Comparison of BSGI and MRI as Approaches to Evaluating Residual Tumor Status after Neoadjuvant Chemotherapy in Chinese Women with Breast Cancer
Abstract
:1. Background
2. Methods
2.1. General Information
2.2. MRI
2.3. BSGI
2.4. Pathological Assessment
2.5. Chemotherapy Regimens
2.6. Statistical Analysis
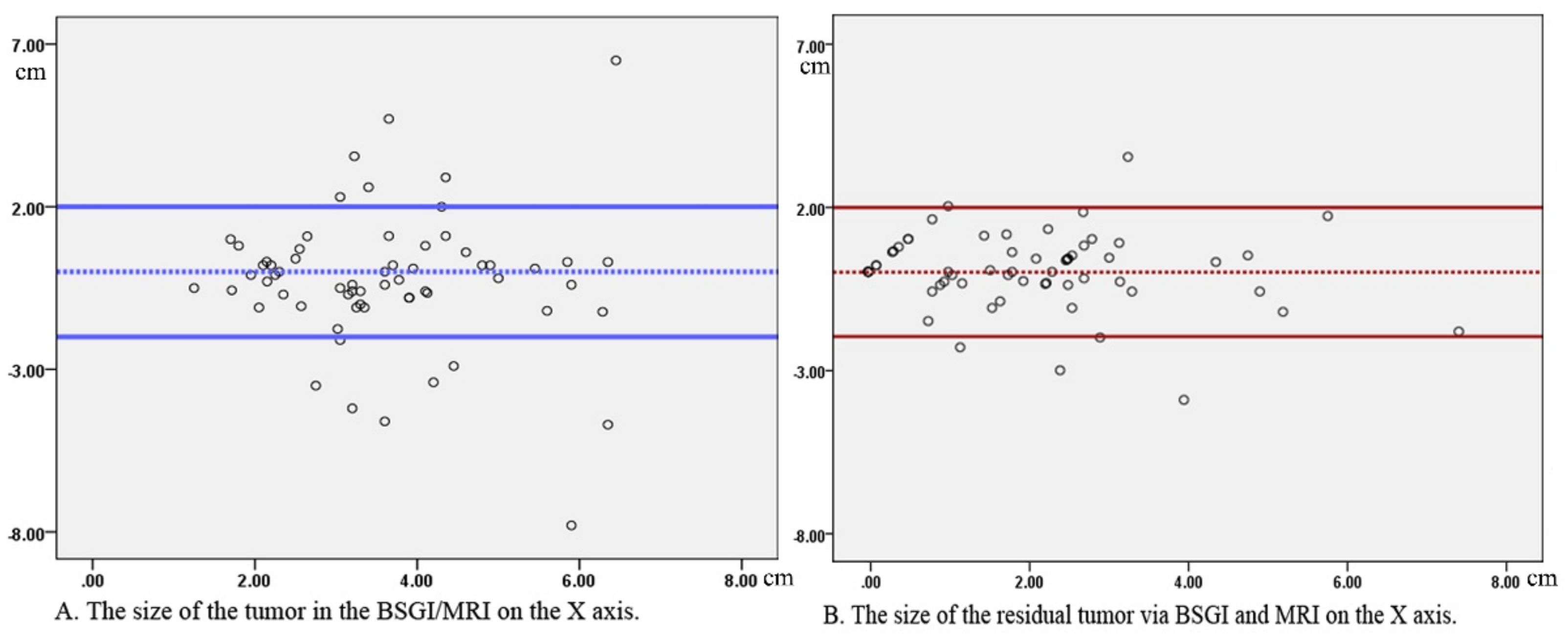
3. Results
3.1. Patient Characteristics
3.2. Residual Tumor Detection Following NAC
3.3. False-CR, False-PR, and False-PD BSGI Findings
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A Systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef] [Green Version]
- Labrosse, J.; Osdoit, M.; Hamy, A.S.; Coussy, F.; Pierga, J.Y.; Reyal, F.; Laas, E. Adjuvant chemotherapy for breast cancer after preoperative chemotherapy: A propensity score matched analysis. PLoS ONE 2020, 15, e0234173. [Google Scholar] [CrossRef]
- Ejlertsen, B. Adjuvant chemotherapy in early breast cancer. Dan. Med. J. 2016, 63, B5222. [Google Scholar]
- Kaufmann, M.; Von Minckwitz, G.; Mamounas, E.P.; Cameron, D.; Carey, L.A.; Cristofanilli, M.; Denkert, C.; Eiermann, W.; Gnant, M.; Harris, J.R.; et al. Recommendations from an International Consensus Conference on the Current Status and Future of Neoadjuvant Systemic Therapy in Primary Breast Cancer. Ann. Surg. Oncol. 2012, 19, 1508–1516. [Google Scholar] [CrossRef]
- Palshof, F.K.; Lanng, C.; Kroman, N.; Benian, C.; Vejborg, I.; Bak, A.; Talman, M.L.; Balslev, E.; Tvedskov, T.F. Prediction of pathologic complete response in breast cancer patients comparing magnetic resonance imaging with ultrasound in neoadjuvant setting. Ann. Surg. Oncol. 2021. [Google Scholar] [CrossRef]
- Dialani, V.; Chadashvili, T.; Slanetz, P.J. Role of imaging in neoadjuvant therapy for breast cancer. Ann. Surg. Oncol. 2015, 22, 1416–1424. [Google Scholar] [CrossRef]
- Scheel, J.R.; Kim, E.; Partridge, S.C.; Lehman, C.D.; Rosen, M.A.; Bernreuter, W.K.; Pisano, E.D.; Marques, H.S.; Morris, E.A.; Weatherall, P.T.; et al. MRI, clinical examination, and mammography for preoperative assessment of residual disease and pathologic complete response after neoadjuvant chemotherapy for breast cancer: ACRIN 6657 trial. AJR Am. J. Roentgenol. 2018, 210, 1376–1385. [Google Scholar] [CrossRef]
- Negrão, E.M.S.; Souza, J.A.; Marques, E.F.; Bitencourt, A.G.V. Breast cancer phenotype influences MRI response evaluation after neoadjuvant chemotherapy. Eur. J. Radiol. 2019, 120, 108701. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhan, H.; Sun, D.; Zhang, Y. Comparison of BSGI, MRI, mammography, and ultrasound for the diagnosis of breast lesions and their correlations with specific molecular subtypes in Chinese women. BMC Med. Imaging 2020, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhan, H.; Sun, D. Comparison of 99mTc-MIBI scintigraphy, ultrasound, and mammography for the diagnosis of BI-RADS 4 category lesions. BMC Cancer 2020, 20, 463. [Google Scholar] [CrossRef]
- Goldsmith, S.J.; Parsons, W.; Guiberteau, M.J.; Stern, L.H.; Lanzkowsky, L.; Weigert, J.; Heston, T.F.; Jones, E.C.; Buscombe, J.; Stabin, M.G. SNM practice guideline for breast scintigraphy with breast-specific gamma-cameras 1.0. J. Nucl. Med. Technol. 2010, 38, 219–224. [Google Scholar] [CrossRef]
- Xing, D.; Lv, Y.; Sun, B.; Xie, H.; Dong, J.; Hao, C.; Chen, Q.; Chi, X. Diagnostic value of contrast-enhanced spectral mammography in comparison to magnetic resonance imaging in breast lesions. J. Comput. Assist. Tomogr. 2019, 43, 245–251. [Google Scholar] [CrossRef]
- Lee, H.S.; Ko, B.S.; Ahn, S.H.; Son, B.H.; Lee, J.W.; Kim, H.J.; Yu, J.H.; Kim, S.B.; Jung, K.H.; Ahn, J.H.; et al. Diagnostic performance of breast-specific gamma imaging in the assessment of residual tumor after neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res. Treat. 2014, 145, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Plemmons, J.; Hoang, K.; Chaudhuri, D.; Kelley, A.; Cunningham, T.; Hoefer, R. Breast-specific gamma imaging versus MRI: Comparing the diagnostic performance in assessing treatment response after neoadjuvant chemotherapy in patients with breast cancer. AJR Am. J. Roentgenol. 2019, 212, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Atkins, J.J.; Appleton, C.M.; Fisher, C.S.; Gao, F.; Margenthaler, J.A. Which imaging modality is superior for prediction of response to neoadjuvant chemotherapy in patients with triple negative breast cancer? J. Oncol. 2013, 2013, 964863. [Google Scholar] [CrossRef]
- Ogston, K.N.; Miller, I.D.; Payne, S.; Hutcheon, A.W.; Sarkar, T.K.; Smith, I.; Schofield, A.; Heys, S.D. A new histological grading system to assess response of breast cancers to primary chemotherapy: Prognostic significance and survival. Breast 2003, 12, 320–327. [Google Scholar] [CrossRef]
- Sampalis, F.S.; Denis, R.; Picard, D.; Fleiszer, D.; Martin, G.; Nassif, E.; Sampalis, J.S. International prospective evaluation of scintimammography with 99mTechnetium sestamibi. Am. J. Surg. 2003, 185, 544–549. [Google Scholar] [CrossRef]
- Weigert, J.M.; Bertrand, M.L.; Lanzkowsky, L.; Stern, L.H.; Kieper, D. Results of a multicenter patient registry to determine the clinical impact of breast-specific gamma imaging, a molecular breast imaging technique. AJR Am. J. Roentgenol. 2012, 198, W69–W75. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Kim, I.J.; Bae, Y.T.; Kim, Y.K.; Kim, D.S. Comparison of quantitative and visual analysis of Tc-99m MIBI scintimammography for detection of primary breast cancer. Eur. J. Radiol. 2005, 53, 192–198. [Google Scholar] [CrossRef]
- Chen, X.; He, C.; Han, D.; Zhou, M.; Wang, Q.; Tian, J.; Li, L.; Xu, F.; Zhou, E.; Yang, K. The predictive value of Ki-67 before neoadjuvant chemotherapy for breast cancer: A systematic review and meta-analysis. Futur. Oncol. 2017, 13, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Ács, B.; Zámbó, V.; Vízkeleti, L.; Szász, A.M.; Madaras, L.; Szentmártoni, G.; Tőkés, T.; Molnár, B.Á.; Molnár, I.A.; Vári-Kakas, S.; et al. Ki-67 as a controversial predictive and prognostic marker in breast cancer patients treated with neoadjuvant chemotherapy. Diagn. Pathol. 2017, 12, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Vecchio, S.; Salvatore, M. 99mTc-MIBI in the evaluation of breast cancer biology. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, S88–S96. [Google Scholar] [CrossRef]
- Arun, B.; Kilic, G.; Yen, C.; Foster, B.; Yardley, D.; Gaynor, R.; Ashfaq, R. Correlation of bcl-2 and p53 expression in primary breast tumors and corresponding metastatic lymph nodes. Cancer 2003, 98, 2554–2559. [Google Scholar] [CrossRef]
- Ziyaie, D.; Hupp, T.R.; Thompson, A.M. P53 and breast cancer. Breast 2000, 9, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Nakamura, K.; Kubo, A.; Enomoto, K.; Ikeda, T.; Kubota, T.; Matsuzaki, S.W.; Kitajima, M. Preoperative evaluation of the chemosensitivity of breast cancer by means of double phase 99mTc-MIBI scintimammography. Ann. Nucl. Med. 1998, 12, 307–312. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, W.; Yang, H.W.; Liu, J.L. Clinical usefulness of breast-specific gamma imaging as an adjunct modality to mammography for diagnosis of breast cancer: A systemic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Suman, V.J.; Davidson, N.E.; Martino, S.; Kaufman, P.A.; Lingle, W.L.; Flynn, P.J.; Ingle, J.N.; Visscher, D.; Jenkins, R.B. HER2 Testing by Local, Central, and Reference Laboratories in Specimens from the North Central Cancer Treatment Group N9831 Intergroup Adjuvant Trial. J. Clin. Oncol. 2006, 24, 3032–3038. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, I.A.; Park, S.Y.; Seo, A.N.; Lim, B.; Chai, Y.; Song, I.H.; Kim, N.E.; Kim, J.Y.; Yu, J.H.; et al. Two histopathologically different diseases: Hormone receptor-positive and hormone receptor-negative tumors in HER2-positive breast cancer. Breast Cancer Res. Treat. 2014, 145, 615–623. [Google Scholar] [CrossRef]
- Gweon, H.M.; Jeong, J.; Son, E.J.; Youk, J.H.; Kim, J.A.; Ko, K.H. The clinical significance of accompanying NME on preoperative MR imaging in breast cancer patients. PLoS ONE 2017, 12, e0178445. [Google Scholar] [CrossRef] [Green Version]
- Pekar, G.; Hofmeyer, S.; Tabar, L.; Tarjan, M.; Chen, T.H.; Yen, A.M.; Chiu, S.Y.; Hellberg, D.; Gere, M.; Tot, T. Multifocal breast cancer documented in large-format histology sections: Long-term follow-up results by molecular phenotypes. Cancer Am. Cancer Soc. 2013, 119, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Morrow, M. Personalizing extent of breast cancer surgery according to molecular subtypes. Breast 2013, 22, S106–S109. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Noh, W.C.; Kim, H.A.; Kim, E.K.; Park, K.W.; Lee, S.S.; Choi, J.H.; Han, K.W.; Byun, B.H.; Lim, I.; et al. The relationship between estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 expression of breast cancer and the retention index in dual phase 18F-FDG PET/CT. Nucl. Med. Mol. Imaging 2016, 50, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Kim, S.J.; Kim, I.J.; Pak, K.; Kim, B.S.; Shin, S. Factors associated with 18F-Fluorodeoxyglucose uptake in T1 and T2 invasive ductal carcinoma of the breast. Nucl. Med. Mol. Imaging 2016, 50, 240–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitencourt, A.G.; Graziano, L.; Osório, C.A.; Guatelli, C.S.; Souza, J.A.; Mendonça, M.H.; Marques, E.F. MRI Features of mucinous cancer of the breast: Correlation with pathologic findings and other imaging methods. AJR Am. J. Roentgenol. 2016, 206, 238–246. [Google Scholar] [CrossRef]
- Li, H.; Yao, L.; Jin, P.; Hu, L.; Li, X.; Guo, T.; Yang, K. MRI and PET/CT for evaluation of the pathological response to neoadjuvant chemotherapy in breast cancer: A systematic review and meta-analysis. Breast 2018, 40, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.L.; Pan, S.M.; Ren, J.; Yang, Z.X.; Jiang, G.Q. Role of magnetic resonance imaging in detection of pathologic complete remission in breast cancer patients treated with neoadjuvant chemotherapy: A meta-analysis. Clin. Breast Cancer 2017, 17, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Berg, W.A.; Madsen, K.S.; Schilling, K.; Tartar, M.; Pisano, E.D.; Larsen, L.H.; Narayanan, D.; Kalinyak, J.E. Comparative effectiveness of positron emission mammography and MRI in the contralateral breast of women with newly diagnosed breast cancer. AJR Am. J. Roentgenol. 2012, 198, 219–232. [Google Scholar] [CrossRef]

| Variable | Value |
|---|---|
| Age | 52.8 (range 25–74) |
| Side of lesion | |
| Left breast | 39 (53.4) |
| Right breast | 34 (46.6) |
| Pathologic type | |
| IDC | 68 (93.2) |
| Invasive lobular carcinoma | 2 (2.7) |
| Others a | 3 (4.1) |
| NAC regimen | |
| TEC | 25 (34.2) |
| EC | 24 (32.9) |
| TCH | 11 (15.1) |
| EC/TH | 8 (11.0) |
| TH | 3 (4.1) |
| AC T | 2 (2.7) |
| Positive node status | 18 (24.7) |
| Miller-payne classification | |
| G 1 | 11 (15.1) |
| G 2 | 13 (17.8) |
| G 3 | 19 (26.0) |
| G 4 | 13 (17.8) |
| G 5 | 17 (23.3) |
| Residual tumor size (cm) | 1.85 ± 1.88 |
| Estrogen receptor | |
| Positive | 37 (50.7) |
| Negative | 36 (49.3) |
| Progesterone receptor | |
| Positive | 29 (39.7) |
| Negative | 44 (60.3) |
| HER2/neu | |
| Positive | 40 (54.8) |
| Negative | 33 (45.2) |
| Ki-67 | |
| >14% | 42 (57.5) |
| ≤14% | 31 (42.5) |
| Molecular subtype | |
| Luminal A | 12 (16.4) |
| Luminal B | 33 (45.2) |
| Her-2 (+) | 22 (30.1) |
| Triple-negative | 6 (8.3) |
| Total | 73 (100) |
| Group | Before NAC | Post NAC | t Value | p |
|---|---|---|---|---|
| pCR | 3.25 ± 0.96 | 1.49 ± 0.30 | 3.997 | 0.044 |
| npCR | 3.26 ± 1.18 | 2.61 ± 1.79 | 2.153 | 0.543 |
| t value | 0.053 | 1.465 | ||
| P | 0.501 | 0.180 |
| Modality | Mean ± SD | Median | Range | t | p | |
|---|---|---|---|---|---|---|
| Before NAC | MRI BSGI | 3.49 ± 1.63 3.77 ± 1.73 | 3.19 3.61 | 1.03–9.67 1.21–9.83 | −1.049 | 0.298 |
| After NAC | MRI BSGI | 1.92 ± 1.59 1.90 ± 1.73 | 2.06 2.30 | 0.22–6.58 0.59–8.33 | 0.126 | 0.900 |
| N | Sensitivity (%) | |||
|---|---|---|---|---|
| BSGI | MRI | p | ||
| Residual cellularity | ||||
| ≤10% | 21 | 16 (76.2) a | 17 (80.9) b | 0.707 |
| >10% | 35 | 33 (94.3) | 34 (97.1) | 0.555 |
| Residual tumor size | ||||
| ≤15 mm | 25 | 19 (79.2) c | 20 (80.0) cd | 0.733 |
| >15 mm | 31 | 30 (96.8) | 31 (100.0) | 0.313 |
| Molecular subtype | ||||
| Luminal A | 11 | 10 (90.9) | 9 (81.8) | 0.534 |
| Luminal B | 27 | 23 (85.2) | 26 (96.3) | 0.159 |
| Her-2 (+) | 14 | 13 (92.9) | 12 (85.7) | 0.541 |
| Triple-negative | 4 | 3 (75.0) | 4 (100) | 0.285 |
| ER Express | ||||
| Positive | 37 | 31 (83.8) | 33 (89.2) | 0.496 |
| Negative | 19 | 18 (94.7) | 17 (89.5) | 0.547 |
| PR Express | ||||
| Positive | 25 | 22 (88.0) | 24 (96.0) | 0.297 |
| Negative | 31 | 27 (87.1) | 27 (87.1) | 1 |
| HER-2 Express | ||||
| Positive | 41 | 35 (85.4) | 37 (90.2) | 0.5 |
| Negative | 15 | 14 (93.3) | 14 (93.3) | 1 |
| Ki-67 | ||||
| >14% | 42 | 10 (23.8) | 13 (31.0) | 0.463 |
| ≤14% | 31 | 7 (16.7) d | 0 (0) | 0.005 |
| Invasiveness | ||||
| Invasive residual | 49 | 42 (85.7) | 45 (91.8) | 0.337 |
| In situ residual | 7 | 7 (100) | 6 (85.7) | 0.299 |
| Total | 56 | 49 (87.5) | 51 (91.1) | 0.541 |
| Case No. | Age | Subtype | Chemotherapy Regimens | M-P Grade | Residual Size (cm) | MRI (cm) | MRI Evaluation | BSGI (cm) | T/N | BSGI Evaluation |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | HER-2 | EC | 1 | 1.0 | 2.6 | PD | 2.4 | 1.97 | PR |
| 2 | 44 | Luminal A | TEC | 2 | 1.0 | 1.7 | PD | 1.8 | 1.91 | PR |
| 3 | 51 | Luminal B | TEC | 3 | 1.0 | 1 | PR | 0 | 1 | CR |
| 4 | 64 | Luminal B | TCH | 3 | 0.7 | 1 | PR | 0 | 1 | CR |
| 5 | 52 | Luminal B | TEC | 3 | 0.5 | 0.76 | PR | 0 | 1 | CR |
| 6 | 57 | Luminal A | TEC | 3 | 0.3 | 1 | SD | 0 | 1 | CR |
| 7 | 67 | Luminal A | TCH | 3 | 1.2 | 2 | SD | 0 | 1 | CR |
| 8 | 46 | Luminal A | TEC | 3 | 1.6 | 2.4 | SD | 0 | 1 | CR |
| 9 | 65 | Luminal A | TCH | 3 | 0.4 | 0.9 | SD | 0 | 1 | CR |
| 10 | 45 | Luminal B | TEC | 3 | 4.5 | 0.5 | PR | 0 | 1 | CR |
| 11 | 74 | Luminal B | EC | 3 | 2.0 | 3.1 | SD | 2.1 | 2.76 | PR |
| 12 | 49 | HER-2 | TCH | 4 | 1.0 | 0 | CR | 0 | 1 | CR |
| 13 | 49 | HER-2 | TCH | 4 | 0.5 | 0 | CR | 0 | 1 | CR |
| 14 | 39 | Luminal B | TEC | 4 | 0.5 | 0 | CR | 0 | 1 | CR |
| 15 | 59 | HER-2 | TEC | 4 | 0.1 | 1.6 | PR | 0 | 1 | CR |
| 16 | 59 | HER-2 | TEC | 4 | 0.1 | 0.6 | PR | 0 | 1 | CR |
| 17 | 48 | HER-2 | TCH | 4 | 0.1 | 2.2 | PR | 0 | 1 | CR |
| 18 | 53 | HER-2 | TCH | 5 | 2 | non-mass enhancement | PD | 4.5 | 2.41 | PD |
| 19 | 70 | Luminal A | TEC | 5 | 0.1 | 1.9 | PR | 2.3 | 1.38 | PR |
| 20 | 60 | HER-2 | EC/ TH | 5 | a small focus of residual DCIS | 2.0 | PR | 1.3 | 1.26 | PR |
| 21 | 54 | HER-2 | TCH | 5 | 0 | 2.3 | PR | 1.0 | 1.3 | PR |
| 22 | 54 | HER-2 | EC | 5 | 0 | 0.6 | PR | 0.9 | 2.36 | SD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhan, H.; Zhang, Y.; He, G.; Wang, H.; Zhang, Q.; Zheng, L. Comparison of BSGI and MRI as Approaches to Evaluating Residual Tumor Status after Neoadjuvant Chemotherapy in Chinese Women with Breast Cancer. Diagnostics 2021, 11, 1846. https://doi.org/10.3390/diagnostics11101846
Liu H, Zhan H, Zhang Y, He G, Wang H, Zhang Q, Zheng L. Comparison of BSGI and MRI as Approaches to Evaluating Residual Tumor Status after Neoadjuvant Chemotherapy in Chinese Women with Breast Cancer. Diagnostics. 2021; 11(10):1846. https://doi.org/10.3390/diagnostics11101846
Chicago/Turabian StyleLiu, Hongbiao, Hongwei Zhan, Ying Zhang, Gangqiang He, Hui Wang, Qiaoxia Zhang, and Lili Zheng. 2021. "Comparison of BSGI and MRI as Approaches to Evaluating Residual Tumor Status after Neoadjuvant Chemotherapy in Chinese Women with Breast Cancer" Diagnostics 11, no. 10: 1846. https://doi.org/10.3390/diagnostics11101846
APA StyleLiu, H., Zhan, H., Zhang, Y., He, G., Wang, H., Zhang, Q., & Zheng, L. (2021). Comparison of BSGI and MRI as Approaches to Evaluating Residual Tumor Status after Neoadjuvant Chemotherapy in Chinese Women with Breast Cancer. Diagnostics, 11(10), 1846. https://doi.org/10.3390/diagnostics11101846






