The Value of a Complete Blood Count (CBC) for Sepsis Diagnosis and Prognosis
Abstract
:1. Introduction
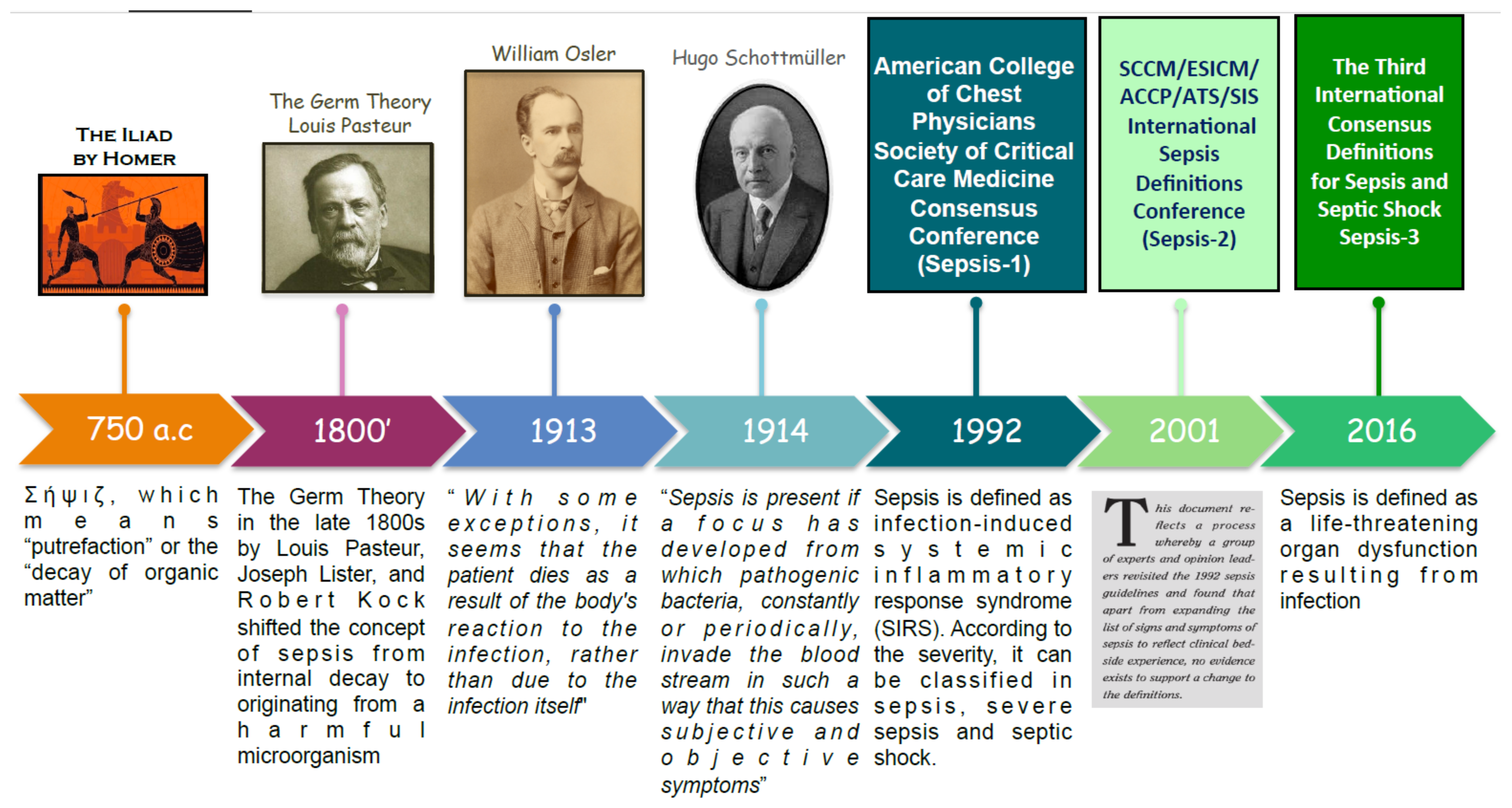
2. Sepsis Definition and Pathogenesis
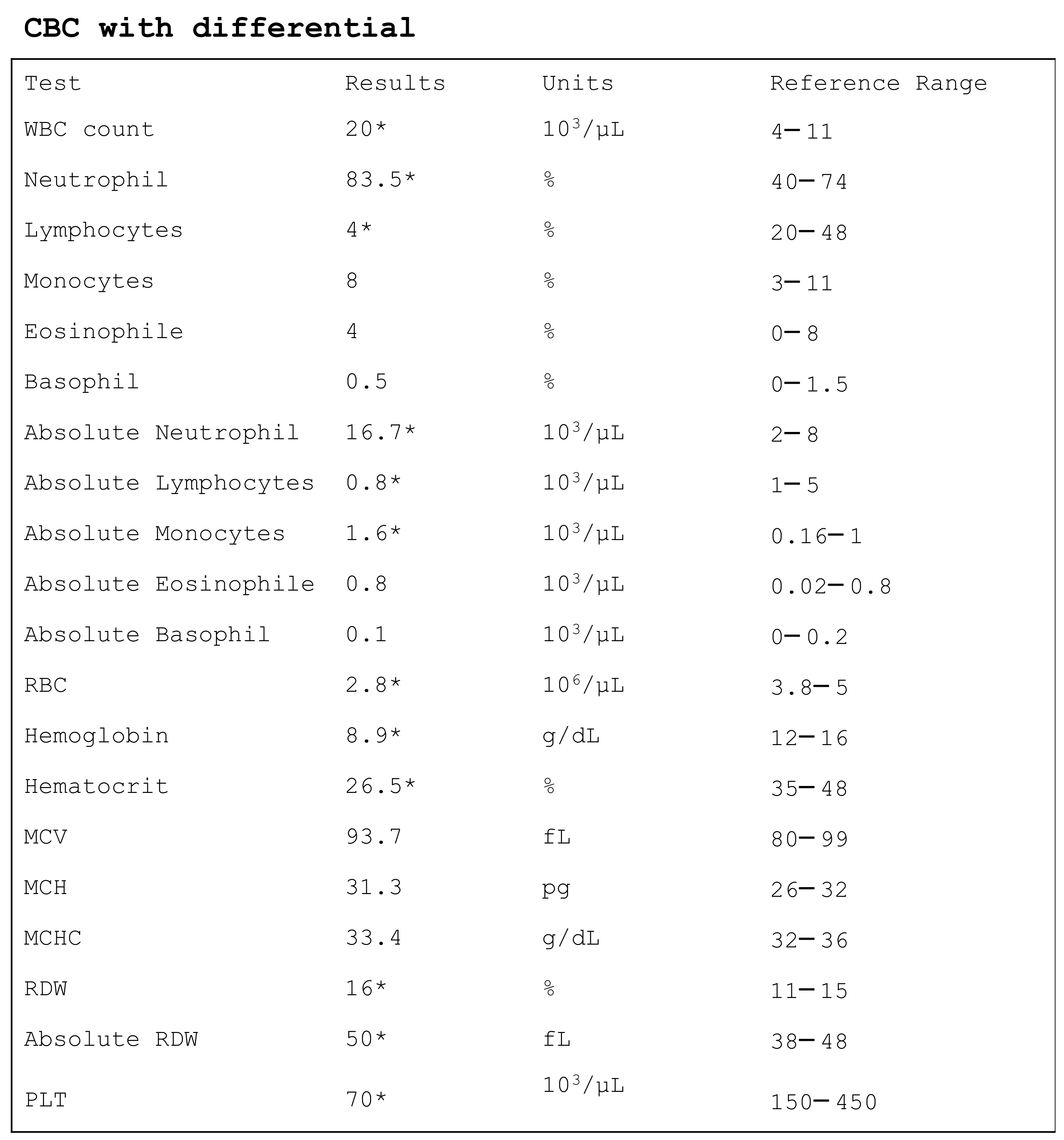
3. Basic Complete Blood Count
3.1. White Blood Cells
3.1.1. Lymphocytes
3.1.2. Monocytes
3.1.3. Neutrophils
3.1.4. Eosinophils
3.1.5. Basophils
3.2. Red Blood Cells
3.2.1. Hemoglobin
3.2.2. Hematocrit
3.2.3. MCV
3.2.4. MCH and MCHC
3.2.5. RDW
3.3. Platelets
4. CBC Parameters Ratios
4.1. Neutrophil-to-Lymphocyte Ratio
4.2. Monocyte-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Mean Platelet Volume-to-Platelet Count
5. Cell Population Data
5.1. Monocyte Distribution Width
5.2. Mean Neutrophil Volume and Mean Monocyte Volume
5.3. Neutrophil Fluorescence Intensity and Monocyte Internal Structure
5.4. Immature Granulocytes
5.5. Immature Platelet Fraction
5.6. Delta Neutrophil Index
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Markwart, R.; Saito, H.; Harder, T.; Tomczyk, S.; Cassini, A.; Fleischmann-Struzek, C.; Reichert, F.; Eckmanns, T.; Allegranzi, B. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: A systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1536–1551. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.J.; Tsalik, E.L.; van Velkinburgh, J.C.; Glickman, S.W.; Rice, B.J.; Wang, C.; Chen, B.; Carin, L.; Suarez, A.; Mohney, R.P.; et al. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci. Transl. Med. 2013, 5, 195ra95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhart, K.; Daniels, R.; Kissoon, N.; Machado, F.R.; Schachter, R.D.; Finfer, S. Recognizing Sepsis as a Global Health Priority—A WHO Resolution. N. Engl. J. Med. 2017, 377, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Shashikumar, S.P.; Stanley, M.D.; Sadiq, I.; Li, Q.; Holder, A.; Clifford, G.D.; Nemati, S. Early sepsis detection in critical care patients using multiscale blood pressure and heart rate dynamics. J. Electrocardiol. 2017, 50, 739–743. [Google Scholar] [CrossRef]
- Kataria, Y.; Remick, D. Sepsis Biomarkers. Methods Mol. Biol. 2021, 2321, 177–189. [Google Scholar] [CrossRef]
- Agnello, L.; Bivona, G.; Parisi, E.; Lucido, G.D.; Iacona, A.; Ciaccio, A.M.; Giglio, R.V.; Ziino, O.; Ciaccio, M. Presepsin and Midregional Proadrenomedullin in Pediatric Oncologic Patients with Febrile Neutropenia. Lab. Med. 2020, 51, 585–591. [Google Scholar] [CrossRef]
- Bellia, C.; Agnello, L.; Lo Sasso, B.; Bivona, G.; Raineri, M.S.; Giarratano, A.; Ciaccio, M. Mid-regional pro-adrenomedullin predicts poor outcome in non-selected patients admitted to an intensive care unit. Clin. Chem. Lab. Med. 2019, 57, 549–555. [Google Scholar] [CrossRef]
- Velissaris, D.; Zareifopoulos, N.; Karamouzos, V.; Karanikolas, E.; Pierrakos, C.; Koniari, I.; Karanikolas, M. Presepsin as a Diagnostic and Prognostic Biomarker in Sepsis. Cureus 2021, 13, e15019. [Google Scholar] [CrossRef]
- Funk, D.J.; Parrillo, J.E.; Kumar, A. Sepsis and septic shock: A history. Crit. Care Clin. 2009, 25, 83–101. [Google Scholar] [CrossRef]
- Yipp, B.G.; Winston, B.W. Sepsis without SIRS is still sepsis. Ann. Transl. Med. 2015, 3, 294. [Google Scholar] [CrossRef]
- Rittirsch, D.; Flierl, M.A.; Ward, P.A. Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 2008, 8, 776–787. [Google Scholar] [CrossRef] [Green Version]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [Green Version]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.L.; Ramsay, G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003, 29, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Nedeva, C.; Menassa, J.; Puthalakath, H. Sepsis: Inflammation Is a Necessary Evil. Front. Cell Dev. Biol. 2019, 7, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, J.; Horie, S.; Laffey, J.G. Role of the adaptive immune response in sepsis. Intensive Care Med. Exp. 2020, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.Y.; Park, J.S.; Park, Y.B.; Lee, S.K.; Ha, Y.J.; Lee, S.W. Delta neutrophil index as a marker for differential diagnosis between flare and infection in febrile systemic lupus erythematosus patients. Lupus 2013, 22, 1102–1109. [Google Scholar] [CrossRef]
- Pyo, J.Y.; Ha, Y.J.; Song, J.J.; Park, Y.B.; Lee, S.K.; Lee, S.W. Delta neutrophil index contributes to the differential diagnosis between acute gout attack and cellulitis within 24 hours after hospitalization. Rheumatology 2017, 56, 795–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis-Pathophysiology and Therapeutic Concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Perl, M.; Venet, F.; Lomas-Neira, J.; Swan, R.; Chung, C.S. Apoptosis in sepsis: Mechanisms, clinical impact and potential therapeutic targets. Curr. Pharm. Des. 2008, 14, 1853–1859. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Swanson, P.E.; Cobb, J.P.; Jacobson, A.; Buchman, T.G.; Karl, I.E. Apoptosis in lymphoid and parenchymal cells during sepsis: Findings in normal and T- and B-cell-deficient mice. Crit. Care Med. 1997, 25, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Tinsley, K.W.; Karl, I.E. Role of apoptotic cell death in sepsis. Scand. J. Infect. Dis. 2003, 35, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Boomer, J.S.; Shuherk-Shaffer, J.; Hotchkiss, R.S.; Green, J.M. A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit. Care 2012, 16, R112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boomer, J.S.; To, K.; Chang, K.C.; Takasu, O.; Osborne, D.F.; Walton, A.H.; Bricker, T.L.; Jarman, S.D.; Kreisel, D., 2nd; Krupnick, A.S.; et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011, 306, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Wesche, D.E.; Lomas-Neira, J.L.; Perl, M.; Chung, C.S.; Ayala, A. Leukocyte apoptosis and its significance in sepsis and shock. J. Leukoc. Biol. 2005, 78, 325–337. [Google Scholar] [CrossRef]
- Venet, F.; Davin, F.; Guignant, C.; Larue, A.; Cazalis, M.A.; Darbon, R.; Allombert, C.; Mougin, B.; Malcus, C.; Poitevin-Later, F.; et al. Early assessment of leukocyte alterations at diagnosis of septic shock. Shock 2010, 34, 358–363. [Google Scholar] [CrossRef]
- Drewry, A.M.; Samra, N.; Skrupky, L.P.; Fuller, B.M.; Compton, S.M.; Hotchkiss, R.S. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 2014, 42, 383–391. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.P.; Chang, H.T.; Lo, S.C.; Chang, L.Y.; Lin, S.Y.; Cheng, A.; Huang, Y.T.; Chen, C.C.; Lee, M.R.; Chen, Y.J.; et al. Severe lymphopenia is associated with elevated plasma interleukin-15 levels and increased mortality during severe sepsis. Shock 2015, 43, 569–575. [Google Scholar] [CrossRef]
- Sheikh Motahar Vahedi, H.; Bagheri, A.; Jahanshir, A.; Seyedhosseini, J.; Vahidi, E. Association of Lymphopenia with Short Term Outcomes of Sepsis Patients; A Brief Report. Arch. Acad. Emerg. Med. 2019, 7, e14. [Google Scholar] [PubMed]
- Hohlstein, P.; Gussen, H.; Bartneck, M.; Warzecha, K.T.; Roderburg, C.; Buendgens, L.; Trautwein, C.; Koch, A.; Tacke, F. Prognostic Relevance of Altered Lymphocyte Subpopulations in Critical Illness and Sepsis. J. Clin. Med. 2019, 8, 353. [Google Scholar] [CrossRef] [Green Version]
- Hortová-Kohoutková, M.; Lázničková, P.; Bendíčková, K.; De Zuani, M.; Andrejčinová, I.; Tomášková, V.; Suk, P.; Šrámek, V.; Helán, M.; Frič, J. Differences in monocyte subsets are associated with short-term survival in patients with septic shock. J. Cell. Mol. Med. 2020, 24, 12504–12512. [Google Scholar] [CrossRef]
- Radzyukevich, Y.V.; Kosyakova, N.I.; Prokhorenko, I.R. Participation of Monocyte Subpopulations in Progression of Experimental Endotoxemia (EE) and Systemic Inflammation. J. Immunol. Res. 2021, 2021, 1762584. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Ożańska, A.; Szymczak, D.; Rybka, J. Pattern of human monocyte subpopulations in health and disease. Scand. J. Immunol. 2020, 92, e12883. [Google Scholar] [CrossRef]
- Ferreira da Mota, N.V.; Brunialti, M.; Santos, S.S.; Machado, F.R.; Assuncao, M.; Azevedo, L.; Salomao, R. Immunophenotyping of Monocytes During Human Sepsis Shows Impairment in Antigen Presentation: A Shift Toward Nonclassical Differentiation and Upregulation of FCγRi-Receptor. Shock 2020, 50, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Lee, J.H.; Jo, Y.H.; Hwang, J.E.; Kim, J. Circulating Monocyte Counts and its Impact on Outcomes in Patients With Severe Sepsis Including Septic Shock. Shock 2019, 51, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.K.; Rupert, J. Evaluation of Patients with Leukocytosis. Am. Fam. Physician 2015, 92, 1004–1011. [Google Scholar] [PubMed]
- Witter, A.R.; Okunnu, B.M.; Berg, R.E. The Essential Role of Neutrophils during Infection with the Intracellular Bacterial Pathogen Listeria monocytogenes. J. Immunol. 2016, 197, 1557–1565. [Google Scholar] [CrossRef] [Green Version]
- Resende, C.B.; Borges, I.; Gonçalves, W.A.; Carneiro, R.; Rezende, B.M.; Pinho, V.; Nobre, V.; Teixeira, M.M. Neutrophil activity in sepsis: A systematic review. Braz. J. Med. Biol. Res. 2020, 53, e7851. [Google Scholar] [CrossRef]
- Gao, L.; Shi, Q.; Li, H.; Guo, Q.; Yan, J.; Zhou, L. Prognostic value of the combined variability of mean platelet volume and neutrophil percentage for short-term clinical outcomes of sepsis patients. Postgrad. Med. 2021, 133, 604–612. [Google Scholar] [CrossRef]
- Nakata, A. Psychosocial job stress and immunity: A systematic review. Methods Mol. Biol. 2012, 934, 39–75. [Google Scholar] [CrossRef] [PubMed]
- Belok, S.H.; Bosch, N.A.; Klings, E.S.; Walkey, A.J. Evaluation of leukopenia during sepsis as a marker of sepsis-defining organ dysfunction. PLoS ONE 2021, 16, e0252206. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Tripathi, A.K.; Mishra, S.; Amzarul, M.; Vaish, A.K. Physiological changes in hematological parameters during pregnancy. Indian J. Hematol. Blood 2012, 28, 144–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagata, M.; Nakagome, K.; Soma, T. Mechanisms of eosinophilic inflammation. Asia Pac. Allergy 2020, 10, e14. [Google Scholar] [CrossRef] [PubMed]
- Zappert, J. Ueber das vorkommen der eosinophilen zellen in menschlichen blute. Z. Klin. Med. 1893, 23, 227–308. [Google Scholar]
- Lavoignet, C.E.; Le Borgne, P.; Chabrier, S.; Bidoire, J.; Slimani, H.; Chevrolet-Lavoignet, J.; Lefebvre, F.; Jebri, R.; Sengler, L.; Bilbault, P. White blood cell count and eosinopenia as valuable tools for the diagnosis of bacterial infections in the ED. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1523–1532. [Google Scholar] [CrossRef]
- Abidi, K.; Khoudri, I.; Belayachi, J.; Madani, N.; Zekraoui, A.; Zeggwagh, A.A.; Abouqal, R. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit. Care 2008, 12, R59. [Google Scholar] [CrossRef] [Green Version]
- Shaaban, H.; Daniel, S.; Sison, R.; Slim, J.; Perez, G. Eosinopenia: Is it a good marker of sepsis in comparison to procalcitonin and C-reactive protein levels for patients admitted to a critical care unit in an urban hospital? J. Crit. Care 2010, 25, 570–575. [Google Scholar] [CrossRef]
- Anand, D.; Ray, S.; Bhargava, S.; Srivastava, L.M.; Garg, A.; Gafoor, I.; Singh, R.; Dhar, D. Exploration of eosinopenia as a diagnostic parameter to differentiate sepsis from systemic inflammatory response syndrome: Results from an observational study. Indian J. Crit. Care Med. 2016, 20, 285–290. [Google Scholar] [CrossRef]
- Tinoco-Sánchez, M.; Suárez-Cuenca, J.A.; Rubio-Guerra, A.F. Usefulness of eosinopenia as prognostic marker of severity in sepsis. Med. Interna Mex. 2017, 33, 572–579. [Google Scholar]
- Varghese, J.; Devadas, K.; Cyriac, R.; Vinayakumar, N.; Hareendran, A.; Iqbal, A. Low eosinophil count, a predictor of 28 day mortality in a cohort of cirrhosis patients with sepsis. J. Gastroenterol. Hepatol. 2019, 34, 495. [Google Scholar]
- Lin, Y.; Rong, J.; Zhang, Z. Silent existence of eosinopenia in sepsis: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 471. [Google Scholar] [CrossRef]
- Karasuyama, H.; Miyake, K.; Yoshikawa, S.; Kawano, Y.; Yamanishi, Y. How do basophils contribute to Th2 cell differentiation and allergic responses? Int. Immunol. 2018, 30, 391–396. [Google Scholar] [CrossRef]
- Karasuyama, H.; Obata, K.; Wada, T.; Tsujimura, Y.; Mukai, K. Newly appreciated roles for basophils in allergy and protective immunity. Allergy 2011, 66, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Rivera, J. Paradigm Shifts in Mast Cell and Basophil Biology and Function: An Emerging View of Immune Regulation in Health and Disease. Methods Mol. Biol. 2020, 2163, 3–31. [Google Scholar] [CrossRef] [PubMed]
- Piliponsky, A.M.; Shubin, N.J.; Lahiri, A.K.; Truong, P.; Clauson, M.; Niino, K.; Tsuha, A.L.; Nedospasov, S.A.; Karasuyama, H.; Reber, L.L.; et al. Basophil-derived tumor necrosis factor can enhance survival in a sepsis model in mice. Nat. Immunol. 2019, 20, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Sinha, H.; Maitra, S.; Anand, R.K.; Aggarwal, R.; Rewari, V.; Subramaniam, R.; Trikha, A.; Arora, M.K.; Batra, R.K.; Saxena, R.; et al. Epidemiology and Prognostic Utility of Cellular Components of Hematological System in Sepsis. Indian J. Crit. Care Med. 2021, 25, 660–667. [Google Scholar] [CrossRef]
- Anderson, H.L.; Brodsky, I.E.; Mangalmurti, N.S. The Evolving Erythrocyte: Red Blood Cells as Modulators of Innate Immunity. J. Immunol. 2018, 201, 1343–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minasyan, H. Erythrocyte and blood antibacterial defense. Eur. J. Microbiol. Immunol. 2014, 4, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Minasyan, H. Sepsis: Mechanisms of bacterial injury to the patient. Scand. J. Trauma Resusc. Emerg. Med. 2019, 27, 19. [Google Scholar] [CrossRef] [Green Version]
- Effenberger-Neidnicht, K.; Hartmann, M. Mechanisms of Hemolysis During Sepsis. Inflammation 2018, 41, 1569–1581. [Google Scholar] [CrossRef]
- Vincent, J.L.; Baron, J.F.; Reinhart, K.; Gattinoni, L.; Thijs, L.; Webb, A.; Meier-Hellmann, A.; Nollet, G.; Peres-Bota, D.; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA 2002, 288, 1499–1507. [Google Scholar] [CrossRef] [Green Version]
- Bateman, R.M.; Sharpe, M.D.; Singer, M.; Ellis, C.G. The Effect of Sepsis on the Erythrocyte. Int. J. Mol. Sci. 2017, 18, 1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Docherty, A.B.; Turgeon, A.F.; Walsh, T.S. Best practice in critical care: Anaemia in acute and critical illness. Transfus. Med. 2018, 28, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Van Beest, P.A.; Hofstra, J.J.; Schultz, M.J.; Boerma, E.C.; Spronk, P.E.; Kuiper, M.A. The incidence of low venous oxygen saturation on admission to the intensive care unit: A multi-center observational study in The Netherlands. Crit. Care 2008, 12, R33. [Google Scholar] [CrossRef] [Green Version]
- Cable, C.A.; Razavi, S.A.; Roback, J.D.; Murphy, D.J. RBC Transfusion Strategies in the ICU: A Concise Review. Crit. Care Med. 2019, 47, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Yealy, D.M.; Kellum, J.A.; Huang, D.T.; Barnato, A.E.; Weissfeld, L.A.; Pike, F.; Terndrup, T.; Wang, H.E.; Hou, P.C.; LoVecchio, F.; et al. A randomized trial of protocol-based care for early septic shock. N. Engl. J. Med. 2014, 370, 1683–1693. [Google Scholar] [CrossRef] [Green Version]
- Lippi, G.; Cervellin, G.; Sanchis-Gomar, F. Red blood cell distribution width: A marker of anisocytosis potentially associated with atrial fibrillation. World J. Cardiol. 2019, 11, 292–304. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 2015, 52, 86–105. [Google Scholar] [CrossRef]
- Krishna, V.; Pillai, G.; Velickakathu Sukumaran, S. Red Cell Distribution Width As a Predictor of Mortality in Patients With Sepsis. Cureus 2021, 13, e12912. [Google Scholar] [CrossRef] [PubMed]
- Huda, A.Q.; Karim, M.R.; Mahmud, M.A.; Islam, M.S.; Haque, M.F.; Islam, M.R.; Hossain, M.A. Use of Acute Physiology and Chronic Health Evaluation (APACHE)-II and Red Cell Distribution Width (RDW) for Assessment of Mortality of Patients with Sepsis in ICU. Mymensingh Med. J. 2017, 26, 585–591. [Google Scholar] [PubMed]
- Li, Y.; She, Y.; Fu, L.; Zhou, R.; Xiang, W.; Luo, L. Association Between Red Cell Distribution Width and Hospital Mortality in Patients with Sepsis. J. Int. Med. Res. 2021, 49, 3000605211004221. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.W.; Liu, D.; Chen, J.M.; Li, W.J.; Gao, C.J. Fluctuation in red cell distribution width predicts disseminated intravascular coagulation morbidity and mortality in sepsis: A retrospective single-center study. Minerva Anestesiol. 2021, 87, 52–64. [Google Scholar] [CrossRef]
- Jo, Y.H.; Kim, K.; Lee, J.H.; Kang, C.; Kim, T.; Park, H.M.; Kang, K.W.; Kim, J.; Rhee, J.E. Red cell distribution width is a prognostic factor in severe sepsis and septic shock. Am. J. Emerg. Med. 2013, 31, 545–548. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, J.T.; Kim, E.J.; Han, J.H.; Han, J.S.; Choi, J.Y.; Han, S.H.; Yoo, T.H.; Kim, Y.S.; Kang, S.W.; et al. An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock. Crit. Care 2013, 17, R282. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.Q.; Zhang, L.; Yan, L.; Li, P.; Ouyang, P.H.; Lippi, G.; Hu, Z.D. Red blood cell distribution width predicts long-term outcomes in sepsis patients admitted to the intensive care unit. Clin. Chim. Acta 2018, 487, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Solé-Violán, J.; Ferreres, J.; Labarta, L.; Díaz, C.; González, O.; García, D.; Jiménez, A.; et al. Red blood cell distribution width during the first week is associated with severity and mortality in septic patients. PLoS ONE 2014, 9, e105436. [Google Scholar] [CrossRef] [Green Version]
- Özdoğan, H.K.; Karateke, F.; Özyazıcı, S.; Özdoğan, M.; Özaltun, P.; Kuvvetli, A.; Gökler, C.; Ersoy, Z. The predictive value of red cell distribution width levels on mortality in intensive care patients with community-acquired intra-abdominal sepsis. Ulusal Travma ve Acil Cerrahi Dergisi 2015, 21, 352–357. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Wei, Y.; Chen, D.; Jin, J.; Chen, H. Prognostic value of an inflammatory biomarker-based clinical algorithm in septic patients in the emergency department: An observational study. Int. Immunopharmacol. 2020, 80, 106145. [Google Scholar] [CrossRef]
- Chen, K.F.; Liu, S.H.; Li, C.H.; Wu, C.C.; Chaou, C.H.; Tzeng, I.S.; Hsieh, Y.H.; Blaney, G.N.; Liu, Z.Y.; Han, S.T.; et al. Development and validation of a parsimonious and pragmatic CHARM score to predict mortality in patients with suspected sepsis. Am. J. Emerg. Med. 2017, 35, 640–646. [Google Scholar] [CrossRef]
- Fontana, V.; Spadaro, S.; Bond, O.; Cavicchi, F.Z.; Annoni, F.; Donadello, K.; Vincent, J.L.; De Backer, D.; Taccone, F.S. No relationship between red blood cell distribution width and microcirculatory alterations in septic patients. Clin. Hemorheol. Microcirc. 2017, 66, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, R.; Shakya, Y.M.; Shrestha, T.M.; Neupane, R.P. The utility of red cell distribution width to predict mortality of septic patients in a tertiary hospital of Nepal. BMC Emerg. Med. 2020, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.D.; Lippi, G.; Montagnana, M. Diagnostic and prognostic value of red blood cell distribution width in sepsis: A narrative review. Clin. Biochem. 2020, 77, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Chen, J.; Lan, Q.F.; Ma, X.J.; Zhang, S.Y. Diagnostic values of red cell distribution width, platelet distribution width and neutrophil-lymphocyte count ratio for sepsis. Exp. Ther. Med. 2016, 12, 2215–2219. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Park, C.J.; Lee, B.R.; Nam, K.S.; Kim, M.J.; Han, M.Y.; Kim, Y.J.; Cho, Y.U.; Jang, S. Sepsis affects most routine and cell population data (CPD) obtained using the Sysmex XN-2000 blood cell analyzer: Neutrophil-related CPD NE-SFL and NE-WY provide useful information for detecting sepsis. Int. J. Lab. Hematol. 2015, 37, 190–198. [Google Scholar] [CrossRef]
- Laukemann, S.; Kasper, N.; Kulkarni, P.; Steiner, D.; Rast, A.C.; Kutz, A.; Felder, S.; Haubitz, S.; Faessler, L.; Huber, A.; et al. Can We Reduce Negative Blood Cultures With Clinical Scores and Blood Markers? Results From an Observational Cohort Study. Medicine 2015, 94, e2264. [Google Scholar] [CrossRef]
- McDonald, B.; Dunbar, M. Platelets and Intravascular Immunity: Guardians of the Vascular Space During Bloodstream Infections and Sepsis. Front. Immunol. 2019, 10, 2400. [Google Scholar] [CrossRef]
- Guo, L.; Rondina, M.T. The Era of Thromboinflammation: Platelets Are Dynamic Sensors and Effector Cells During Infectious Diseases. Front. Immunol. 2019, 10, 2204. [Google Scholar] [CrossRef] [Green Version]
- Assinger, A.; Schrottmaier, W.C.; Salzmann, M.; Rayes, J. Platelets in Sepsis: An Update on Experimental Models and Clinical Data. Front. Immunol. 2019, 10, 1687. [Google Scholar] [CrossRef]
- Ma, A.C.; Kubes, P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J. Thromb. Haemost. 2008, 6, 415–420. [Google Scholar] [CrossRef]
- Dewitte, A.; Lepreux, S.; Villeneuve, J.; Rigothier, C.; Combe, C.; Ouattara, A.; Ripoche, J. Blood platelets and sepsis pathophysiology: A new therapeutic prospect in critically [corrected] ill patients? Ann. Intensive Care 2017, 7, 115. [Google Scholar] [CrossRef] [Green Version]
- Levi, M. Platelets in Critical Illness. Semin. Thromb. Hemost. 2016, 42, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Claushuis, T.A.; van Vught, L.A.; Scicluna, B.P.; Wiewel, M.A.; Klein Klouwenberg, P.M.; Hoogendijk, A.J.; Ong, D.S.; Cremer, O.L.; Horn, J.; Franitza, M.; et al. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood 2016, 127, 3062–3072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vardon-Bounes, F.; Gratacap, M.P.; Groyer, S.; Ruiz, S.; Georges, B.; Seguin, T.; Garcia, C.; Payrastre, B.; Conil, J.M.; Minville, V. Kinetics of mean platelet volume predicts mortality in patients with septic shock. PLoS ONE 2019, 14, e0223553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vardon-Bounes, F.; Ruiz, S.; Gratacap, M.P.; Garcia, C.; Payrastre, B.; Minville, V. Platelets Are Critical Key Players in Sepsis. Int. J. Mol. Sci. 2019, 20, 3494. [Google Scholar] [CrossRef] [Green Version]
- Giustozzi, M.; Ehrlinder, H.; Bongiovanni, D.; Borovac, J.A.; Guerreiro, R.A.; Gąsecka, A.; Papakonstantinou, P.E.; Parker, W. Coagulopathy and sepsis: Pathophysiology, clinical manifestations and treatment. Blood Rev. 2021, 100864, in press. [Google Scholar] [CrossRef] [PubMed]
- Liberski, P.S.; Szewczyk, M.; Krzych, Ł.J. Haemogram-Derived Indices for Screening and Prognostication in Critically Ill Septic Shock Patients: A Case-Control Study. Diagnostics 2020, 10, 638. [Google Scholar] [CrossRef]
- Taneja, R.; Parodo, J.; Jia, S.H.; Kapus, A.; Rotstein, O.D.; Marshall, J.C. Delayed neutrophil apoptosis in sepsis is associated with maintenance of mitochondrial transmembrane potential and reduced caspase-9 activity. Crit. Care Med. 2004, 32, 1460–1469. [Google Scholar] [CrossRef]
- Rehman, F.U.; Khan, A.; Aziz, A.; Iqbal, M.; Mahmood, S.; Ali, N. Neutrophils to Lymphocyte Ratio: Earliest and Efficacious Markers of Sepsis. Cureus 2020, 12, e10851. [Google Scholar] [CrossRef]
- Meshaal, M.S.; Nagi, A.; Eldamaty, A.; Elnaggar, W.; Gaber, M.; Rizk, H. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as independent predictors of outcome in infective endocarditis (IE). Egypt Heart J. 2019, 71, 13. [Google Scholar] [CrossRef]
- Can, E.; Hamilcikan, Ş.; Can, C. The Value of Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio for Detecting Early-onset Neonatal Sepsis. J. Pediatr. Hematol. Oncol. 2018, 40, e229–e232. [Google Scholar] [CrossRef]
- De Jager, C.P.; Wever, P.C.; Gemen, E.F.; Kusters, R.; van Gageldonk-Lafeber, A.B.; van der Poll, T.; Laheij, R.J. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS ONE 2012, 7, e46561. [Google Scholar] [CrossRef]
- Dursun, A.; Ozsoylu, S.; Akyildiz, B.N. Neutrophil-to-lymphocyte ratio and mean platelet volume can be useful markers to predict sepsis in children. Pak. J. Med. Sci. 2018, 34, 918–922. [Google Scholar] [CrossRef]
- Velissaris, D.; Pantzaris, N.D.; Bountouris, P.; Gogos, C. Correlation between neutrophil-to-lymphocyte ratio and severity scores in septic patients upon hospital admission. A series of 50 patients. Rom. J. Intern. Med. 2018, 56, 153–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Fu, Z.; Huang, W.; Huang, K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am. J. Emerg. Med. 2020, 38, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, C.; Sabarimurugan, S.; Madurantakam, R.M.; Lakhotiya, K.; Samiappan, S.; Baxi, S.; Nachimuthu, R.; Gothandam, K.M.; Jayaraj, R. Prognostic significance of blood inflammatory biomarkers NLR, PLR, and LMR in cancer-A protocol for systematic review and meta-analysis. Medicine 2019, 98, e14834. [Google Scholar] [CrossRef]
- Farkas, J.D. The complete blood count to diagnose septic shock. J. Thorac. Dis. 2020, 12, S16–S21. [Google Scholar] [CrossRef]
- Djordjevic, D.; Rondovic, G.; Surbatovic, M.; Stanojevic, I.; Udovicic, I.; Andjelic, T.; Zeba, S.; Milosavljevic, S.; Stankovic, N.; Abazovic, D.; et al. Neutrophil-to-Lymphocyte Ratio, Monocyte-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Mean Platelet Volume-to-Platelet Count Ratio as Biomarkers in Critically Ill and Injured Patients: Which Ratio to Choose to Predict Outcome and Nature of Bacteremia? Mediat. Inflamm. 2018, 2018, 3758068. [Google Scholar] [CrossRef] [Green Version]
- Oh, G.H.; Chung, S.P.; Park, Y.S.; Hong, J.H.; Lee, H.S.; Chung, H.S.; You, J.S.; Park, J.W.; Park, I. Mean Platelet Volume to Platelet Count Ratio as a Promising Predictor of Early Mortality in Severe Sepsis. Shock 2017, 47, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Huang, X.; Zhang, W. Platelet-to-lymphocyte ratio as a prognostic predictor of mortality for sepsis: Interaction effect with disease severity—A retrospective study. BMJ Open 2019, 9, e022896. [Google Scholar] [CrossRef] [PubMed]
- Ates, S.; Oksuz, H.; Dogu, B.; Bozkus, F.; Ucmak, H.; Yanıt, F. Can mean platelet volume and mean platelet volume/platelet count ratio be used as a diagnostic marker for sepsis and systemic inflammatory response syndrome? Saudi Med. J. 2015, 36, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Urrechaga, E.; Bóveda, O.; Aguirre, U. Improvement in detecting sepsis using leukocyte cell population data (CPD). Clin. Chem. Lab. Med. 2019, 57, 918–926. [Google Scholar] [CrossRef]
- Hausfater, P.; Robert Boter, N.; Morales Indiano, C.; Cancella de Abreu, M.; Marin, A.M.; Pernet, J.; Quesada, D.; Castro, I.; Careaga, D.; Arock, M.; et al. Monocyte distribution width (MDW) performance as an early sepsis indicator in the emergency department: Comparison with CRP and procalcitonin in a multicenter international European prospective study. Crit. Care 2021, 25, 227. [Google Scholar] [CrossRef] [PubMed]
- Piva, E.; Zuin, J.; Pelloso, M.; Tosato, F.; Fogar, P.; Plebani, M. Monocyte distribution width (MDW) parameter as a sepsis indicator in intensive care units. Clin. Chem. Lab. Med. 2021, 59, 1307–1314. [Google Scholar] [CrossRef]
- Woo, A.; Oh, D.K.; Park, C.J.; Hong, S.B. Monocyte distribution width compared with C-reactive protein and procalcitonin for early sepsis detection in the emergency department. PLoS ONE 2021, 16, e0250101. [Google Scholar] [CrossRef]
- Agnello, L.; Bivona, G.; Vidali, M.; Scazzone, C.; Giglio, R.V.; Iacolino, G.; Iacona, A.; Mancuso, S.; Ciaccio, A.M.; Lo Sasso, B.; et al. Monocyte distribution width (MDW) as a screening tool for sepsis in the Emergency Department. Clin. Chem. Lab. Med. 2020, 58, 1951–1957. [Google Scholar] [CrossRef]
- Agnello, L.; Iacona, A.; Lo Sasso, B.; Scazzone, C.; Pantuso, M.; Giglio, R.V.; Gambino, C.M.; Ciaccio, A.M.; Bivona, G.; Vidali, M.; et al. A new tool for sepsis screening in the Emergency Department. Clin. Chem. Lab. Med. 2021, 59, 1600–1605. [Google Scholar] [CrossRef]
- Agnello, L.; Iacona, A.; Maestri, S.; Lo Sasso, B.; Giglio, R.V.; Mancuso, S.; Ciaccio, A.M.; Vidali, M.; Ciaccio, M. Independent Validation of Sepsis Index for Sepsis Screening in the Emergency Department. Diagnostics 2021, 11, 1292. [Google Scholar] [CrossRef] [PubMed]
- Agnello, L.; Lo Sasso, B.; Vidali, M.; Scazzone, C.; Gambino, C.M.; Giglio, R.V.; Ciaccio, A.M.; Bivona, G.; Ciaccio, M. Validation of monocyte distribution width decisional cutoff for sepsis detection in the acute setting. Int. J. Lab. Hematol. 2021, 43, O183–O185. [Google Scholar] [CrossRef]
- Agnello, L.; Sasso, B.L.; Giglio, R.V.; Bivona, G.; Gambino, C.M.; Cortegiani, A.; Ciaccio, A.M.; Vidali, M.; Ciaccio, M. Monocyte distribution width as a biomarker of sepsis in the intensive care unit: A pilot study. Ann. Clin. Biochem. 2021, 58, 70–73. [Google Scholar] [CrossRef]
- Crouser, E.D.; Parrillo, J.E.; Martin, G.S.; Huang, D.T.; Hausfater, P.; Grigorov, I.; Careaga, D.; Osborn, T.; Hasan, M.; Tejidor, L. Monocyte distribution width enhances early sepsis detection in the emergency department beyond SIRS and qSOFA. J. Intensive Care 2020, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Parrillo, J.E.; Seymour, C.; Angus, D.C.; Bicking, K.; Tejidor, L.; Magari, R.; Careaga, D.; Williams, J.; Closser, D.R.; et al. Improved Early Detection of Sepsis in the ED With a Novel Monocyte Distribution Width Biomarker. Chest 2017, 152, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Gupta, P.K.; Lingaiah, R.; Mukhopadhyay, A.K. Volume, conductivity, and scatter parameters of leukocytes as early markers of sepsis and treatment response. J. Lab. Physicians 2019, 11, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Mardi, D.; Fwity, B.; Lobmann, R.; Ambrosch, A. Mean cell volume of neutrophils and monocytes compared with C-reactive protein, interleukin-6 and white blood cell count for prediction of sepsis and nonsystemic bacterial infections. Int. J. Lab. Hematol. 2010, 32, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Mammen, J.; Choudhuri, J.; Paul, J.; Sudarsan, T.I.; Josephine, T.; Mahasampath, G.; Jeyaseelan, V.; Nair, S.C.; Peter, J.V. Cytomorphometric Neutrophil and Monocyte Markers May Strengthen the Diagnosis of Sepsis. J. Intensive Care Med. 2018, 33, 656–662. [Google Scholar] [CrossRef]
- Lee, A.J.; Kim, S.G. Mean cell volumes of neutrophils and monocytes are promising markers of sepsis in elderly patients. Blood Res. 2013, 48, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Buoro, S.; Seghezzi, M.; Vavassori, M.; Dominoni, P.; Apassiti Esposito, S.; Manenti, B.; Mecca, T.; Marchesi, G.; Castellucci, E.; Azzarà, G.; et al. Clinical significance of cell population data (CPD) on Sysmex XN-9000 in septic patients with our without liver impairment. Ann. Transl. Med. 2016, 4, 418. [Google Scholar] [CrossRef] [Green Version]
- Urrechaga, E.; Bóveda, O.; Aguirre, U. Role of leucocytes cell population data in the early detection of sepsis. J. Clin. Pathol. 2018, 71, 259–266. [Google Scholar] [CrossRef]
- Urrechaga, E. Reviewing the value of leukocytes cell population data (CPD) in the management of sepsis. Ann. Transl. Med. 2020, 8, 953. [Google Scholar] [CrossRef]
- Park, D.H.; Park, K.; Park, J.; Park, H.H.; Chae, H.; Lim, J.; Oh, E.J.; Kim, Y.; Park, Y.J.; Han, K. Screening of sepsis using leukocyte cell population data from the Coulter automatic blood cell analyzer DxH800. Int. J. Lab. Hematol. 2011, 33, 391–399. [Google Scholar] [CrossRef]
- Ayres, L.S.; Sgnaolin, V.; Munhoz, T.P. Immature granulocytes index as early marker of sepsis. Int. J. Lab. Hematol. 2019, 41, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Karon, B.S.; Tolan, N.V.; Wockenfus, A.M.; Block, D.R.; Baumann, N.A.; Bryant, S.C.; Clements, C.M. Evaluation of lactate, white blood cell count, neutrophil count, procalcitonin and immature granulocyte count as biomarkers for sepsis in emergency department patients. Clin. Biochem. 2017, 50, 956–958. [Google Scholar] [CrossRef] [PubMed]
- Tauseef, A.; Zafar, M.; Arshad, W.; Thirumalareddy, J.; Sood, A.; Farooque, U.; Nair, S.; Mirza, M. Role of immature platelet fraction (IPF) in sepsis patients: A systematic review. J. Fam. Med. Prim. Care 2021, 10, 2148–2152. [Google Scholar] [CrossRef]
- Ahn, C.; Kim, W.; Lim, T.H.; Cho, Y.; Choi, K.S.; Jang, B.H. The delta neutrophil index (DNI) as a prognostic marker for mortality in adults with sepsis: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 6621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.W.; Yoon, J.H.; Jin, S.J.; Kim, S.B.; Ku, N.S.; Jeong, S.J.; Han, S.H.; Choi, J.Y.; Kim, J.M.; Song, Y.G. Delta neutrophil index as a prognostic marker of early mortality in gram negative bacteremia. Infect. Chemother. 2014, 46, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Celik, I.H.; Arifoglu, I.; Arslan, Z.; Aksu, G.; Bas, A.Y.; Demirel, N. The value of delta neutrophil index in neonatal sepsis diagnosis, follow-up and mortality prediction. Early Hum. Dev. 2019, 131, 6–9. [Google Scholar] [CrossRef]
- Riedel, S.; Carroll, K.C. Early Identification and Treatment of Pathogens in Sepsis: Molecular Diagnostics and Antibiotic Choice. Clin. Chest Med. 2016, 37, 191–207. [Google Scholar] [CrossRef]
- Agnello, L.; Bellia, C.; Di Gangi, M.; Lo Sasso, B.; Calvaruso, L.; Bivona, G.; Scazzone, C.; Dones, P.; Ciaccio, M. Utility of serum procalcitonin and C-reactive protein in severity assessment of community-acquired pneumonia in children. Clin. Biochem. 2016, 49, 47–50. [Google Scholar] [CrossRef]
- Giulia, B.; Luisa, A.; Concetta, S.; Bruna, L.S.; Chiara, B.; Marcello, C. Procalcitonin and community-acquired pneumonia (CAP) in children. Clin. Chim. Acta 2015, 451, 215–218. [Google Scholar] [CrossRef] [Green Version]
- Covington, E.W.; Roberts, M.Z.; Dong, J. Procalcitonin Monitoring as a Guide for Antimicrobial Therapy: A Review of Current Literature. Pharmacotherapy 2018, 38, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Sreekantan Nair, A.; Illango, J.; Siddiqui, N.; Gor, R.; Fernando, R.W.; Hamid, P. The Advancement in Detecting Sepsis and Its Outcome: Usefulness of Procalcitonin in Diagnosing Sepsis and Predicting Fatal Outcomes in Patients Admitted to Intensive Care Unit. Cureus 2021, 13, e14439. [Google Scholar] [CrossRef] [PubMed]
- Ciaccio, M.; Lo Sasso, B.; Scazzone, C.; Gambino, C.M.; Ciaccio, A.M.; Bivona, G.; Piccoli, T.; Giglio, R.V.; Agnello, L. COVID-19 and Alzheimer’s Disease. Brain Sci. 2021, 11, 305. [Google Scholar] [CrossRef]
- Lippi, G. Sepsis biomarkers: Past, present and future. Clin. Chem. Lab. Med. 2019, 57, 1281–1283. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Alteration | Clinical Usefulness | |
|---|---|---|---|
| Basic | WBC | ↑ | Diagnosis |
| Neutrophils | ↑ | Prognosis | |
| Lymphocytes | ↓ | Prognosis | |
| Monocytes | ↑↓ | Controversial | |
| Eosinophil | ↓ | Diagnosis | |
| Basophil | ↓ | Prognosis | |
| RBC | ↓ | None | |
| Hemoglobin | ↓ | Guide RBC transfusion | |
| Hematocrit | ↓ | Target for RBC transfusion | |
| MCV | - | - | |
| MCH | - | - | |
| MCHC | - | - | |
| RDW | ↑ | Prognosis | |
| Platelets | ↓ | Diagnosis and prognosis | |
| CPD | MDW | ↑ | Diagnosis |
| MNV | ↑ | Diagnosis | |
| MMV | ↑ | Diagnosis | |
| NE-SFL | ↑ | Diagnosis and prognosis | |
| MO-X | ↑ | Diagnosis and prognosis | |
| IPF | ↑ | Diagnosis and prognosis | |
| DNI | ↑ | Prognosis and monitoring therapy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agnello, L.; Giglio, R.V.; Bivona, G.; Scazzone, C.; Gambino, C.M.; Iacona, A.; Ciaccio, A.M.; Lo Sasso, B.; Ciaccio, M. The Value of a Complete Blood Count (CBC) for Sepsis Diagnosis and Prognosis. Diagnostics 2021, 11, 1881. https://doi.org/10.3390/diagnostics11101881
Agnello L, Giglio RV, Bivona G, Scazzone C, Gambino CM, Iacona A, Ciaccio AM, Lo Sasso B, Ciaccio M. The Value of a Complete Blood Count (CBC) for Sepsis Diagnosis and Prognosis. Diagnostics. 2021; 11(10):1881. https://doi.org/10.3390/diagnostics11101881
Chicago/Turabian StyleAgnello, Luisa, Rosaria Vincenza Giglio, Giulia Bivona, Concetta Scazzone, Caterina Maria Gambino, Alessandro Iacona, Anna Maria Ciaccio, Bruna Lo Sasso, and Marcello Ciaccio. 2021. "The Value of a Complete Blood Count (CBC) for Sepsis Diagnosis and Prognosis" Diagnostics 11, no. 10: 1881. https://doi.org/10.3390/diagnostics11101881
APA StyleAgnello, L., Giglio, R. V., Bivona, G., Scazzone, C., Gambino, C. M., Iacona, A., Ciaccio, A. M., Lo Sasso, B., & Ciaccio, M. (2021). The Value of a Complete Blood Count (CBC) for Sepsis Diagnosis and Prognosis. Diagnostics, 11(10), 1881. https://doi.org/10.3390/diagnostics11101881









