, V.; Raju, K. Common Internal Iliac Vein Joining Inferior Vena Cava—A Rare Anatomical Variation: Anatomic and Surgical Relevance. Indian J. Surg. 2020, 82, 701–703. [Google Scholar] [CrossRef]
- Kose, M.F.; Turan, T.; Karasu, Y.; Gundogdu, B.; Boran, N.; Tulunay, G. Anomalies of Major Retroperitoneal Vascular Structure. Int. J. Gynecol. Cancer 2011, 21, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Jasani, V.; Jaffray, D. The anatomy of the iliolumbar vein: A cadaver study. J. Bone Joint Surg. Br. 2002, 84, 1046–1049. [Google Scholar] [CrossRef]
- Lolis, E.; Panagouli, E.; Venieratos, D. Study of the ascending lumbar and iliolumbar veins: Surgical anatomy, clinical implications and review of the literature. Ann. Anat. 2011, 193, 516–529. [Google Scholar] [CrossRef]
- Unruh, K.P.; Camp, C.L.; Zietlow, S.P.; Huddleston, P.M. Anatomical variations of the iliolumbar vein with application to the anterior retroperitoneal approach to the lumbar spine: A cadaver study. Clin. Anat. 2008, 21, 666–673. [Google Scholar] [CrossRef]
- Venieratos, D.; Panagouli, E.; Lolis, E. Variations of the iliac and pelvic venous systems with special attention to the drainage patterns of the ascending lumbar and iliolumbar veins. Ann. Anat. 2012, 194, 396–403. [Google Scholar] [CrossRef]
- Hamid, M.; Toussaint, P.J.; Delmas, V.; Gillot, C.; Coutaux, A.; Plaisant, O. Anatomical and radiological evidence for the iliolumbar vein as an inferior lumbar venous system. Clin. Anat. 2007, 20, 545–552. [Google Scholar] [CrossRef]
- Sivakumar, G.; Paluzzi, A.; Freeman, B. Avulsion of ascending lumbar and iliolumbar veins in anterior spinal surgery: An anatomical study. Clin. Anat. 2007, 20, 553–555. [Google Scholar] [CrossRef]
- Lotz, P.R.; Seeger, J. Normal variation in iliac venous anatomy. AJR 1982, 138, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Djedovic, G.; Putz, D. Case report: Description of a venous annulus of the external iliac vein. Ann. Anat. 2006, 188, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Naito, M.; Yakura, T.; Kumazaki, T.; Itoh, M.; Nakano, T. A case of an additional right external iliac vein surrounding the right external iliac artery and lacking the right common iliac vein. Anat. Sci. Int. 2015, 91, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.; Childers, J.; Nezhat, F.; Nezhat, C.H.; Seidman, D.S. Major retroperitoneal vascular injury during laparoscopic surgery. Hum. Reprod. 1997, 12, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Herraiz Roda, J.L.; Llueca, A.; Maazouzi, Y.; Piquer Simó, D.; Guijarro Colomer, M.; Sentís Masllorens, J. Complications of laparoscopic lymphadenectomy for gynecologic malignancies. Experience of 372 patients. Res. Rep. Gynaecol. Obstet. 2017, 1, 12–16. [Google Scholar]
- Ghassemi, A.; Furkert, R.; Prescher, A.; Riediger, D.; Knobe, M.; O’dey, D.; Gerressen, M. Variants of the supplying vessels of the vascularized iliac bone graft and their relationship to important surgical landmarks. Clin. Anat. 2013, 26, 509–521. [Google Scholar] [CrossRef]
- Elsy, B.; Alghamdi, A.; Osman, L. Bilateral branching variants of internal and external iliac arteries—Cadaveric study Case Report. Eur. J. Anat. 2020, 24, 63–68. [Google Scholar]
- Vidal, V.; Monnet, O.; Jacquier, A.; Bartoli, J.-M.; Tropiano, P. Accessory Iliac Vein. J. Spinal Disord Tech. 2010, 23, 398–403. [Google Scholar] [CrossRef]
- Shin, M.; Lee, J.B.; Park, S.B.; Park, H.J.; Kim, Y.S. Multidetector computed tomography of iliac vein variation: Prevalence and classification. Surg. Radiol. Anat. 2014, 37, 303–309. [Google Scholar] [CrossRef]
- Morita, S.; Saito, N.; Mitsuhashi, N. Variations in internal iliac veins detected using multidetector computed tomography. Acta Radiol. 2007, 48, 1082–1085. [Google Scholar] [CrossRef]
- Chong, G.O.; Lee, Y.H.; Hong, D.G.; Cho, Y.L.; Lee, Y.S. Anatomical variations of the internal iliac veins in the presacral area: Clinical implications during sacral colpopepxy or extended pelvic lymphadenectomy. Clin. Anat. 2014, 28, 661–664. [Google Scholar] [CrossRef]
- Nayak, S.B. Dangerous twisted communications between external and internal iliac veins which might rupture during catheterization. Anat. Cell. Biol. 2018, 51, 309–311. [Google Scholar] [CrossRef]
- Kanjanasilp, P.; Ng, J.L.; Kajohnwongsatit, K.; Thiptanakit, C.; Limvorapitak, T.; Sahakitrungruang, C. Anatomical Variations of Iliac Vein Tributaries and Their Clinical Implications During Complex Pelvic Surgeries. Dis. Colon Rectum. 2019, 62, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Liu, P.; Chen, C.; Chen, L.; Li, P.; Li, W.; Gong, S.; Xv, Y.; Chen, R.; Tang, L. Reconstruction of three-dimensional vascular models for lymphadenectomy before surgery. Minim. Invasive Ther. Allied Technol. 2020, 29, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Sanna, B.; Henry, B.M.; Vikse, J.; Skinningsrud, B.; Pękala, J.R.; Walocha, J.A.; Cirocchi, R.; Tomaszewski, K.A. The prevalence and morphology of the corona mortis (Crown of death): A meta-analysis with implications in abdominal wall and pelvic surgery. Injury 2018, 49, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Darmanis, S.; Lewis, A.; Mansoor, A.; Bircher, M. Corona mortis: An anatomical study with clinical implications in approaches to the pelvis and acetabulum. Clin. Anat. 2007, 20, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Berberoĝlu, M.; Uz, A.; Özmen, M.M.; Bozkurt, C.; Erkuran, C.; Taner, S.; Tekin, A.; Tekdemir, I. Corona mortis: An anatomic study in seven cadavers and an endoscopic study in 28 patients. Surg. Endosc. 2001, 15, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S. Early experience with laparoscopic pelvic lymphadenectomy in women with gynecologic malignancy. J. Am. Assoc. Gynecol. Laparosc. 1999, 6, 59–63. [Google Scholar] [CrossRef]
- Selçuk, İ.; Tatar, İ.; Fırat, A.; Huri, E.; Güngör, T. Is corona mortis a historical myth? A perspective from a gynecologic oncologist. J. Turk. Ger. Gynecol. Assoc. 2018, 19, 171–172. [Google Scholar] [CrossRef]
- Anagnostopoulou, S.; Kostopanagiotou, G.; Paraskeuopoulos, T.; Chantzi, C.; Lolis, E.; Saranteas, T. Anatomic Variations of the Obturator Nerve in the Inguinal Region. Reg. Anesth. Pain Med. 2009, 34, 33–39. [Google Scholar] [CrossRef]
- Anloague, P.A.; Huijbregts, P. Anatomical Variations of the Lumbar Plexus: A Descriptive Anatomy Study with Proposed Clinical Implications. J. Man Manip. Ther. 2009, 17, 107E–114E. [Google Scholar] [CrossRef]
- Turgut, M.; Protas, M.; Gardner, B.; Oskouian, R.; Loukas, M.; Tubbs, R. The accessory obturator nerve: An anatomical study with literature analysis. Anatomy 2017, 11, 121–127. [Google Scholar] [CrossRef]
- Archana, B.J.; Nagaraj, D.N.; Pradeep, P.; Subhash, L. Anatomical Variations of Accessory Obturator Nerve: Cadaveric Study with Proposed Clinical Implications. Int. J. Anat. Res. 2016, 4, 2158–2161. [Google Scholar] [CrossRef]
- Gindha, G.S.; Arora, D.; Kaushal, S.; Chhabra, U. Variations in origin of the genitofemoral nerve from the lumbar plexuses in north Indian population (a cadaveric study). MOJ Anat. Physiol. 2015, 1, 72–76. [Google Scholar] [CrossRef]
- Paul, L.; Shastri, D. Anatomical variations in formation and branching pattern of the border nerves of lumbar Region. Natl. J. Clin. Anat. 2019, 8, 57–61. [Google Scholar] [CrossRef]
- Deepti, A.; Shyam, S.T.; Subhash, K.; Usha, C. Morphology of Lumbar Plexus and Its Clinicalsignificance. Int. J. Anat. Res. 2016, 4, 2007–2014. [Google Scholar] [CrossRef]
- Cardosi, R.; Cox, C.; Hoffman, M. Postoperative neuropathies after major pelvic surgery. Obstet. Gynecol. 2002, 100, 240–244. [Google Scholar] [CrossRef] [PubMed]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostov, S.; Kornovski, Y.; Slavchev, S.; Ivanova, Y.; Dzhenkov, D.; Dimitrov, N.; Yordanov, A. Pelvic Lymphadenectomy in Gynecologic Oncology—Significance of Anatomical Variations. Diagnostics 2021, 11, 89. https://doi.org/10.3390/diagnostics11010089
Kostov S, Kornovski Y, Slavchev S, Ivanova Y, Dzhenkov D, Dimitrov N, Yordanov A. Pelvic Lymphadenectomy in Gynecologic Oncology—Significance of Anatomical Variations. Diagnostics. 2021; 11(1):89. https://doi.org/10.3390/diagnostics11010089
Chicago/Turabian StyleKostov, Stoyan, Yavor Kornovski, Stanislav Slavchev, Yonka Ivanova, Deyan Dzhenkov, Nikolay Dimitrov, and Angel Yordanov. 2021. "Pelvic Lymphadenectomy in Gynecologic Oncology—Significance of Anatomical Variations" Diagnostics 11, no. 1: 89. https://doi.org/10.3390/diagnostics11010089
APA StyleKostov, S., Kornovski, Y., Slavchev, S., Ivanova, Y., Dzhenkov, D., Dimitrov, N., & Yordanov, A. (2021). Pelvic Lymphadenectomy in Gynecologic Oncology—Significance of Anatomical Variations. Diagnostics, 11(1), 89. https://doi.org/10.3390/diagnostics11010089






