Validation of Coronary Angiography-Derived Vessel Fractional Flow Reserve in Heart Transplant Patients with Suspected Graft Vasculopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Invasive Procedure
2.3. Angiography-Derived FFR Computation
2.4. Study Objectives
2.5. Statistical Analysis
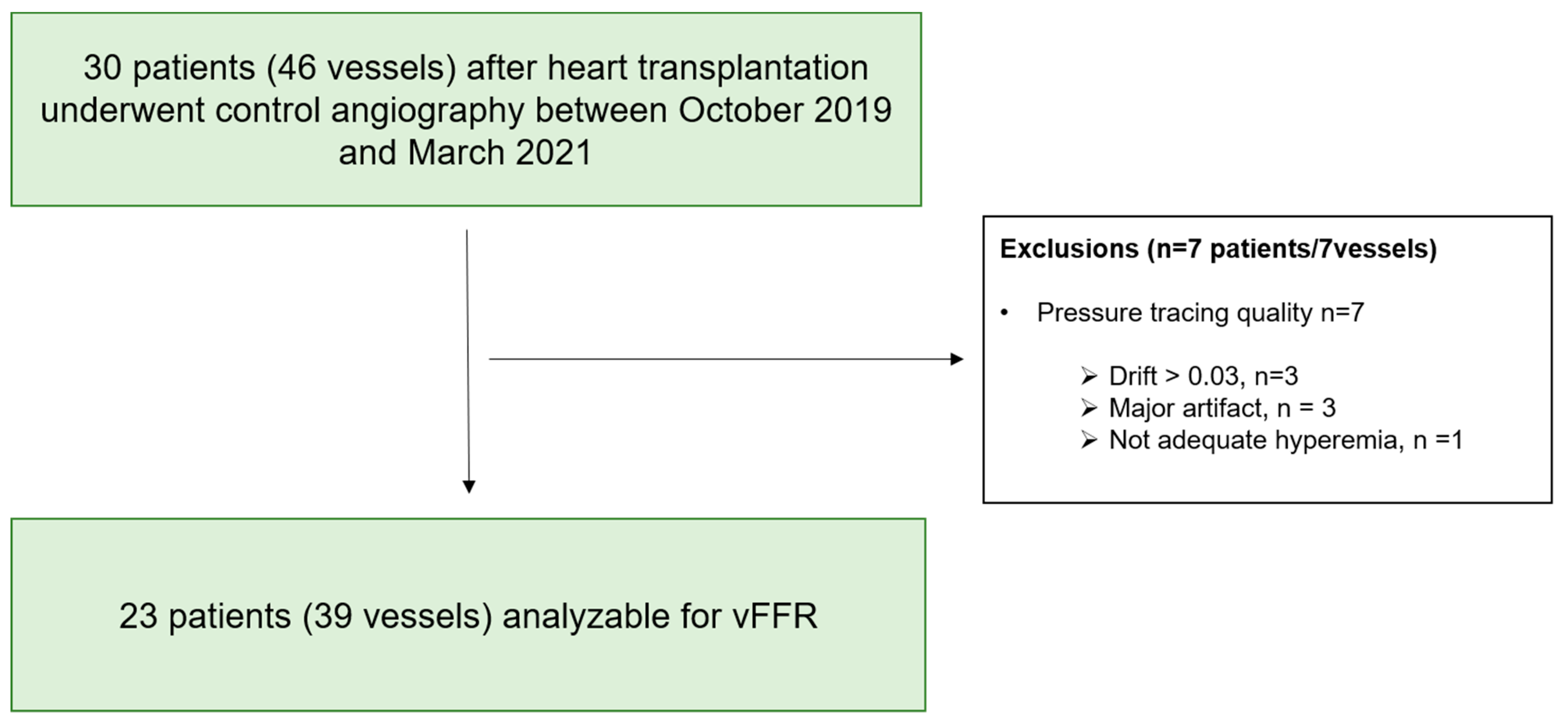
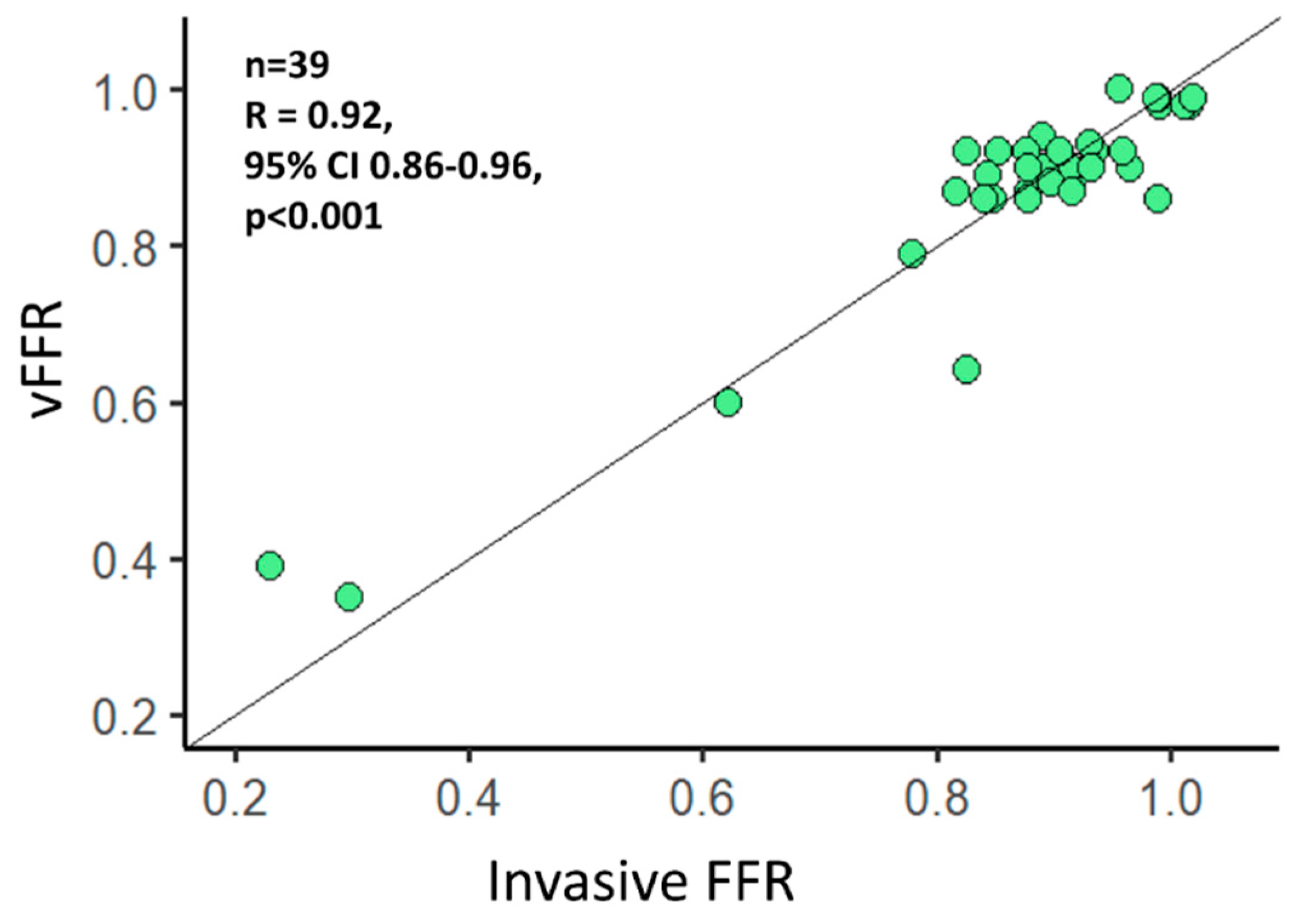
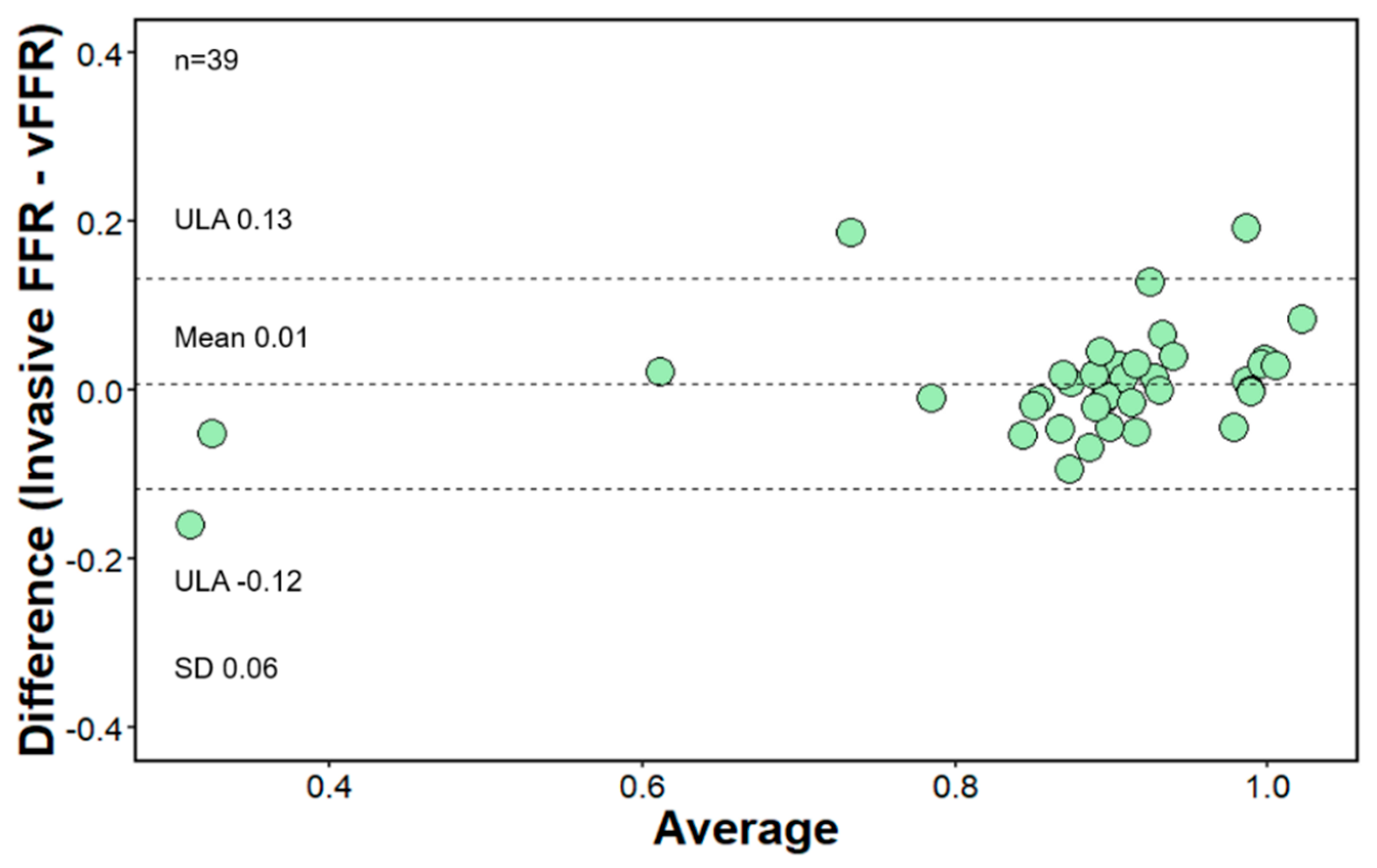
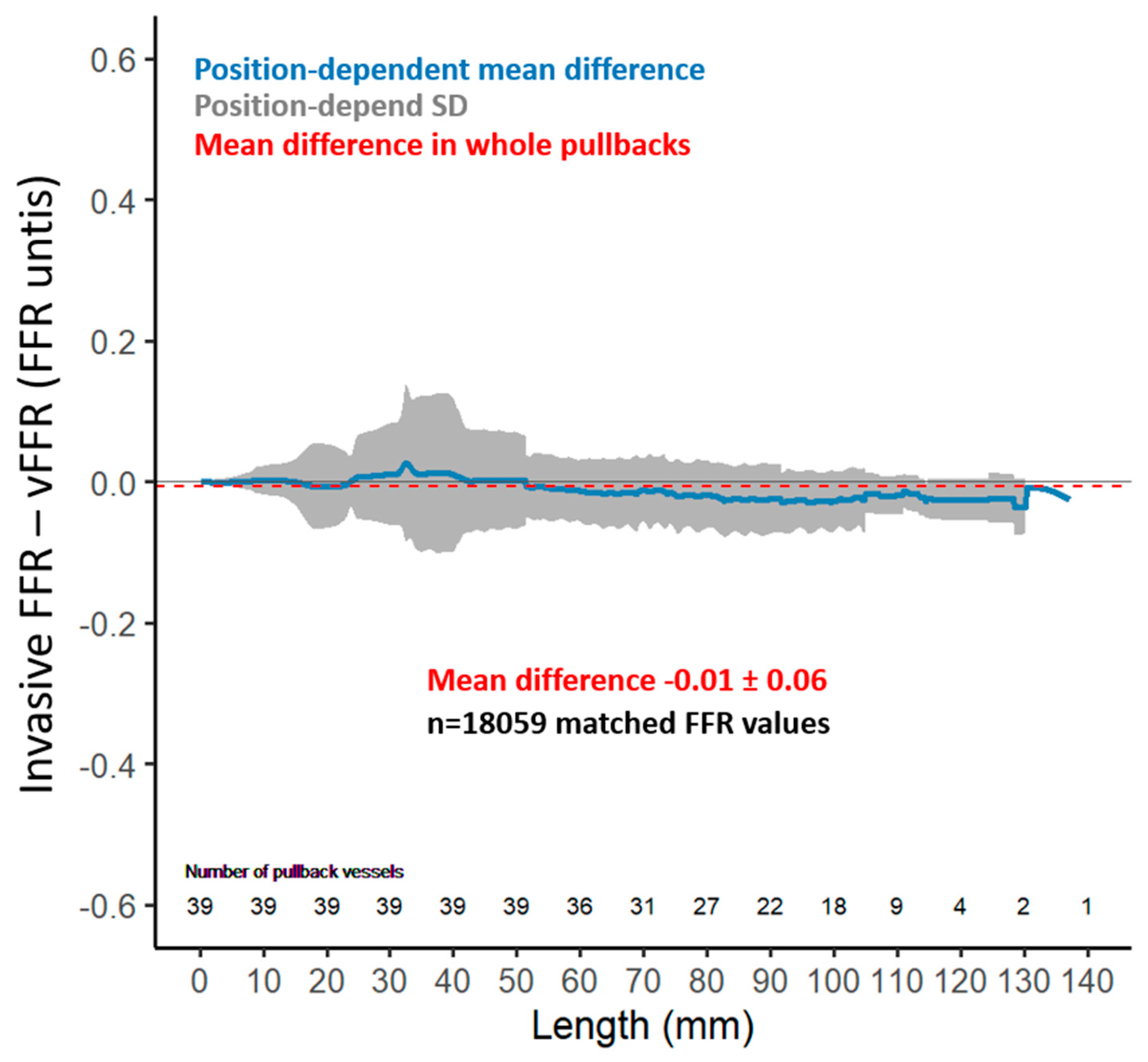
3. Results
Functional Evaluation
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lund, L.H.; Khush, K.K.; Cherikh, W.S.; Goldfarb, S.; Kucheryavaya, A.Y.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; Chambers, D.C.; Yusen, R.D.; et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report-2017; Focus Theme: Allograft ischemic time. J. Heart Lung Transplant. 2017, 36, 1037–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chih, S.; Chong, A.Y.; Mielniczuk, L.M.; Bhatt, D.L.; Beanlands, R.S. Allograft Vasculopathy: The Achilles’ Heel of Heart Transplantation. J. Am. Coll. Cardiol. 2016, 68, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.D.; Silva Soto, D.A.; Feher, J.F.A.; Rafiroiu, D.; Lungu, A.; Varma, S.; Lawford, P.V.; Hose, D.R.; Gunn, J.P. Fast Virtual Fractional Flow Reserve Based Upon Steady-State Computational Fluid Dynamics Analysis: Results From the VIRTU-Fast Study. JACC Basic Transl. Sci. 2017, 2, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Masdjedi, K.; van Zandvoort, L.J.C.; Balbi, M.M.; Gijsen, F.J.H.; Ligthart, J.M.R.; Rutten, M.C.M.; Lemmert, M.E.; Wilschut, J.M.; Diletti, R.; de Jaegere, P.; et al. Validation of a three-dimensional quantitative coronary angiography-based software to calculate fractional flow reserve: The FAST study. EuroIntervention 2020, 16, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Nagumo, S.; Gallinoro, E.; Candreva, A.; Mizukami, T.; Monizzi, G.; Kodeboina, M.; Verstreken, S.; Dierckx, R.; Heggermont, W.; Bartunek, J.; et al. Vessel Fractional Flow Reserve and Graft Vasculopathy in Heart Transplant Recipients. J. Interv. Cardiol. 2020, 2020, 9835151. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Crespo-Leiro, M.G.; Dipchand, A.; Ensminger, S.M.; Hiemann, N.E.; Kobashigawa, J.A.; Madsen, J.; Parameshwar, J.; Starling, R.C.; Uber, P.A. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J. Heart Lung Transplant. 2010, 29, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Toth, G.G.; Johnson, N.P.; Jeremias, A.; Pellicano, M.; Vranckx, P.; Fearon, W.F.; Barbato, E.; Kern, M.J.; Pijls, N.H.; De Bruyne, B. Standardization of Fractional Flow Reserve Measurements. J. Am. Coll. Cardiol. 2016, 68, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Stehlik, J.; Edwards, L.B.; Kucheryavaya, A.Y.; Benden, C.; Christie, J.D.; Dobbels, F.; Kirk, R.; Rahmel, A.O.; Hertz, M.I. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report—2011. J. Heart Lung Transplant. 2011, 30, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Hirohata, A.; Nakamura, M.; Waseda, K.; Honda, Y.; Lee, D.P.; Vagelos, R.H.; Hunt, S.A.; Valantine, H.A.; Yock, P.G.; Fitzgerald, P.J.; et al. Changes in coronary anatomy and physiology after heart transplantation. Am. J. Cardiol. 2007, 99, 1603–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuzcu, E.M.; Kapadia, S.R.; Sachar, R.; Ziada, K.M.; Crowe, T.D.; Feng, J.; Magyar, W.A.; Hobbs, R.E.; Starling, R.C.; Young, J.B.; et al. Intravascular ultrasound evidence of angiographically silent progression in coronary atherosclerosis predicts long-term morbidity and mortality after cardiac transplantation. J. Am. Coll. Cardiol. 2005, 45, 1538–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fearon, W.F.; Nakamura, M.; Lee, D.P.; Rezaee, M.; Vagelos, R.H.; Hunt, S.A.; Fitzgerald, P.J.; Yock, P.G.; Yeung, A.C. Simultaneous assessment of fractional and coronary flow reserves in cardiac transplant recipients: Physiologic Investigation for Transplant Arteriopathy (PITA Study). Circulation 2003, 108, 1605–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fearon, W.F.; Hirohata, A.; Nakamura, M.; Luikart, H.; Lee, D.P.; Vagelos, R.H.; Hunt, S.A.; Valantine, H.A.; Fitzgerald, P.J.; Yock, P.G.; et al. Discordant changes in epicardial and microvascular coronary physiology after cardiac transplantation: Physiologic Investigation for Transplant Arteriopathy II (PITA II) study. J. Heart Lung Transplant. 2006, 25, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Khush, K.; Luikart, H.; Okada, K.; Lim, H.S.; Kobayashi, Y.; Honda, Y.; Yeung, A.C.; Valantine, H.; Fearon, W.F. Invasive Assessment of Coronary Physiology Predicts Late Mortality After Heart Transplantation. Circulation 2016, 133, 1945–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variables | Overall |
|---|---|
| Total number of patients, n | 23 |
| Age receptor at angiography, years (mean (SD)) | 59.30 (9.69) |
| Sex, Male, n (%) | 20 (87.0) |
| Dyslipidemia, n (%) | 22 (95.7) |
| Hypertension, n (%) | 9 (39.1) |
| Diabetes mellitus, n (%) | 10 (43.5) |
| Current smoker, n (%) | 1 (4.3) |
| History of ischemic cardiomyopathy, n (%) | 11 (47.8) |
| Other cardiomyopathies, n (%) | 12 (52.2) |
| Time between heart transplant and coronary angiography, years (median [IQR]) | 5.24 [1.20, 11.25] |
| CAV ≥ 1 grade, n (%) | 13 (56.5) |
| Variables | Overall |
|---|---|
| Total number of vessels, n | 39 |
| Type of vessel, n (%) | |
| LAD, n (%) | 24 (61.5) |
| LCX, n (%) | 11 (28.2) |
| RCA, n (%) | 4 (10.3) |
| Invasive FFR, (mean (SD)) | 0.88 (0.17) |
| Invasive FFR ≤ 0.80, n (%) | 4 (10.3) |
| Lesion length, mm, (mean (SD)) | 23.0 (14.3) |
| Minimal lumen diameter, mm, (mean (SD)) | 1.94 (0.69) |
| Diameter stenosis, %, (mean (SD)) | 32.5 (16.0) |
| Reference vessel diameter, mm, (mean (SD)) | 2.90 (0.81) |
| vFFR, (mean (SD)) | 0.87 (0.14) |
| vFFR value ≤ 0.80, n (%) | 5 (12.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mileva, N.; Nagumo, S.; Gallinoro, E.; Sonck, J.; Verstreken, S.; Dierkcx, R.; Heggermont, W.; Bartunek, J.; Goethals, M.; Heyse, A.; et al. Validation of Coronary Angiography-Derived Vessel Fractional Flow Reserve in Heart Transplant Patients with Suspected Graft Vasculopathy. Diagnostics 2021, 11, 1750. https://doi.org/10.3390/diagnostics11101750
Mileva N, Nagumo S, Gallinoro E, Sonck J, Verstreken S, Dierkcx R, Heggermont W, Bartunek J, Goethals M, Heyse A, et al. Validation of Coronary Angiography-Derived Vessel Fractional Flow Reserve in Heart Transplant Patients with Suspected Graft Vasculopathy. Diagnostics. 2021; 11(10):1750. https://doi.org/10.3390/diagnostics11101750
Chicago/Turabian StyleMileva, Niya, Sakura Nagumo, Emanuele Gallinoro, Jeroen Sonck, Sofie Verstreken, Riet Dierkcx, Ward Heggermont, Jozef Bartunek, Marc Goethals, Alex Heyse, and et al. 2021. "Validation of Coronary Angiography-Derived Vessel Fractional Flow Reserve in Heart Transplant Patients with Suspected Graft Vasculopathy" Diagnostics 11, no. 10: 1750. https://doi.org/10.3390/diagnostics11101750
APA StyleMileva, N., Nagumo, S., Gallinoro, E., Sonck, J., Verstreken, S., Dierkcx, R., Heggermont, W., Bartunek, J., Goethals, M., Heyse, A., Barbato, E., De Bruyne, B., Collet, C., & Vanderheyden, M. (2021). Validation of Coronary Angiography-Derived Vessel Fractional Flow Reserve in Heart Transplant Patients with Suspected Graft Vasculopathy. Diagnostics, 11(10), 1750. https://doi.org/10.3390/diagnostics11101750







