Cardiovascular Magnetic Resonance in Peripartum Cardiomyopathy: Comparison with Idiopathic Dilated Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CMR Examination
2.3. Image Analysis
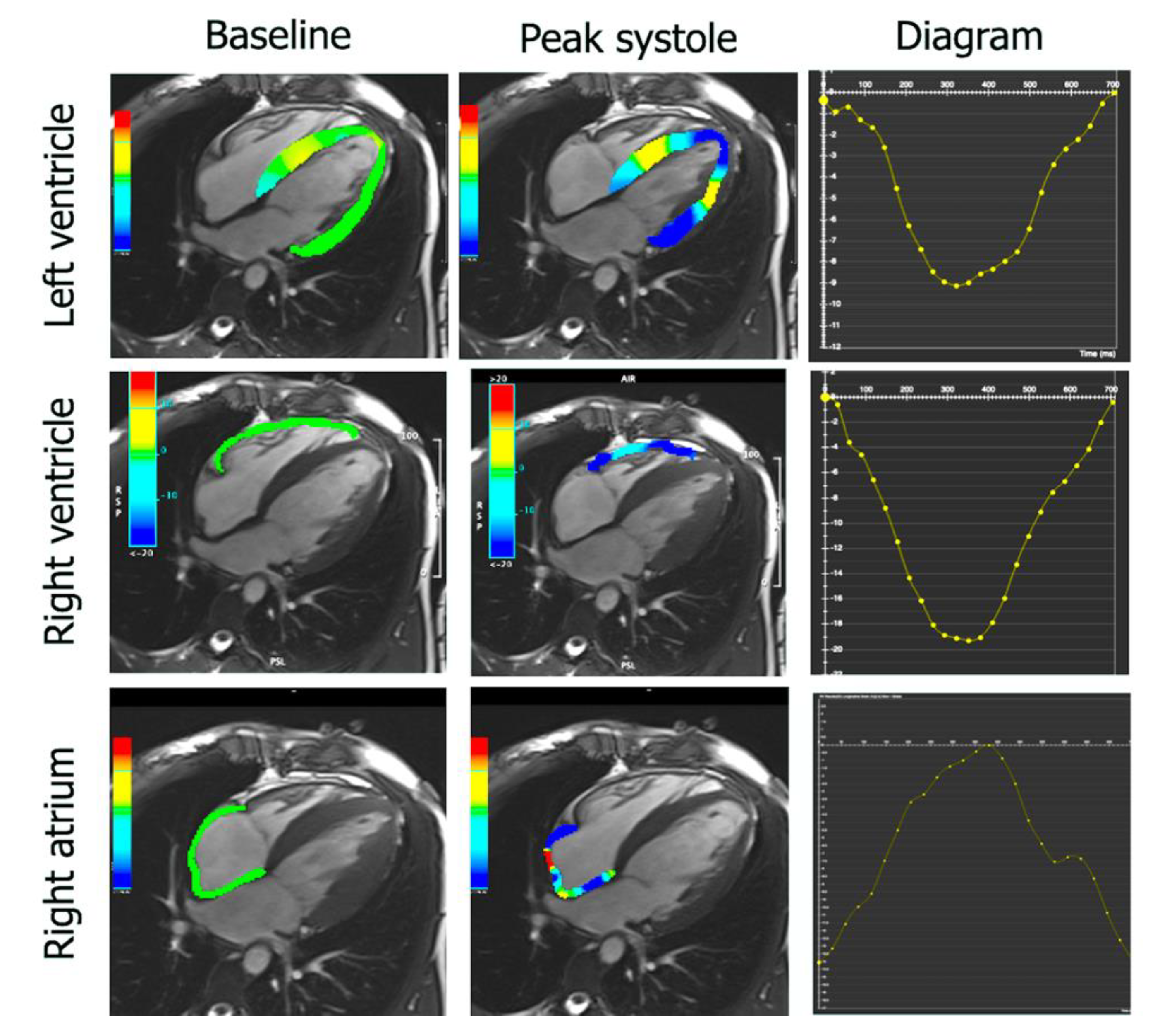
2.4. Feature Tracking
2.5. Statistics
3. Results
4. Discussion
- Patients with PPCM have lower LV stroke volume index and higher RA volume with lower RA total ejection fraction in comparison to women with DCM.
- In patients with PPCM, some indices of LV mechanics such as LV GLS and LV GLS rate, as well as LV GRS rate, are less impaired than in DCM women.
- Both PPCM and DCM female patients with LGE have more dilated and impaired left and right ventricles in comparison to patients without LGE on CMR imaging.
- Almost all LV and RV mechanical indices are more impaired in patients with LGE in comparison to patients without LGE in both PPCM and DCM groups.
4.1. CMR for Diagnosis and Prognostication
4.2. LV Fibrosis in PPCM
4.3. LV Fibrosis and Myocardial Mechanics
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bollen, I.A.; Ehler, E.; Fleischanderl, K.; Bouwman, F.; Kempers, L.; Ricke-Hoch, M.; Hilfiker-Kleiner, D.; Dos Remedios, C.G.; Krüger, M.; Vink, A.; et al. Myofilament Remodeling and Function Is More Impaired in Peripartum Cardiomyopathy Compared with Dilated Cardiomyopathy and Ischemic Heart Disease. Am. J. Pathol. 2017, 187, 2645–2658. [Google Scholar] [CrossRef]
- van Hoeven, K.; Kitsis, R.N.; Katz, S.D.; Factor, S.M. Peripartum versus idiopathic dilated cardiomyopathy in young women—A comparison of clinical, pathologic and prognostic features. Int. J. Cardiol. 1993, 40, 57–65. [Google Scholar] [CrossRef]
- Pillarisetti, J.; Kondur, A.; Alani, A.; Reddy, M.; Vacek, J.; Weiner, C.P.; Ellerbeck, E.; Schreiber, T.; Lakkireddy, D. Peripartum Cardiomyopathy. J. Am. Coll. Cardiol. 2014, 63, 2831–2839. [Google Scholar] [CrossRef]
- Kryczka, K.E.; Demkow, M.; Dzielinska, Z. Peripartum cardiomyopathy—A cardiovascular disease in pregnancy and puerperi-um. The actual state of knowledge, challenges, and perspectives. Ginekol. Pol. 2020, 92, 147–152. [Google Scholar] [CrossRef]
- Lane, R.E.; Cowie, M.R.; Chow, A.W. Prediction and prevention of sudden cardiac death in heart failure. Heart 2005, 91, 674–680. [Google Scholar] [CrossRef]
- Pierce, J.A. Familial Occurrence of Postpartal Heart Failure. Arch. Intern. Med. 1963, 111, 651–655. [Google Scholar] [CrossRef]
- Ware, J.; Julie, D.; Mazaika, E.; Yasso, C.M.; DeSouza, T.; Cappola, T.P.; Tsai, E.; Hilfiker-Kleiner, D.; Kamiya, C.A.; Mazzarotto, F.; et al. Shared Genetic Predisposition in Peripartum and Dilated Cardiomyopathies. N. Engl. J. Med. 2016, 374, 233–241. [Google Scholar] [CrossRef]
- Van Spaendonck-Zwarts, K.Y.; Posafalvi, A.; Berg, M.V.D.; Hilfiker-Kleiner, D.; Bollen, I.A.; Sliwa, K.; Alders, M.; Almomani, R.; Van Langen, I.M.; Van Der Meer, P.; et al. Titin gene mutations are common in families with both peripartum cardiomyopathy and dilated cardiomyopathy. Eur. Heart J. 2014, 35, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Petryka-Mazurkiewicz, J.; Kryczka, K.; Marona, M.; Kuriata, J.; Sitkowska-Rysiak, E.; Konopka, A.; Marczak, M.; Kołsut, P.; Kuśmierczyk, M.; Demkow, M.; et al. Biventricular assist device–induced recovery from acute heart failure in peripartum cardiomyopathy on cardiac magnetic resonance imaging. Kardiol. Pol. 2020, 78, 1284–1285. [Google Scholar] [CrossRef]
- Mazurkiewicz, Ł.; Petryka, J.; Śpiewak, M.; Miłosz-Wieczorek, B.; Małek, Ł.A.; Jasińska, A.; Jarmus, E.; Marczak, M.; Misko, J.; Grzybowski, J. Clinical and prognostic relevancy of left ventricular trabeculation assessed by cardiac magnetic resonance in patients with dilated cardiomyopathy. Kardiol. Pol. 2017, 75, 794–803. [Google Scholar] [CrossRef]
- Mouquet, F.; Lions, C.; De Groote, P.; Bouabdallaoui, N.; Willoteaux, S.; Dagorn, J.; Deruelle, P.; Lamblin, N.; Bauters, C.; Beregi, J.P. Characterisation of peripartum cardiomyopathy by cardiac magnetic resonance imaging. Eur. Radiol. 2008, 18, 2765–2769. [Google Scholar] [CrossRef]
- Sliwa, K.; Hilfiker-Kleiner, D.; Petrie, M.C.; Mebazaa, A.; Pieske, B.; Buchmann, E.; Regitz-Zagrosek, V.; Schaufelberger, M.; Tavazzi, L.; Van Veldhuisen, D.J.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2010, 12, 767–778. [Google Scholar] [CrossRef]
- Iles, L.M.; Ellims, A.H.; Llewellyn, H.; Hare, J.L.; Kaye, D.M.; McLean, C.A.; Taylor, A. Histological validation of cardiac magnetic resonance analysis of regional and diffuse interstitial myocardial fibrosis. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 14–22. [Google Scholar] [CrossRef]
- Mazurkiewicz, Ł.; Petryka, J.; Spiewak, M.; Miłosz-Wieczorek, B.; Werys, K.; Małek, Ł.A.; Polanska-Skrzypczyk, M.; Ojrzynska, N.; Kubik, A.; Marczak, M.; et al. Biventricular mechanics in prediction of severe myocardial fibrosis in patients with dilated cardiomyopathy: CMR study. Eur. J. Radiol. 2017, 91, 71–81. [Google Scholar] [CrossRef]
- Mazurkiewicz, Ł.; Ziółkowska, L.; Petryka, J.; Śpiewak, M.; Łukasz, M.; Kubik, A.; Marczak, M.; Misko, J.; Brzezińska-Rajszys, G. Biatrial performance in children with hypertrophic cardiomyopathy: CMR study. Eur. Radiol. 2018, 28, 5148–5159. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Ismail, T.F.; Jabbour, A.; Alpendurada, F.; Guha, K.; Ismail, N.A.; Raza, S.; Khwaja, J.; Brown, T.D.; Morarji, K.; et al. The Prevalence and Prognostic Significance of Right Ventricular Systolic Dysfunction in Nonischemic Dilated Cardiomyopathy. Circulation 2013, 128, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Röntgen, P.; Vogel-Claussen, J.; Schwab, J.; Westenfeld, R.; Ehlermann, P.; Berliner, D.; Podewski, E.; Hilfiker-Kleiner, D.; Bauersachs, J. Prognostic implication of right ventricular involvement in peripartum cardiomyopathy: A cardiovascular magnetic resonance study. ESC Heart Fail. 2015, 2, 139–149. [Google Scholar] [CrossRef]
- Spiewak, M.; Małek, Ł.A.; Petryka, J.; Mazurkiewicz, L.; Marczak, M.; Biernacka, E.K.; Kowalski, M.; Hoffman, P.; Demkow, M.; Miśko, J.; et al. Determinants of left- and right?ventricular ejection fractions in patients with repaired tetralogy of Fallot: A cardiac magnetic resonance imaging study. Pol. Arch. Intern. Med. 2013, 123, 539–546. [Google Scholar] [CrossRef][Green Version]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [CrossRef]
- Anderson, J.; Horne, B.; Pennell, D. Atrial Dimensions in Health and Left Ventricular Disease Using Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2005, 7, 671–675. [Google Scholar] [CrossRef]
- Pritchett, A.M.; Mahoney, D.W.; Jacobsen, S.; Rodeheffer, R.J.; Karon, B.L.; Redfield, M.M. Diastolic dysfunction and left atrial volume: A population-based study. J. Am. Coll. Cardiol. 2005, 45, 87–92. [Google Scholar] [CrossRef]
- Gulati, A.; Ismail, T.F.; Jabbour, A.; Ismail, N.; Morarji, K.; Ali, A.; Raza, S.; Khwaja, J.; Brown, T.D.; Liodakis, E.; et al. Clinical utility and prognostic value of left atrial volume assessment by cardiovascular magnetic resonance in non-ischaemic dilated cardiomyopathy. Eur. J. Heart Fail. 2013, 15, 660–670. [Google Scholar] [CrossRef]
- Doherty, N.E.; Seelos, K.C.; Suzuki, J.-I.; Caputo, G.R.; O’Sullivan, M.; Sobol, S.M.; Cavero, P.; Chatterjee, K.; Parmley, W.W.; Higgins, C.B. Application of cine nuclear magnetic resonance imaging for sequential evaluation of response to angiotensin-converting enzyme inhibitor therapy in dilated cardiomyopathy. J. Am. Coll. Cardiol. 1992, 19, 1294–1302. [Google Scholar] [CrossRef]
- Bellenger, N.; Davies, L.C.; Francis, J.; Coats, A.S.; Pennell, D. Reduction in Sample Size for Studies of Remodeling in Heart Failure by the Use of Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2000, 2, 271–278. [Google Scholar] [CrossRef]
- Marmursztejn, J.; Vignaux, O.; Goffinet, F.; Cabanes, L.; Duboc, D. Delayed-enhanced cardiac magnetic resonance imaging features in peripartum cardiomyopathy. Int. J. Cardiol. 2009, 137, e63–e64. [Google Scholar] [CrossRef]
- Arora, N.P.; Mahajan, N.; Mohamad, T.; Kottam, A.; Afonso, L.C.; Danrad, R.; Li, T. Cardiac Magnetic Resonance Imaging in Peripartum Cardiomyopathy. Am. J. Med. Sci. 2014, 347, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Leurent, G.; Baruteau, A.; Larralde, A.; Ollivier, R.; Schleich, J.; Boulmier, D.; Bedossa, M.; Langella, B.; Le Breton, H. Contribution of cardiac MRI in the comprehension of peripartum cardiomyopathy pathogenesis. Int. J. Cardiol. 2009, 132, e91–e93. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Weiss, R.G.; Thiemann, D.R.; Kitagawa, K.; Schmidt, A.; Dalal, D.; Lai, S.; Bluemke, D.; Gerstenblith, G.; Marbán, E.; et al. Late Gadolinium Enhancement by Cardiovascular Magnetic Resonance Heralds an Adverse Prognosis in Nonischemic Cardiomyopathy. J. Am. Coll. Cardiol. 2008, 51, 2414–2421. [Google Scholar] [CrossRef]
- Mewton, N.; Liu, C.Y.; Croisille, P.; Bluemke, D.; Lima, J.A. Assessment of Myocardial Fibrosis with Cardiovascular Magnetic Resonance. J. Am. Coll. Cardiol. 2011, 57, 891–903. [Google Scholar] [CrossRef]
- Ntusi, N.B.A.; Chin, A. Characterisation of peripartum cardiomyopathy by cardiac magnetic resonance imaging. Eur. Radiol. 2009, 19, 1324–1325. [Google Scholar] [CrossRef]
- Buss, S.J.; Breuninger, K.; Lehrke, S.; Voss, A.; Galuschky, C.; Lossnitzer, D.; Andre, F.; Ehlermann, P.; Franke, J.; Taeger, T.; et al. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, M.; Kagiyama, N.; Hasselberg, N.E.; Blauwet, L.A.; Briller, J.; Cooper, L.; Fett, J.D.; Hsich, E.; Wells, G.; McNamara, D.; et al. Global Left Ventricular Strain at Presentation Is Associated with Subsequent Recovery in Patients with Peripartum Cardiomyopathy. J. Am. Soc. Echocardiogr. 2019, 32, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Briasoulis, A.; Mocanu, M.; Marinescu, K.; Qaqi, O.; Palla, M.; Telila, T.; Afonso, L. Longitudinal systolic strain profiles and outcomes in peripartum cardiomyopathy. Echocardiography 2016, 33, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Mondillo, S.; Righini, F.M.; Lisi, M.; Dokollari, A.; Lindqvist, P.; Maccherini, M.; Henein, M. Left Ventricular Deformation and Myocardial Fibrosis in Patients With Advanced Heart Failure Requiring Transplantation. J. Card. Fail. 2016, 22, 901–907. [Google Scholar] [CrossRef] [PubMed]
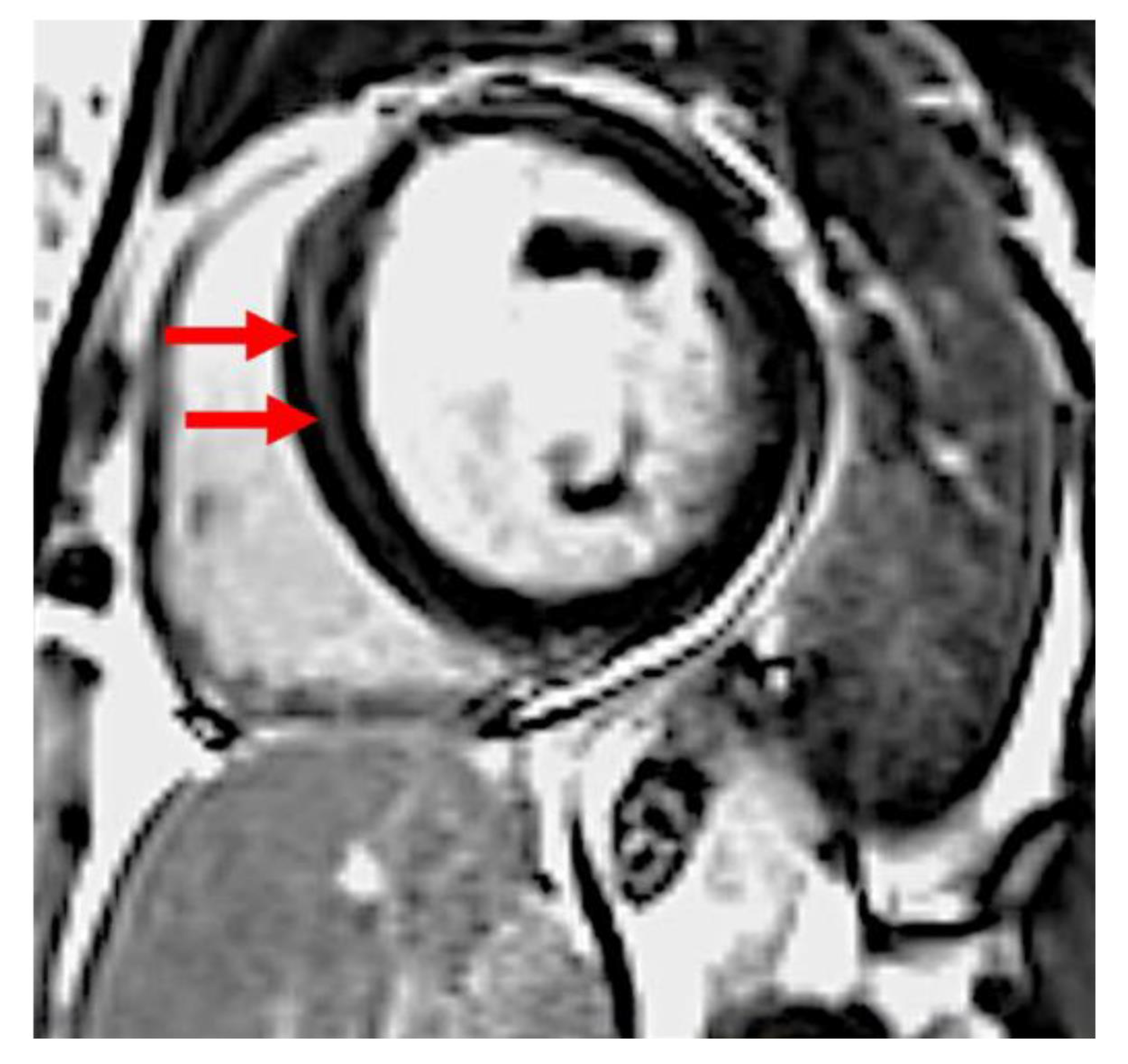
| PPCM n = 21 | Female DCM n = 30 | p | |
|---|---|---|---|
| Age (years) | 30.5 ± 5.9 | 41.5 ± 16.8 | 0.03 |
| BSA (m2) | 1.76 ± 0.19 | 1.77 ± 0.16 | 0.91 |
| Diabetes n (%) | 0 (0%) | 2 (7%) | 0.23 |
| Arterial hypertension (%) | 3 (14%) | 2 (7%) | 0.17 |
| NYHA class | 2.97 | 2.20 | 0.001 |
| HR (beats/min) | 83.1 ± 15.9 | 69.5 ± 11.4 | 0.002 |
| SBP (mmHg) | 107.3 ± 19.2 | 110.9 ± 14.1 | 0.47 |
| DBP (mmHg) | 69.2 ± 15.0 | 68.9 ± 8.9 | 0.93 |
| Troponin T (ng/mL) | 10.7 (0.001–6800) | 5.45 (0.001–23.7) | 0.34 |
| NT-proBNP (pg/mL) | 3007 (27–35,000) | 2167 (79–3036) | 0.048 |
| B-blocker n (%) | 17 (81%) | 30 (100%) | 0.02 |
| ACE-I/ARB n (%) | 20 (95%) | 30 (100%) | 0.16 |
| Aldosterone antagonist n (%) | 15 (71%) | 20 (66%) | 0.39 |
| Left ventricle | |||
| LVEDV (mL) | 245.5 ± 97.4 | 266.9 ± 61.6 | 0.36 |
| LVESV (mL) | 167.1 ± 89.7 | 178.2 ± 68.6 | 0.63 |
| LVSV (mL) | 78.6 ± 22.0 | 88.5 ± 20.3 | 0.12 |
| LVEF (%) | 35.9 ± 13.7 | 35.2 ± 11.2 | 0.85 |
| LVM (g) | 125.9 ± 33.2 | 126.1 ± 36.2 | 0.99 |
| LVEDVI (mL/m2) | 138.3 ± 50.1 | 151.7 ± 35.5 | 0.29 |
| LVESVI (mL/m2) | 93.7 ± 48.6 | 101.3 ± 39.1 | 0.56 |
| LVSVI (mL/m2) | 44.5 ± 10.5 | 50.3 ± 11.8 | 0.04 |
| LVMI (g/m2) | 72.2 ± 16.8 | 71.2 ± 19.7 | 0.86 |
| Right ventricle | |||
| RVEDV (mL) | 162.9 ± 44.7 | 152.1 ± 39.8 | 0.39 |
| RVESV (mL) | 96.2 ± 43.8 | 93.2 ± 45.1 | 0.82 |
| RVSV (mL) | 66.8 ± 23.4 | 59.1 ± 20.2 | 0.24 |
| RVEF (%) | 42.6 ± 13.2 | 41.1 ± 14.3 | 0.74 |
| RVEDVI (mL/m2) | 92.7 ± 23.4 | 85.9 ± 23.0 | 0.33 |
| RVESVI (mL/m2) | 54.8 ± 25.3 | 52.0 ± 23.4 | 0.70 |
| RVSVI (mL/m2) | 37.6 ± 11.5 | 34.1 ± 13.6 | 0.37 |
| Left atrium | |||
| LA area (cm2) | 26.1 ± 7.5 | 27.2 ± 5.4 | 0.57 |
| LAV max (mL) | 93.1 ± 34.7 | 93.9 ± 21.2 | 0.93 |
| LAV min (mL) | 50.2 ± 28.5 | 46.2 ± 19.8 | 0.61 |
| LAV pac (mL) | 74.6 ± 35.8 | 73.5 ± 24.2 | 0.91 |
| Total LAEF (%) | 48.9 ± 13.6 | 52.3 ± 12.1 | 0.41 |
| Passive LAEF (%) | 23.0 ± 12.0 | 23.0 ± 12.1 | 0.99 |
| Active LAEF (%) | 33.9 ± 12.6 | 37.8 ± 14.6 | 0.40 |
| Right atrium | |||
| RA area | 22.3 ± 4.2 | 19.8 ± 3.6 | 0.01 |
| RAV max (mL) | 82.0 ± 22.0 | 74.3 ± 24.2 | 0.26 |
| RAV min (mL) | 47.2 ± 16.0 | 37.2 ± 8.2 | <0.01 |
| RAV pac (mL) | 68.7 ± 18.3 | 58.4 ± 13.9 | 0.02 |
| Total RAEF (%) | 42.4 ± 10.0 | 48.6 ± 9.8 | 0.02 |
| Passive RAEF (%) | 15.6 ± 8.7 | 20.0 ± 12.5 | 0.24 |
| Active RAEF (%) | 31.7 ± 10.3 | 35.6 ± 8.0 | 0.19 |
| LGE | |||
| LGE n (%) | 9 (43%) | 18 (60%) | 0.22 |
| LGE % (IQR) | 1.7% (0–2.9%) | 2.3% (0–4.5%) | 0.63 |
| PPCM n = 21 | Female DCM n = 30 | p | |
|---|---|---|---|
| Left ventricle | |||
| LV GRS (%) | 14.1 ± 6.4 | 13.9 ± 4.9 | 0.90 |
| LV GCS (%) | −11.0 ± 4.6 | −9.8 ± 3.3 | 0.32 |
| LV GLS (%) | −9.6 ± 3.9 | −7.7 ± 2.6 | 0.03 |
| LV GRS rate (s−1) | 0.91 ± 0.44 | 0.76 ± 0.21 | 0.22 |
| LV GCS rate (s−1) | −0.64 ± 0.26 | −0.53 ± 0.13 | 0.04 |
| LV GLS rate (s−1) | −0.55 ± 0.14 | −0.45 ± 0.12 | <0.01 |
| Right ventricle | |||
| RV GRS (%) | 14.1 ± 6.2 | 13.2 ± 8.9 | 0.70 |
| RV GCS (%) | −9.1 ± 3.8 | −8.3 ± 6.4 | 0.62 |
| RV GLS (%) | −14.7 ± 7.1 | 13.8 ± 6.8 | 0.68 |
| RV GRS rate (s−1) | 1.01 ± 0.49 | 0.83 ± 0.47 | 0.21 |
| RV GCS rate (s−1) | −0.54 ± 0.28 | −0.48 ± 0.26 | 0.49 |
| RV GLS rate (s−1) | −1.01 ± 0.40 | −0.86 ± 0.31 | 0.20 |
| Left atrium | |||
| LA GLS total (%) | 17.5 ± 9.2 | 19.8 ± 9.1 | 0.43 |
| LA GLS passive (%) | 9.3 ± 5.1 | 10.4 ± 4.1 | 0.46 |
| LA GLS active (%) | 8.2 ± 6.3 | 8.7 ± 4.4 | 0.74 |
| LA SRS (s−1) | 0.72 ± 0.35 | 0.75 ± 0.40 | 0.80 |
| LA SRE (s−1) | −0.66 ± 0.41 | −0.83 ± 0.50 | 0.25 |
| LA SRA (s−1) | −0.70 ± 0.40 | −0.62 ± 0.36 | 0.57 |
| Right atrium | |||
| RA GLS total (%) | 26.6 ± 9.6 | 26.5 ± 9.1 | 0.96 |
| RA GLS passive (%) | 11.2 ± 6.3 | 11.4 ± 5.8 | 0.94 |
| RA GLS active (%) | 15.4 ± 8.1 | 13.2 ± 5.8 | 0.32 |
| RA SRS (s−1) | 1.18 ± 0.40 | 1.14 ± 0.42 | 0.80 |
| RA SRE (s−1) | −0.59 ± 0.32 | −0.72 ± 0.48 | 0.34 |
| RA SRA (s−1) | −1.00 ± 0.67 | −0.89 ± 0.39 | 0.58 |
| PPCM without LGE n = 12 (67%) | PPCM with LGE n = 9 (43%) | p | DCM without LGE n = 12 (40%) | DCM with LGE n = 18 (60%) | p | |
|---|---|---|---|---|---|---|
| Age (years) | 29.9 ± 6.3 | 31.4 ± 5.7 | ns | 44.4 ± 14.8 | 39.6 ± 18.6 | 0.44 |
| Left ventricle | ||||||
| LVEDV (mL) | 201.9 ± 81.1 | 307.7 ± 88.1 | 0.022 | 236.0 ± 63.3 | 287.4 ± 54.3 | 0.02 |
| LVESV (mL) | 122.6 ± 70.0 | 230.6 ± 78.2 | 0.009 | 141.5 ± 56.9 | 202.7 ± 67.2 | 0.01 |
| LVSV (mL) | 79.5 ± 25.6 | 77.3 ± 17.5 | 0.84 | 94.3 ± 15.8 | 84.7 ± 22.9 | 0.20 |
| LVEF (%) | 42.5 ± 13.0 | 26.4 ± 8.1 | 0.007 | 41.2 ± 8.3 | 31.2 ± 11.4 | <0.01 |
| LVM (g) | 121.3 ± 38.3 | 132.4 ± 25.6 | 0.51 | 110.7 ± 31.9 | 136.3 ± 36.9 | 0.04 |
| LVEDVI (mL/m2) | 114.7 ± 39.8 | 172.0 ± 45.5 | 0.015 | 129.5 ± 30.5 | 166.6 ± 31.8 | <0.01 |
| LVESVI (mL/m2) | 69.2 ± 37.1 | 128.7 ± 42.3 | 0.008 | 77.3 ± 28.1 | 117.3 ± 38.1 | <0.01 |
| LVSVI (mL/m2) | 45.3 ± 11.9 | 43.3 ± 8.8 | 0.71 | 52.2 ± 9.1 | 49.1 ± 13.6 | 0.49 |
| LVMI (g/m2) | 69.2 ± 17.6 | 76.4 ± 15.7 | 0.40 | 60.3 ± 13.8 | 78.4 ± 20.2 | <0.01 |
| Right ventricle | ||||||
| RVEDV (mL) | 146.9 ± 45.1 | 185.7 ± 35.2 | 0.08 | 134.8 ± 41.7 | 163.6 ± 36.2 | 0.04 |
| RVESV (mL) | 75.0 ± 27.9 | 126.4 ± 46.2 | 0.012 | 70.3 ± 35.1 | 108.4 ± 46.2 | 0.01 |
| RVSV (mL) | 72.0 ± 25.5 | 59.4 ± 19.5 | 0.29 | 64.5 ± 21.3 | 55.4 ± 20.3 | 0.23 |
| RVEF (%) | 49.1 ± 8.8 | 33.4 ± 13.5 | 0.011 | 49.2 ± 13.7 | 35.8 ± 16.3 | 0.02 |
| RVEDVI (mL/m2) | 83.8 ± 22.9 | 105.4 ± 18.7 | 0.057 | 74.0 ± 23.5 | 93.9 ± 20.1 | 0.02 |
| RVESVI (mL/m2) | 42.7 ± 16.6 | 72.0 ± 26.5 | 0.013 | 38.3 ± 18.0 | 61.1 ± 22.9 | <0.01 |
| RVSVI (mL/m2) | 40.4 ± 12.3 | 33.6 ± 5.4 | 0.24 | 35.8 ± 14.1 | 32.9 ± 14.1 | 0.56 |
| Left atrium | ||||||
| LA area (cm2) | 21.4 ± 4.7 | 32.7 ± 5.4 | 0.0003 | 26.5 ± 5.3 | 27.7 ± 5.8 | 0.57 |
| LAV max (mL) | 73.5 ± 28.2 | 115.6 ± 29.8 | 0.015 | 90.6 ± 17.8 | 96.1 ± 24.6 | 0.53 |
| LAV min (mL) | 30.7 ± 14.5 | 72.4 ± 25.1 | 0.001 | 37.8 ± 16.7 | 51.5 ± 21.4 | 0.09 |
| LAV pac (mL) | 51.5 ± 23.7 | 101.1 ± 30.2 | 0.004 | 68.0 ± 24.4 | 77.0 ± 25.9 | 0.37 |
| Total LAEF (%) | 58.3 ± 10.1 | 38.1 ± 8.6 | 0.001 | 59.3 ± 11.7 | 47.9 ± 11.3 | 0.02 |
| Passive LAEF (%) | 31.3 ± 8.6 | 13.5 ± 8.2 | 0.001 | 26.6 ± 14.4 | 20.6 ± 12.3 | 0.25 |
| Active LAEF (%) | 39.0 ± 14.2 | 28.2 ± 8.8 | 0.11 | 44.4 ± 12.8 | 33.7 ± 15.5 | 0.07 |
| Right atrium | ||||||
| RA area | 21.7 ± 4.9 | 23.3 ± 3.1 | 0.46 | 22.2 ± 3.1 | 18.0 ± 2.9 | <0.01 |
| RAV max (mL) | 84.0 ± 26.3 | 79.7 ± 18.8 | 0.73 | 82.1 ± 16.9 | 69.4 ± 21.6 | 0.11 |
| RAV min (mL) | 48.0 ± 16.9 | 46.3 ± 16.8 | 0.84 | 40.0 ± 5.9 | 35.5 ± 9.6 | 0.18 |
| RAV pac (mL) | 67.1 ± 19.1 | 70.6 ± 19.6 | 0.73 | 61.0 ± 14.9 | 56.7 ± 14.5 | 0.49 |
| Total RAEF (%) | 42.1 ± 11.1 | 42.8 ± 9.8 | 0.91 | 50.3 ± 9.3 | 47.5 ± 10.8 | 0.01 |
| Passive RAEF (%) | 19.2 ± 5.8 | 11.4 ± 10.4 | 0.09 | 25.0 ± 15.1 | 16.8 ± 11.0 | 0.01 |
| Active RAEF (%) | 28.7 ± 11.0 | 35.2 ± 9.3 | 0.24 | 33.3 ± 6.4 | 37.0 ± 9.2 | 0.46 |
| PPCM without LGE n = 12 (67%) | PPCM with LGE n = 9 (43%) | p | DCM without LGE n = 12 (40%) | DCM with LGE n = 18 (60%) | p | |
|---|---|---|---|---|---|---|
| Left ventricle | ||||||
| LV GRS (%) | 17.9 ± 5.8 | 9.2 ± 3.4 | 0.002 | 16.9 ± 2.7 | 11.9 ± 5.2 | <0.01 |
| LV GCS (%) | −13.3 ± 4.5 | −8.0 ± 2.9 | 0.013 | −11.9 ± 2.0 | −8.5 ± 3.5 | <0.01 |
| LV GLS (%) | −12.3 ± 3.4 | −6.6 ± 1.9 | 0.002 | −9.1 ± 1.7 | −6.8 ± 2.8 | 0.01 |
| LV GRS rate (s−1) | 1.1 ± 0.43 | 0.62 ± 0.28 | 0.015 | 0.86 ± 0.21 | 0.70 ± 0.21 | 0.04 |
| LV GCS rate (s−1) | −0.78 ± 0.22 | −0.46 ± 0.21 | 0.011 | −0.62 ± 0.11 | −0.47 ± 0.13 | <0.01 |
| LV GLS rate (s−1) | −0.61 ± 0.16 | −0.47 ± 0.04 | 0.053 | −0.42 ± 0.09 | −0.46 ± 0.14 | 0.38 |
| Right ventricle | ||||||
| RV GRS (%) | 17.9 ± 5.3 | 9.3 ± 3.6 | 0.002 | 17.1 ± 6.2 | 10.6 ± 10.0 | 0.03 |
| RV GCS (%) | −11.1 ± 2.3 | −6.5 ± 3.3 | 0.015 | −11.7 ± 5.3 | −6.1 ± 6.6 | 0.01 |
| RV GLS (%) | −17.9 ± 7.3 | −11.0 ± 5.5 | 0.06 | −16.6 ± 6.7 | −12.0 ± 7.0 | 0.09 |
| RV GRS rate (s−1) | 1.2 ± 0.52 | 0.74 ± 0.36 | 0.044 | 1.10 ± 0.48 | 0.64 ± 0.40 | 0.01 |
| RV GCS rate (s−1) | −0.62 ± 0.3 | −0.44 ± 0.23 | 0.21 | −0.62 ± 0.19 | −0.39 ± 0.27 | 0.01 |
| RV GLS rate (s−1) | −1.09 ± 0.42 | −0.92 ± 0.41 | 0.45 | −1.01 ± 0.32 | −0.78 ± −0.30 | 0.06 |
| Left atrium | ||||||
| LA GLS total (%) | 22.7 ± 7.9 | 10.8 ± 6.6 | 0.006 | 26.3 ± 7.2 | 15.8 ± 8.4 | <0.01 |
| LA GLS passive (%) | 11.1 ± 4.9 | 7.0 ± 4.8 | 0.11 | 12.0 ± 3.0 | 9.4 ± 4.7 | 0.12 |
| LA GLS active (%) | 12.5 ± 5.2 | 2.7 ± 2.3 | <0.001 | 11.9 ± 2.1 | 6.8 ± 4.6 | <0.01 |
| LA SRS (s−1) | 0.91 ± 0.33 | 0.50 ± 0.24 | 0.014 | 0.94 ± 0.45 | 0.64 ± 0.35 | 0.06 |
| LA SRE (s−1) | −0.83 ± −0.43 | −0.45 ± 0.30 | 0.06 | −0.92 ± 0.63 | −0.78 ± 0.45 | 0.50 |
| LA SRA (s−1) | −0.72 ± 0.46 | −0.66 ± 0.37 | 0.77 | −0.74 ± 0.37 | −0.56 ± 0.37 | 0.23 |
| Right atrium | ||||||
| RA GLS total (%) | 27.1 ± 9.0 | 26.0 ± 11.4 | 0.83 | 32.0 ± 9.9 | 23.1 ± 7.5 | 0.01 |
| RA GLS passive (%) | 10.5 ± 5.3 | 12.2 ± 7.9 | 0.62 | 15.1 ± 6.6 | 9.1 ± 4.3 | <0.01 |
| RA GLS active (%) | 16.7 ± 10.0 | 13.8 ± 5.5 | 0.51 | 11.9 ± 4.0 | 14.0 ± 7.0 | 0.37 |
| RA SRS (s−1) | 1.2 ± 0.41 | 1.1 ± 0.42 | 0.54 | 1.4 ± 0.42 | 0.99 ± 0.39 | 0.02 |
| RA SRE (s−1) | −0.65 ± 0.29 | −0.50 ± 0.37 | 0.39 | −0.88 ± 0.69 | −0.62 ± 0.32 | 0.25 |
| RA SRA (s−1) | −0.79 ± 0.49 | −1.2 ± 0.85 | 0.20 | −0.79 ± 0.42 | −0.96 ± 0.41 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petryka-Mazurkiewicz, J.; Kryczka, K.; Mazurkiewicz, Ł.; Miłosz-Wieczorek, B.; Śpiewak, M.; Marczak, M.; Henzel, J.; Grzybowski, J.; Demkow, M.; Dzielińska, Z. Cardiovascular Magnetic Resonance in Peripartum Cardiomyopathy: Comparison with Idiopathic Dilated Cardiomyopathy. Diagnostics 2021, 11, 1752. https://doi.org/10.3390/diagnostics11101752
Petryka-Mazurkiewicz J, Kryczka K, Mazurkiewicz Ł, Miłosz-Wieczorek B, Śpiewak M, Marczak M, Henzel J, Grzybowski J, Demkow M, Dzielińska Z. Cardiovascular Magnetic Resonance in Peripartum Cardiomyopathy: Comparison with Idiopathic Dilated Cardiomyopathy. Diagnostics. 2021; 11(10):1752. https://doi.org/10.3390/diagnostics11101752
Chicago/Turabian StylePetryka-Mazurkiewicz, Joanna, Karolina Kryczka, Łukasz Mazurkiewicz, Barbara Miłosz-Wieczorek, Mateusz Śpiewak, Magdalena Marczak, Jan Henzel, Jacek Grzybowski, Marcin Demkow, and Zofia Dzielińska. 2021. "Cardiovascular Magnetic Resonance in Peripartum Cardiomyopathy: Comparison with Idiopathic Dilated Cardiomyopathy" Diagnostics 11, no. 10: 1752. https://doi.org/10.3390/diagnostics11101752
APA StylePetryka-Mazurkiewicz, J., Kryczka, K., Mazurkiewicz, Ł., Miłosz-Wieczorek, B., Śpiewak, M., Marczak, M., Henzel, J., Grzybowski, J., Demkow, M., & Dzielińska, Z. (2021). Cardiovascular Magnetic Resonance in Peripartum Cardiomyopathy: Comparison with Idiopathic Dilated Cardiomyopathy. Diagnostics, 11(10), 1752. https://doi.org/10.3390/diagnostics11101752





