The Diagnostic Accuracy of LOGIQ S8 and E9 Shear Wave Elastography for Staging Hepatic Fibrosis, in Comparison with Transient Elastography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Endpoint
2.2. Liver Histology and Transient Elastography
2.3. Shear Wave Elastography
2.4. Sample Size Calculation
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
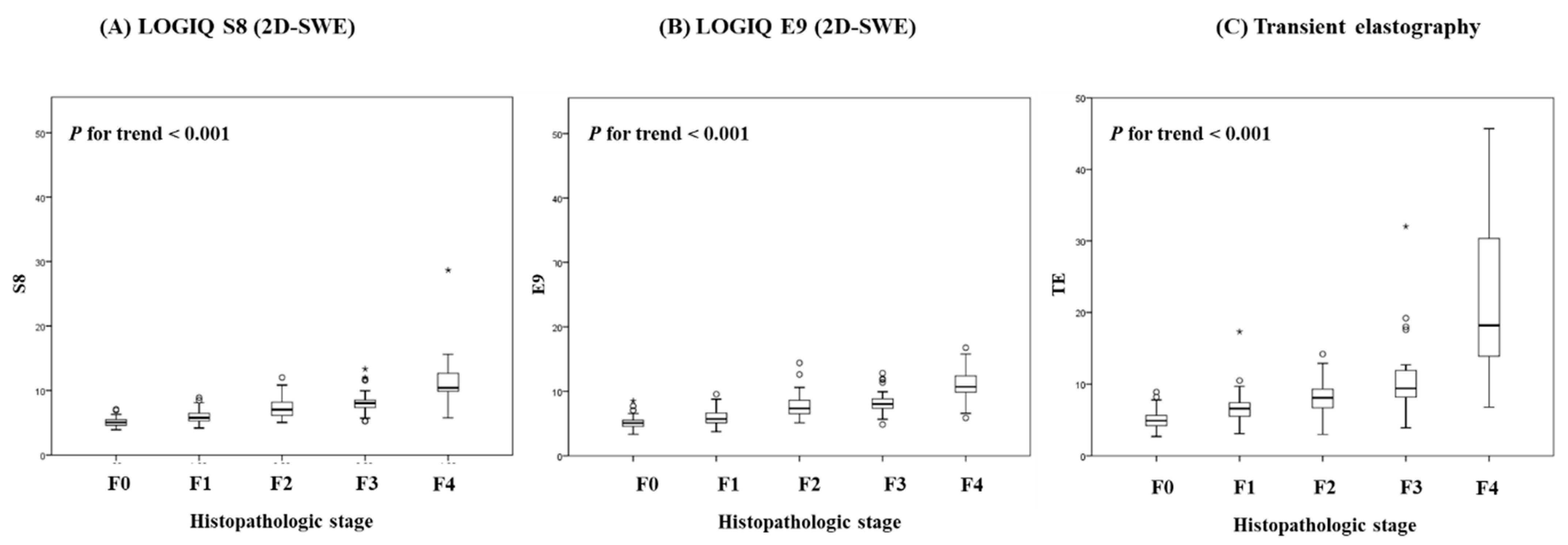
3.2. Diagnostic Performances of S8 2D-SWE, E9 2D-SWE and TE for Distinguishing Liver Fibrosis Stage
3.3. Interobserver Reliability between Two Types of 2D-SWEs, S8 and E9
3.4. Diagnostic Performances in Comparison with Transient Elastography
3.5. Correlation among Values Obtained by APRI Index and Platelet Count and LS by S8, E9 and TE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Marcellin, P.; Kutala, B.K. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018, 38 (Suppl. 1), 2–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.M.; Venkatesh, S.K.; Ehman, R.L.; Jhaveri, K.; Kamath, P.; Ohliger, M.A.; Samir, A.E.; Silva, A.C.; Taouli, B.; Torbenson, M.S.; et al. Evaluation of hepatic fibrosis: A review from the society of abdominal radiology disease focus panel. Abdom. Radiol. 2017, 42, 2037–2053. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Kim, J.I.; Lee, B.; Kim, S.G.; Jung, M.J.; Kim, Y.S.; Jeong, S.W.; Jang, J.Y.; Yoo, J.J. Clinical application of ultrasonography-guided percutaneous liver biopsy and its safety over 18 years. Clin. Mol. Hepatol. 2020, 26, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Cadranel, J.F.; Rufat, P.; Degos, F. Practices of liver biopsy in France: Results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 2000, 32, 477–481. [Google Scholar] [CrossRef]
- Bravo, A.A.; Sheth, S.G.; Chopra, S. Liver biopsy. N. Engl. J. Med. 2001, 344, 495–500. [Google Scholar] [CrossRef]
- Maharaj, B.; Maharaj, R.J.; Leary, W.P.; Cooppan, R.M.; Naran, A.D.; Pirie, D.; Pudifin, D.J. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet 1986, 1, 523–525. [Google Scholar] [CrossRef]
- Bedossa, P.; Dargere, D.; Paradis, V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003, 38, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Guha, I.N.; Parkes, J.; Roderick, P.; Chattopadhyay, D.; Cross, R.; Harris, S.; Kaye, P.; Burt, A.D.; Ryder, S.D.; Aithal, G.P.; et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology 2008, 47, 455–460. [Google Scholar] [CrossRef]
- Vali, Y.; Lee, J.; Boursier, J.; Spijker, R.; Loffler, J.; Verheij, J.; Brosnan, M.J.; Bocskei, Z.; Anstee, Q.M.; Bossuyt, P.M.; et al. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: A systematic review and meta-analysis. J. Hepatol. 2020, 73, 252–262. [Google Scholar] [CrossRef]
- Sandrin, L.; Fourquet, B.; Hasquenoph, J.M.; Yon, S.; Fournier, C.; Mal, F.; Christidis, C.; Ziol, M.; Poulet, B.; Kazemi, F.; et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef]
- Castera, L.; Vergniol, J.; Foucher, J.; Le Bail, B.; Chanteloup, E.; Haaser, M.; Darriet, M.; Couzigou, P.; De Ledinghen, V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005, 128, 343–350. [Google Scholar] [CrossRef]
- Loomba, R.; Wolfson, T.; Ang, B.; Hooker, J.; Behling, C.; Peterson, M.; Valasek, M.; Lin, G.; Brenner, D.; Gamst, A.; et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: A prospective study. Hepatology 2014, 60, 1920–1928. [Google Scholar] [CrossRef] [Green Version]
- Foucher, J.; Chanteloup, E.; Vergniol, J.; Castera, L.; Le Bail, B.; Adhoute, X.; Bertet, J.; Couzigou, P.; de Ledinghen, V. Diagnosis of cirrhosis by transient elastography (FibroScan): A prospective study. Gut 2006, 55, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Vizzutti, F.; Arena, U.; Romanelli, R.G.; Rega, L.; Foschi, M.; Colagrande, S.; Petrarca, A.; Moscarella, S.; Belli, G.; Zignego, A.L.; et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology 2007, 45, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.R.; Yoo, J.J.; Kang, S.H.; Jeong, S.W.; Kim, M.Y.; Cho, Y.K.; Chang, Y.; Kim, S.G.; Jang, J.Y.; Kim, Y.S.; et al. The cut-off value of transient elastography to the value of hepatic venous pressure gradient in alcoholic cirrhosis. Clin. Mol. Hepatol. 2021, 27, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Kettaneh, A.; Marcellin, P.; Douvin, C.; Poupon, R.; Ziol, M.; Beaugrand, M.; de Ledinghen, V. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: A prospective study of 935 patients. J. Hepatol. 2007, 46, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraioli, G.; Friedrich-Rust, M.; Gilja, O.H.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017, 38, e16–e47. [Google Scholar] [CrossRef] [Green Version]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.H.; Cosgrove, D.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: Basic principles and terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, R.G.; Ferraioli, G.; Palmeri, M.L.; Goodman, Z.D.; Garcia-Tsao, G.; Rubin, J.; Garra, B.; Myers, R.P.; Wilson, S.R.; Rubens, D.; et al. Elastography Assessment of Liver Fibrosis: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2015, 276, 845–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, R.G.; Wilson, S.R.; Rubens, D.; Garcia-Tsao, G.; Ferraioli, G. Update to the Society of Radiologists in Ultrasound Liver Elastography Consensus Statement. Radiology 2020, 296, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Sporea, I.; Bende, F.; Popescu, A.; Lupusoru, R.; Fofiu, R.; Sirli, R. Are there different cut-off values for staging liver fibrosis using 2D-SWE implemented on different systems from the same manufacturer? Med. Ultrason. 2020, 22, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Bende, F.; Sporea, I.; Sirli, R.; Nistorescu, S.; Fofiu, R.; Baldea, V.; Popescu, A. The Performance of a 2-Dimensional Shear-Wave Elastography Technique for Predicting Different Stages of Liver Fibrosis Using Transient Elastography as the Control Method. Ultrasound Q. 2020, 37, 97–104. [Google Scholar] [CrossRef]
- Ryu, H.; Ahn, S.J.; Yoon, J.H.; Lee, J.M. Reproducibility of liver stiffness measurements made with two different 2-dimensional shear wave elastography systems using the comb-push technique. Ultrasonography 2019, 38, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Goodman, Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 2007, 47, 598–607. [Google Scholar] [CrossRef] [PubMed]
- de Franchis, R.; Baveno, V.I.F. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Ferraioli, G.; Tinelli, C.; Dal Bello, B.; Zicchetti, M.; Filice, G.; Filice, C.; Liver Fibrosis Study, G. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: A pilot study. Hepatology 2012, 56, 2125–2133. [Google Scholar] [CrossRef]
- Herrmann, E.; de Ledinghen, V.; Cassinotto, C.; Chu, W.C.; Leung, V.Y.; Ferraioli, G.; Filice, C.; Castera, L.; Vilgrain, V.; Ronot, M.; et al. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology 2018, 67, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Park, C.; Yoon, H.M.; Jung, A.Y.; Lee, J.S.; Jung, S.C.; Cho, Y.A. Technical performance of shear wave elastography for measuring liver stiffness in pediatric and adolescent patients: A systematic review and meta-analysis. Eur. Radiol. 2019, 29, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Kuroda, H.; Fujiwara, Y.; Yoshida, Y.; Miyasaka, A.; Kamiyama, N.; Takikawa, Y. Accuracy of 2D shear wave elastography in the diagnosis of liver fibrosis in patients with chronic hepatitis C. J. Clin. Ultrasound 2018, 46, 319–327. [Google Scholar] [CrossRef]
- Furlan, A.; Tublin, M.E.; Yu, L.; Chopra, K.B.; Lippello, A.; Behari, J. Comparison of 2D Shear Wave Elastography, Transient Elastography, and MR Elastography for the Diagnosis of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. AJR Am. J. Roentgenol. 2020, 214, W20–W26. [Google Scholar] [CrossRef]
- Ronot, M.; Ferraioli, G.; Muller, H.P.; Friedrich-Rust, M.; Filice, C.; Vilgrain, V.; Cosgrove, D.; Lim, A.K. Comparison of liver stiffness measurements by a 2D-shear wave technique and transient elastography: Results from a European prospective multi-centre study. Eur. Radiol. 2021, 31, 1578–1587. [Google Scholar] [CrossRef]
- Jiang, W.; Huang, S.; Teng, H.; Wang, P.; Wu, M.; Zhou, X.; Ran, H. Diagnostic accuracy of point shear wave elastography and transient elastography for staging hepatic fibrosis in patients with non-alcoholic fatty liver disease: A meta-analysis. BMJ Open 2018, 8, e021787. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.; Ren, X.; Ni, Z.; Zhan, W. A Noninvasive Method-Shear-Wave Elastography Compared With Transient Elastography in Evaluation of Liver Fibrosis in Patients With Chronic Hepatitis B. Ultrasound Q. 2019, 35, 147–152. [Google Scholar] [CrossRef]
- Leong, W.L.; Lai, L.L.; Nik Mustapha, N.R.; Vijayananthan, A.; Rahmat, K.; Mahadeva, S.; Chan, W.K. Comparing point shear wave elastography (ElastPQ) and transient elastography for diagnosis of fibrosis stage in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2020, 35, 135–141. [Google Scholar] [CrossRef]
- Villani, R.; Cavallone, F.; Romano, A.D.; Bellanti, F.; Serviddio, G. Two-Dimensional Shear Wave Elastography versus Transient Elastography: A Non-Invasive Comparison for the Assessment of Liver Fibrosis in Patients with Chronic Hepatitis C. Diagnostics 2020, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, T.; Wartelle-Bladou, C.; Wong, P.; Sebastiani, G.; Giard, J.M.; Castel, H.; Murphy-Lavallee, J.; Olivie, D.; Ilinca, A.; Sylvestre, M.P.; et al. Prospective comparison of transient, point shear wave, and magnetic resonance elastography for staging liver fibrosis. Eur. Radiol. 2019, 29, 6477–6488. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Paparo, F.; Bacigalupo, L.; Cenderello, G.; Mussetto, I.; De Cesari, M.; Bernardi, S.P.; Cevasco, L.; Forni, G.L.; Cassola, G.; et al. Noninvasive liver fibrosis assessment in chronic viral hepatitis C: Agreement among 1D transient elastography, 2D shear wave elastography, and magnetic resonance elastography. Abdom. Radiol. 2019, 44, 4011–4021. [Google Scholar] [CrossRef]
- Erman, A.; Sathya, A.; Nam, A.; Bielecki, J.M.; Feld, J.J.; Thein, H.H.; Wong, W.W.L.; Grootendorst, P.; Krahn, M.D. Estimating chronic hepatitis C prognosis using transient elastography-based liver stiffness: A systematic review and meta-analysis. J. Viral Hepat. 2018, 25, 502–513. [Google Scholar] [CrossRef]
- Pang, J.X.; Zimmer, S.; Niu, S.; Crotty, P.; Tracey, J.; Pradhan, F.; Shaheen, A.A.; Coffin, C.S.; Heitman, S.J.; Kaplan, G.G.; et al. Liver stiffness by transient elastography predicts liver-related complications and mortality in patients with chronic liver disease. PLoS ONE 2014, 9, e95776. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Variables | n = 195 |
|---|---|
| Age (years) | |
| Mean ± SD | 47.98 ± 13.98 |
| Gender | |
| Male Female | 109 (55.9%) 86 (44.1%) |
| Etiology | |
| HBV | 49 (25.1%) |
| Alcoholic liver disease | 34 (17.4%) |
| NAFLD | 56 (28.7%) |
| HCV | 4 (2.0%) |
| Others | 52 (26.6%) |
| Alcohol Consumption | |
| None | 122 (62.6%) |
| Mild drinking | 36 (18.5%) |
| Moderate drinking | 37 (18.9%) |
| Diabetes | 37 (18.9%) |
| Hypertension | 62 (31.8%) |
| Histologic fibrosis stage | |
| F0 | 44 (22.6%) |
| F1 | 58 (29.7%) |
| F2 | 33 (16.9%) |
| F3 | 25 (12.8%) |
| F4 | 35 (17.9%) |
| Histologic inflammation grade | |
| G0 | 58 (29.7%) |
| G1 | 83 (42.5%) |
| G2 | 47 (24.1%) |
| G3 | 7 (3.5%) |
| SWE S8 (kPa) | 7.22 ± 2.89 |
| SWE E9 (kPa) | 7.28 ± 2.70 |
| Transient elastography (kPa) | 10.39 ± 9.98 |
| Laboratory test | |
| AST (U/L) | 59 ± 40 |
| ALT (U/L) | 70 ± 57 |
| AST/ALT ratio | 1.37 ± 1.37 |
| Platelet (103/mm) | 207 ± 76 |
| Total bilirubin (mg/dL) | 1.21 ± 1.89 |
| Serum albumin (mg/dL) | 3.91 ± 0.51 |
| Serum creatinine (mg/dL) | 0.88 ± 0.27 |
| Prothrombin time (INR) | 1.05 ± 0.12 |
| Histopathologic Stage | 2D-SWE (S8) | 2D-SWE (E9) | TE | |
|---|---|---|---|---|
| Histopathologic stage | 0.841 * | 0.726 * | 0.623 * | |
| 2D-SWE (S8) | 0.841 * | 0.819 * | 0.840 * | |
| 2D-SWE (E9) | 0.726 * | 0.819 * | 0.726 * | |
| TE | 0.623 * | 0.840 * | 0.726 * |
| Fibrosis Stage | ≥F1 (95% CI) | ≥F2 (95% CI) | ≥F3 (95% CI) | F = 4 (95% CI) |
|---|---|---|---|---|
| 2D-SWE (S8) | ||||
| Cut-off, kPa | 5.980 | 6.695 | 7.255 | 9.150 |
| Sensitivity, % | 71.52 | 79.57 | 86.67 | 82.86 |
| Specificity, % | 93.18 | 87.25 | 83.70 | 95.00 |
| PPV, % | 97.30 | 85.06 | 70.27 | 78.38 |
| NPV, % | 48.81 | 82.41 | 93.39 | 96.20 |
| AUROC | 0.887 (0.839–0.929) | 0.908 (0.874–0.940) | 0.905 (0.864–0.954) | 0.931 (0.889–0.964) |
| 2D-SWE (E9) | ||||
| Cut-off, kPa | 5.720 | 6.420 | 7.355 | 8.875 |
| Sensitivity, % | 76.82 | 87.10 | 86.67 | 85.71 |
| Specificity, % | 86.36 | 81.37 | 80.74 | 0.918 |
| PPV, % | 95.08 | 81.00 | 66.67 | 69.77 |
| NPV, % | 52.05 | 87.37 | 93.16 | 96.71 |
| AUROC | 0.854 (0.797–0.903) | 0.910 (0.871–0.944) | 0.897 (0.844–0.939) | 0.931 (0.874–0.969) |
| TE | ||||
| Cut-off, kPa | 6.150 | 7.350 | 8.450 | 10.05 |
| Sensitivity, % | 77.48 | 84.95 | 85.00 | 91.43 |
| Specificity, % | 84.09 | 82.35 | 82.96 | 88.75 |
| PPV, % | 94.35 | 81.44 | 68.92 | 64.00 |
| NPV, % | 52.11 | 85.71 | 92.56 | 97.93 |
| AUROC | 0.867 (0.804–0.913) | 0.887 (0.937–0.928) | 0.907 (0.850–0.953) | 0.951 (0.909–0.981) |
| Histologic Fibrosis Stage | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | Kappa | ||
| S8 fibrosis stage | 0 | 41 (21.03) | 32 (16.41) | 6 (3.08) | 4 (2.05) | 1 (0.51) | 0.635 (0.564–0.706) |
| 1 | 1 (0.51) | 15 (7.69) | 7 (3.59) | 1 (0.51) | |||
| 2 | 2 (1.03) | 3 (1.54) | 6 (3.08) | 1 (0.51) | 1 (0.51) | ||
| 3 | 8 (4.10) | 11 (5.64) | 14 (7.18) | 4 (2.05) | |||
| 4 | 3 (1.54) | 5 (2.56) | 29 (14.87) | ||||
| E9 fibrosis stage | 0 | 38 (19.5) | 29 (14.9) | 4 (2.04) | 2 (1.03) | 0.423 (0.381–0.465) | |
| 1 | 2 (1.03) | 14 (7.14) | 3 (1.54) | 2 (1.03) | 1 (0.51) | ||
| 2 | 2 (1.03) | 7 (3.59) | 10 (5.1) | 2 (1.03) | 1 (0.51) | ||
| 3 | 2 (1.03) | 7 (3.59) | 10 (5.1) | 13 (6.63) | 3 (1.54) | ||
| 4 | 1 (0.51) | 6 (3.08) | 6 (3.08) | 30 (15.4) | |||
| TE fibrosis stage | 0 | 37 (18.9) | 26 (13.33) | 5 (2.56) | 3 (1.53) | 0.422 (0.337–0.511) | |
| 1 | 4 (2.05) | 17 (8.71) | 4 (2.05) | 1 (0.51) | 1 (0.51) | ||
| 2 | 2 (1.02) | 6 (3.07) | 11 (5.64) | 3 (1.53) | 1 (0.51) | ||
| 3 | 1 (0.51) | 7 (3.58) | 8 (4.10) | 8 (4.10) | 1 (0.51) | ||
| 4 | 2 (1.02) | 5 (2.56) | 10 (5.12) | 32 (16.41) | |||
| Aim | 2D-SWE | Cut-Off | Sensitivity | Specificity | AUROC |
|---|---|---|---|---|---|
| TE < 10 kPa | 2D-SWE (S8) | 7.25 | 0.960 | 0.821 | 0.964 |
| 2D-SWE (E9) | 7.73 | 0.841 | 0.960 | 0.961 | |
| TE ≥ 15 kPa | 2D-SWE (S8) | 9.44 | 0.896 | 0.945 | 0.963 |
| 2D-SWE (E9) | 9.35 | 0.931 | 0.927 | 0.963 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, J.-J.; Kim, S.G.; Kim, Y.S. The Diagnostic Accuracy of LOGIQ S8 and E9 Shear Wave Elastography for Staging Hepatic Fibrosis, in Comparison with Transient Elastography. Diagnostics 2021, 11, 1817. https://doi.org/10.3390/diagnostics11101817
Yoo J-J, Kim SG, Kim YS. The Diagnostic Accuracy of LOGIQ S8 and E9 Shear Wave Elastography for Staging Hepatic Fibrosis, in Comparison with Transient Elastography. Diagnostics. 2021; 11(10):1817. https://doi.org/10.3390/diagnostics11101817
Chicago/Turabian StyleYoo, Jeong-Ju, Sang Gyune Kim, and Young Seok Kim. 2021. "The Diagnostic Accuracy of LOGIQ S8 and E9 Shear Wave Elastography for Staging Hepatic Fibrosis, in Comparison with Transient Elastography" Diagnostics 11, no. 10: 1817. https://doi.org/10.3390/diagnostics11101817






