ALADA Dose Optimization in the Computed Tomography of the Temporal Bone: The Diagnostic Potential of Different Low-Dose CT Protocols
Abstract
:1. Introduction
2. Materials and Methods
2.1. CT Scanning
2.2. CBCT Scanning
2.3. Dose Estimation
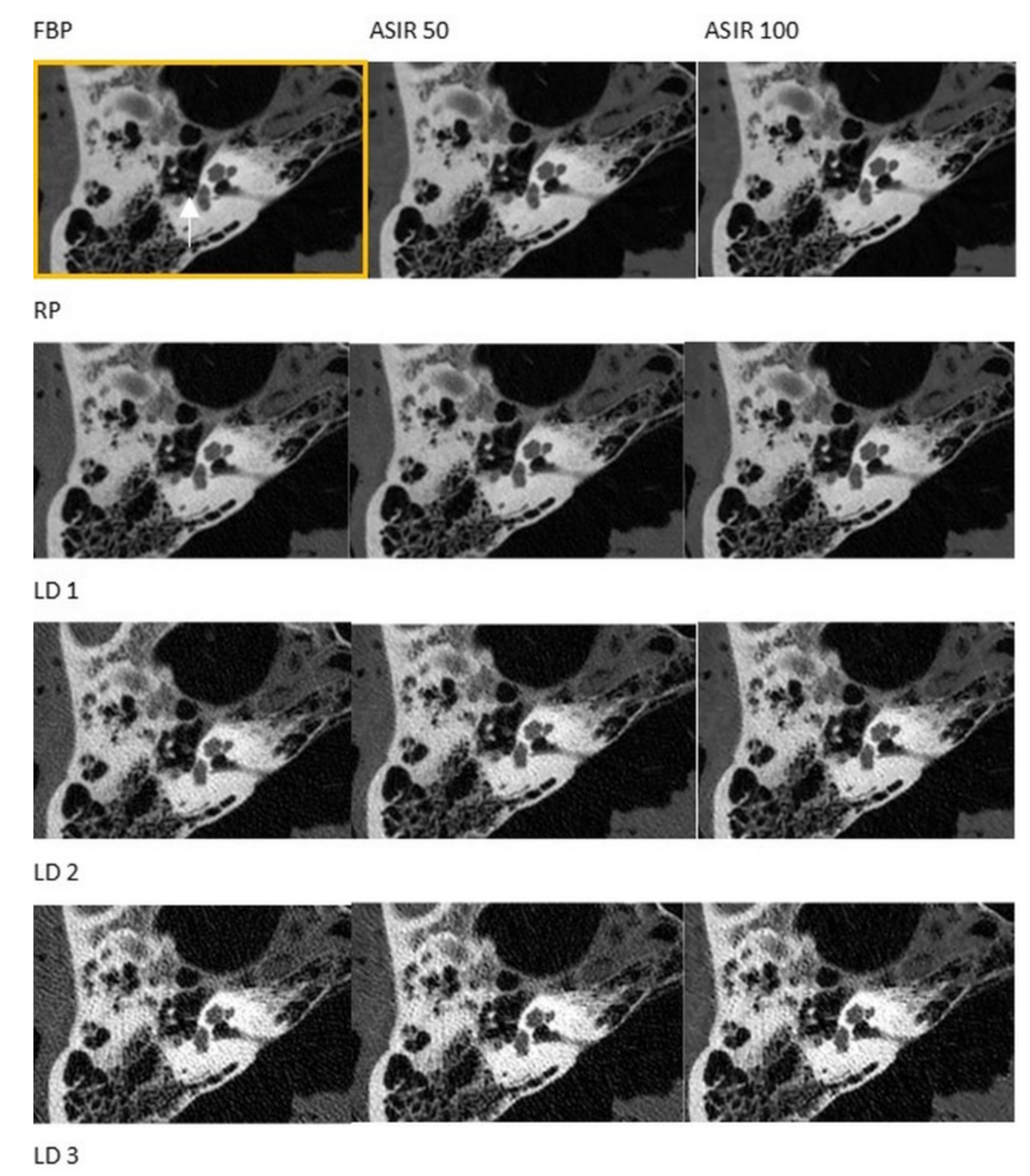
2.4. Analysis of Diagnostic Image Quality
2.5. Statistical analyses
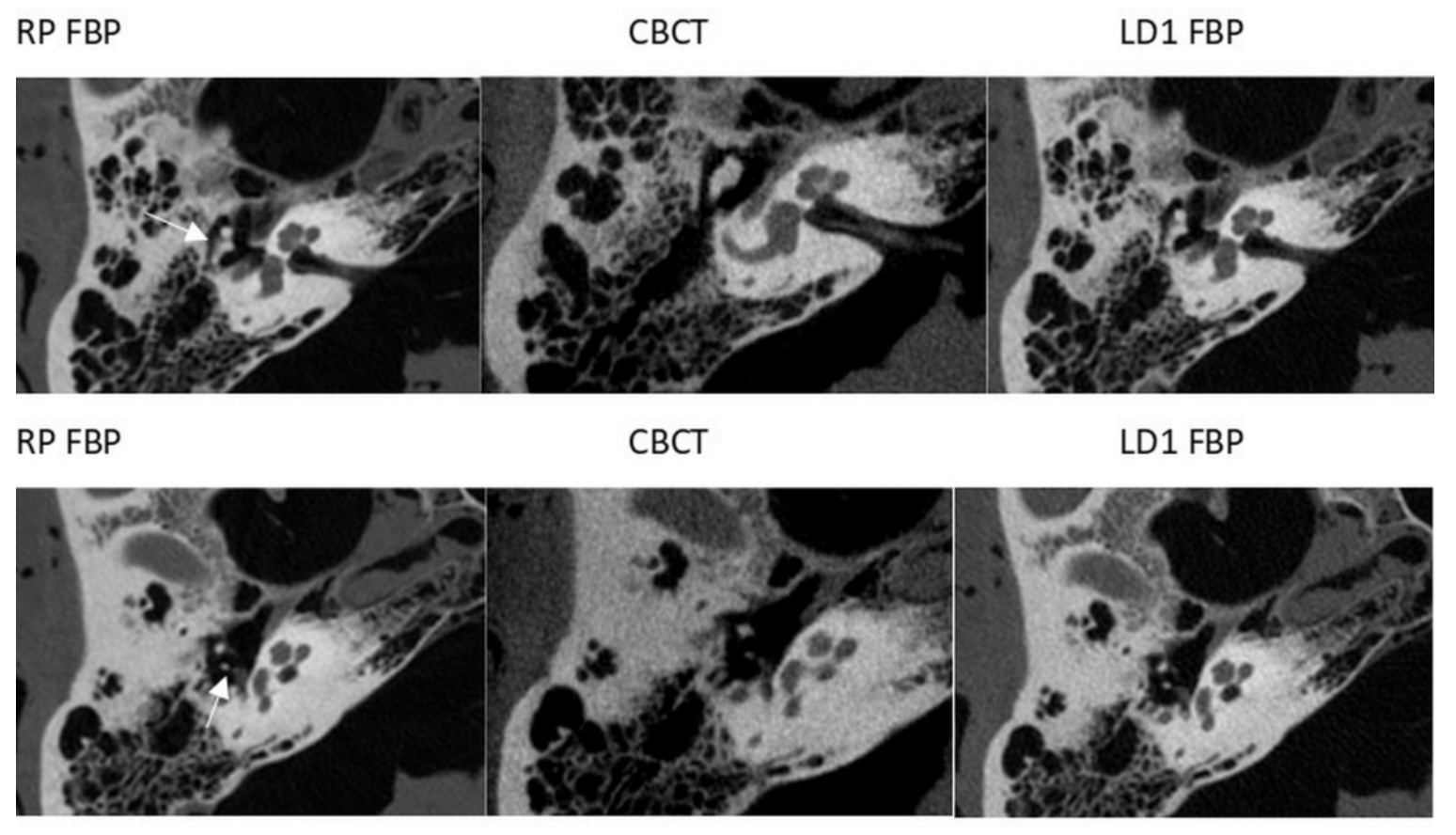
3. Results
3.1. CT
3.2. CBCT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cristalli, G.; Manciocco, V.; Pichi, B.; Marucci, L.; Arcangeli, G.; Telera, S.; Spriano, G. Treatment and outcome of advanced external auditory canal and middle ear squamous cell carcinoma. J. Craniofacial Surg. 2009, 20, 816–821. [Google Scholar] [CrossRef]
- Fujiwara, S.; Toyama, Y.; Miyashita, T.; Osaki, Y.; Inamoto, R.; Hoshikawa, H.; Mori, N. Usefulness of multislice-CT using multiplanar reconstruction in the preoperative assessment of the ossicular lesions in the middle ear diseases. Auris Nasus Larynx 2016, 43, 247–253. [Google Scholar] [CrossRef]
- Molteni, G.; Fabbris, C.; Molinari, G.; Alicandri-Ciufelli, M.; Presutti, L.; Paltrinieri, D.; Marchioni, D. Correlation between pre-operative CT findings and intra-operative features in pediatric cholesteatoma: A retrospective study on 26 patients. Eur. Arch Otorhinolaryngol. 2019, 276, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Meher, R.; Passey, J.C.; Kumar, J.; Gupta, A.; Kharbanda, S. Comparative evaluation of round window niche accessibility pre-operatively on high-resolution computed tomography of the temporal bone with intra-operative findings. J. Laryngol. Otol. 2019, 133, 575–579. [Google Scholar] [CrossRef]
- Touska, P.; Connor, S.E.J. Imaging of the temporal bone. Clin. Radiol. 2020, 75, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Kachniarz, B.; Gilani, S.; Shin, J.J. Risk of malignancy associated with head and neck CT in children: A systematic review. Otolaryngol. Head Neck Surg. 2014, 151, 554–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baysson, H.; Journy, N.; Roue, T.; Ducou-Lepointe, H.; Etard, C.; Bernier, M.O. Exposure to CT scans in childhood and long-term cancer risk: A review of epidemiological studies. Bull. Cancer 2016, 103, 190–198. [Google Scholar] [CrossRef]
- Schonfeld, S.J.; Lee, C.; Berrington de Gonzalez, A. Medical exposure to radiation and thyroid cancer. Clin. Oncol. 2011, 23, 244–250. [Google Scholar] [CrossRef]
- Giannitto, C.; Campoleoni, M.; Maccagnoni, S.; Angileri, A.S.; Grimaldi, M.C.; Giannitto, N.; De Piano, F.; Ancona, E.; Biondetti, P.R.; Esposito, A.A. Unindicated multiphase CT scans in non-traumatic abdominal emergencies for women of reproductive age: A significant source of unnecessary exposure. Radiol. Med. 2018, 123, 185–190. [Google Scholar] [CrossRef]
- Deak, P.D.; Smal, Y.; Kalender, W.A. Multisection CT protocols: Sex- and age-specific conversion factors used to determine effective dose from dose-length product. Radiology 2010, 257, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Mathews, J.D.; Forsythe, A.V.; Brady, Z.; Butler, M.W.; Goergen, S.K.; Byrnes, G.B.; Giles, G.G.; Wallace, A.B.; Anderson, P.R.; Guiver, T.A.; et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: Data linkage study of 11 million Australians. BMJ 2013, 346, f2360. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.K.; Tsai, D.C.; Chang, S.C.; Yuan, M.C.; Chang, S.J.; Chen, H.W.; Leu, H.B. The risk of cataract associated with repeated head and neck CT studies: A nationwide population-based study. AJR Am. J. Roentgenol. 2013, 201, 626–630. [Google Scholar] [CrossRef]
- Andresz, S.; Morgan, J.; Crouail, P.; Vermeersch, F. Conclusions and recommendations from the 17th Workshop of the European ALARA Network ‘ALARA in emergency exposure situations’. J. Radiol. Prot. 2018, 38, 434–439. [Google Scholar] [CrossRef]
- European ALARA Network. Optimization of Radiation Protection. ALARA: A Practical Guidebook, 1st ed.; European ALARA Network: Fontenay-aux-Roses, France, 2019; ISBN 978-2-9569796-0-9. Available online: www.eu-alara.net (accessed on 24 September 2021).
- European Society of Radiology. ESR EuroSafe Imaging-Computed Tomography. Available online: http://www.eurosafeimaging.org/radiation-protection-in-ct (accessed on 24 September 2021).
- Awad, M.F.; Karout, L.; Arnous, G.; Rawashdeh, M.A.; Hneiny, L.; Saade, C. A systematic review on the current status of adult diagnostic reference levels in head, chest and abdominopelvic Computed Tomography. J. Radiol. Prot. Off. J. Soc. Radiol. Prot. 2020, 40, R71–R98. [Google Scholar] [CrossRef]
- Jaju, P.P.; Jaju, S.P. Cone-beam computed tomography: Time to move from ALARA to ALADA. Imaging Sci. Dent. 2015, 45, 263–265. [Google Scholar] [CrossRef] [Green Version]
- Kadesjo, N.; Benchimol, D.; Falahat, B.; Nasstrom, K.; Shi, X.Q. Evaluation of the effective dose of cone beam CT and multislice CT for temporomandibular joint examinations at optimized exposure levels. Dentomaxillofac Radiol. 2015, 44, 20150041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aberle, C.; Ryckx, N.; Treier, R.; Schindera, S. Update of national diagnostic reference levels for adult CT in Switzerland and assessment of radiation dose reduction since 2010. Eur. Radiol. 2020, 30, 1690–1700. [Google Scholar] [CrossRef]
- McHanwell, S.; Brenner, E.; Chirculescu, A.R.M.; Drukker, J.; van Mameren, H.; Mazzotti, G.; Pais, D.; Paulsen, F.; Plaisant, O.; Caillaud, M.M.; et al. The legal and ethical framework governing Body Donation in Europe—A review of current practice and recommendations for good practice. Eur. J. Anat. 2008, 12, 1–24. [Google Scholar]
- Beat, M.; Riederer, S.B.; Brenner, E.; Bueno-López, J.L.; Circulescu, A.R.M.; Davies, D.C.; De Caro, R.; Gerrits, P.O.; McHanwell, S.; Pais, D.; et al. The legal and ethical framework governing Body Donation in Europe—1st update on current practice. Eur. J. Anat. 2012, 16, 1–21. [Google Scholar]
- Platzer, E. Ein neues Konservierungs- und Aufbewahrungssystem für anatomisches Material. Acta Anat. 1978, 102, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Pein, M.K.; Brandt, S.; Plontke, S.K.; Kosling, S. Visualization of subtle temporal bone structures. Comparison of cone beam CT and MDCT. Radiologe 2014, 54, 271–278. [Google Scholar] [CrossRef]
- Al-Ekrish, A.A.; Al-Shawaf, R.; Schullian, P.; Al-Sadhan, R.; Hormann, R.; Widmann, G. Validity of linear measurements of the jaws using ultralow-dose MDCT and the iterative techniques of ASIR and MBIR. Int. J. Comput. Assist. Radiol. Surg. 2016, 11, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Widmann, G.; Fasser, M.; Schullian, P.; Zangerl, A.; Puelacher, W.; Kral, F.; Riechelmann, H.; Jaschke, W.; Bale, R. Substantial dose reduction in modern multi-slice spiral computed tomography (MSCT)-guided craniofacial and skull base surgery. Rofo 2012, 184, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Tada, A.; Sato, S.; Masaoka, Y.; Kanazawa, S. Imaging of the temporal bone in children using low-dose 320-row area detector computed tomography. J. Med. Imaging Radiat. Oncol. 2017, 61, 489–493. [Google Scholar] [CrossRef]
- Niu, Y.T.; Mehta, D.; Zhang, Z.R.; Zhang, Y.X.; Liu, Y.F.; Kang, T.L.; Xian, J.F.; Wang, Z.C. Radiation dose reduction in temporal bone CT with iterative reconstruction technique. AJNR. Am. J. Neuroradiol. 2012, 33, 1020–1026. [Google Scholar] [CrossRef] [Green Version]
- Bauknecht, H.C.; Siebert, E.; Dannenberg, A.; Bohner, G.; Jach, C.; Diekmann, S.; Scheurig, C.; Klingebiel, R. Image quality and radiation exposure in 320-row temporal bone computed tomography. Dentomaxillofac Radiol. 2010, 39, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- Dahmani-Causse, M.; Marx, M.; Deguine, O.; Fraysse, B.; Lepage, B.; Escude, B. Morphologic examination of the temporal bone by cone beam computed tomography: Comparison with multislice helical computed tomography. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Widmann, G.; Zangerl, A.; Schullian, P.; Fasser, M.; Puelacher, W.; Bale, R. Do image modality and registration method influence the accuracy of craniofacial navigation? J. Oral Maxillofac Surg. 2012, 70, 2165–2173. [Google Scholar] [CrossRef]
- Kyriakou, Y.; Kolditz, D.; Langner, O.; Krause, J.; Kalender, W. Digital volume tomography (DVT) and multislice spiral CT (MSCT): An objective examination of dose and image quality. Rofo 2011, 183, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Widmann, G.; Fischer, B.; Berggren, J.P.; Dennhardt, A.; Schullian, P.; Reto, B.; Puelacher, W. Cone Beam Computed Tomography vs. Multislice Computed Tomography in Computer-Aided Design/Computer-Assisted Manufacture Guided Implant Surgery Based on Three-Dimensional Optical Scanning and Stereolithographic Guides: Does Image Modality Matter? Int. J. Oral Maxillofac Implants 2016, 31, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Knorgen, M.; Brandt, S.; Kosling, S. Comparison of quality on digital X-ray devices with 3D-capability for ENT-clinical objectives in imaging of temporal bone and paranasal sinuses. Rofo 2012, 184, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, Y.; Struffert, T.; Dorfler, A.; Kalender, W.A. Basic principles of flat detector computed tomography (FD-CT). Radiologe 2009, 49, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.P.; Reynolds, R.M.; Ramakrishna, R.; Levitt, M.R.; Hopper, R.A.; Lee, A.; Browd, S.R. Low-dose head computed tomography in children: A single institutional experience in pediatric radiation risk reduction: Clinical article. J. Neurosurg. Pediatrics 2013, 12, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Komarraju, A.; Mehta, S.T.; Glacier, C.; Nabaweesi, R.; Choudhary, A.; Ramakrishnaiah, R. Ultra-Low-Dose Computed Tomography Protocol for Preoperative Evaluation in Children With Craniofacial Anomalies. J. Craniofacial Surg. 2021, 32, 130–133. [Google Scholar] [CrossRef]
- Van Aalst, J.; Jeukens, C.R.; Vles, J.S.; van Maren, E.A.; Kessels, A.G.; Soudant, D.L.; Weber, J.W.; Postma, A.A.; Cornips, E.M. Diagnostic radiation exposure in children with spinal dysraphism: An estimation of the cumulative effective dose in a cohort of 135 children from The Netherlands. Arch. Dis. Child. 2013, 98, 680–685. [Google Scholar] [CrossRef]
- Johnson, J.N.; Hornik, C.P.; Li, J.S.; Benjamin, D.K., Jr.; Yoshizumi, T.T.; Reiman, R.E.; Frush, D.P.; Hill, K.D. Cumulative radiation exposure and cancer risk estimation in children with heart disease. Circulation 2014, 130, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Weinrich, J.M.; Maas, K.J.; Starekova, J.; Tahir, E.; Intert, L.; Heinemann, A.; Sehner, S.; Regier, M.; Püschel, K.; Adam, G.; et al. Feasibility of Submillisievert CT of the Skeletal Pelvis Using Iterative Reconstruction: A Human Cadaver Study. AJR. Am. J. Roentgenol. 2019, 213, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Weinrich, J.M.; Regier, M.; Well, L.; Bannas, P.; Nykolyn, O.; Heinemann, A.; Sehner, S.; Behzadi, C.; Püschel, K.; Adam, G.; et al. Feasibility of sub-milliSievert CT of the cervical spine: Initial results in fresh human cadavers. Eur. J. Radiol. 2019, 120, 108697. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Q.; Hu, D.; Shen, Y.; Yang, H.; Chen, C.; Zhou, Z.; Peng, Y. Feasibility study of using one-tenth mSv radiation dose in young children chest CT with 80 kVp and model-based iterative reconstruction. Sci. Rep. 2019, 9, 12481. [Google Scholar] [CrossRef]
- Tanabe, Y.; Kido, T.; Kurata, A.; Kouchi, T.; Hosokawa, T.; Nishiyama, H.; Kawaguchi, N.; Kido, T.; Uetani, T.; Mochizuki, T. Impact of Knowledge-Based Iterative Model Reconstruction on Image Quality and Hemodynamic Parameters in Dynamic Myocardial Computed Tomography Perfusion Using Low-Tube-Voltage Scan: A Feasibility Study. J. Comput. Assist. Tomogr. 2019, 43, 811–816. [Google Scholar] [CrossRef] [PubMed]

| Unit | Measurements |
|---|---|
| kV | 120 |
| mAs | 250 |
| Table speed/Table feed per rotation | 10,625/10,625 |
| Pitch (mm) | 0.531 |
| Collimation | 20 × 10,625 |
| Slice thickness (mm) | 0.625 |
| Recon increment | 0.625 |
| FOV, Scan length | individual (as small as possible) |
| Kernel | BONE2 |
| CTDIvol (reference) (mGy) | 83.72 |
| CTDIvol (120 kV/80 mAs) = LD1 (mGy) | 26.79 |
| CTDIvol (100 kV/35 mAs) = LD2 (mGy) | 7.66 |
| CTDIvol (80 kV/40 mAs) = LD3 (mGy) | 4.82 |
| FBP/Kernel | FBP/BONE2 |
| IRT/Kernel | ASIR 50/BONE2 |
| IRT/Kernel | ASIR 100/BONE2 |
| Protocol | RP | LD1 | LD2 | LD3 |
|---|---|---|---|---|
| kV/mA | 120/250 | 120/80 | 100/35 | 80/40 |
| Scanlength (cm) | 10 | 10 | 10 | 10 |
| DLP (mGy × cm) | 837.2 | 267.9 | 76.6 | 48.2 |
| Conversion factor adult | 0.0019 | 0.0019 | 0.0019 | 0.0018 |
| Effective dose adult (mSv) | 1.59068 | 0.50901 | 0.14554 | 0.08676 |
| Conversion factor 10-year-old (mSv) | 0.0027 | 0.0027 | 0.0027 | 0.0026 |
| Effective dose 10-year-old (mSv) | 2.26044 | 0.72333 | 0.20682 | 0.12532 |
| Conversion factor 5-year-old | 0.0035 | 0.0035 | 0.0035 | 0.0035 |
| Effective dose 5-year-old (mSv) | 2.9302 | 0.93765 | 0.2681 | 0.1687 |
| Conversion factor 1-year-old | 0.0053 | 0.0053 | 0.0054 | 0.0056 |
| Effective dose 1-year-old (mSv) | 4.43716 | 1.41987 | 0.41364 | 0.26992 |
| Conversion factor newborn | 0.0085 | 0.0085 | 0.0088 | 0.0094 |
| Effective dose newborn (mSv) | 7.1162 | 2.27715 | 0.67408 | 0.45308 |
| Imaging Device | Cadaver Head | Side | Shaded Structure |
|---|---|---|---|
| CT | Number 1 | Right | None |
| Left | None | ||
| Number 2 | Right | Tendon of the m. tensor tympani Tendon of the m. stapedius Tympanic membrane Chorda tympani Stapes | |
| Left | Stapes | ||
| CBCT | Number 1 | Right | None |
| Left | None | ||
| Number 2 | Right | None | |
| Left | Tympanic membrane Chorda tympani Modiolus Stapes |
| Protocol | Score FBP | p-Value | Score ASIR 50 | p-Value | Score ASIR 100 | p-Value | |
|---|---|---|---|---|---|---|---|
| Tendon of m. tensor tympani | RP LD1 LD2 LD3 CBCT | 4.0 (1–5) 3.0 (1–4) 2.5 (1–3) 2.0 (1–3) 3.0 (1–4) | n/a 0.083 0.023 * 0.023 * 0.336 | 4.0 (1–5) 3.0 (1–4) 2.5 (1–3) 2.0 (1–3) 3.0 (1–4) | n/a 0.083 0.023 * 0.023 * 0.336 | 4.0 (1–5) 3.0 (1–4) 3.0 (1–3) 2.0 (1–3) 3.0 (1–4) | n/a 0.046 * 0.023 * 0.026 * 0.236 |
| Tendon of m. stapedius | RP LD1 LD2 LD3 CBCT | 2.0 (1–3) 1.5 (1–3) 1.0 (1–2) 1.0 (1–1) 2.5 (1–4) | n/a 0.157 0.059 0.046 * 0.046 * | 2.0 (1–3) 1.5 (1–3) 1.0 (1–2) 1.0 (1–1) 2.5 (1–4) | n/a 0.157 0.059 0.046 * 0.046 * | 2.0 (1–3) 1.5 (1–3) 1.0 (1–2) 1.0 (1–1) 2.5 (1–4) | n/a 0.157 0.059 0.046 * 0.046 * |
| Incudostapedial joint | RP LD1 LD2 LD3 CBCT | 4.0 (3–4) 3.5 (3–4) 3.0 (2–3) 2.0 (1–3) 3.5 (3–4) | n/a 0.083 0.008 * 0.011 * 0.083 | 4.0 (3–4) 3.5 (3–4) 3.0 (2–3) 2.0 (1–3) 3.5 (3–4) | n/a 0.083 0.008 * 0.011 * 0.083 | 4.0 (3–5) 3.5 (3–4) 3.0 (2–3) 2.0 (1–3) 3.5 (3–4) | n/a 0.046 * 0.009 * 0.011 * 0.046 * |
| Incudomalleolar joint | RP LD1 LD2 LD3 CBCT | 5.0 (4–5) 4.0 (4–4) 3.5 (3–4) 3.0 (1–4) 4.0 (3–4) | n/a 0.014 * 0.015 * 0.017 * 0.023 * | 5.0 (4–5) 4.0 (4–5) 3.5 (3–4) 3.0 (1–4) 4.0 (3–4) | n/a 0.025 * 0.015 * 0.017 * 0.023 * | 5.0 (4–5) 4.0 (4–5) 3.5 (3–4) 3.0 (1–4) 4.0 (3–4) | n/a 0.046 * 0.015 * 0.017 * 0.023 * |
| Stapes feet | RP LD1 LD2 LD3 CBCT | 3.0 (1–4) 2.5 (1–4) 1.5 (1–3) 1.5 (1–2) 3.0 (2–3) | n/a 0.083 0.059 0.024 * 0.750 | 3.0 (1–4) 2.5 (1–4) 1.5 (1–3) 1.5 (1–2) 3.0 (2–3) | n/a 0.083 0.059 0.024 * 0.750 | 3.0 (1–5) 2.5 (1–4) 1.5 (1–3) 1.5 (1–2) 3.0 (2–3) | n/a 0.046 * 0.066 0.026 * 1.000 |
| Stapes head | RP LD1 LD2 LD3 CBCT | 4.0 (3–4) 3.0 (2–4) 2.5 (1–3) 2.0 (1–2) 3.0 (3–4) | n/a 0.014 * 0.015 * 0.008 * 0.046 * | 4.0 (3–4) 3.0 (2–4) 2.5 (1–3) 2.0 (1–2) 3.0 (3–4) | n/a 0.014 * 0.015 * 0.008 * 0.046 * | 4.0 (3–4) 3.0 (2–4) 2.5 (1–3) 2.0 (1–2) 3.0 (3–4) | n/a 0.014 * 0.015 * 0.008 * 0.046 * |
| Tympanic membrane | RP LD1 LD2 LD3 CBCT | 4.0 (1–5) 3.0 (1–5) 2.5 (1–4) 2.0 (1–3) 3.0 (1–4) | n/a 0.157 0.020 * 0.014 * 0.206 | 4.0 (1–5) 3.0 (1–5) 2.5 (1–4) 2.0 (1–3) 3.0 (1–4) | n/a 0.157 0.020 * 0.014 * 0.206 | 4.0 (1–5) 3.0 (1–5) 2.5 (1–4) 2.0 (1–3) 3.0 (1–4) | n/a 0.157 0.020 * 0.014 * 0.206 |
| Lamina spiralis ossea | RP LD1 LD2 LD3 CBCT | 2.0 (2–2) 2.0 (2–2) 2.0 (1–2) 1.5 (1–2) 2.0 (2–2) | n/a 1.000 0.157 0.046 * 1.000 | 2.0 (2–2) 2.0 (2–2) 2.0 (1–2) 1.5 (1–2) 2.0 (2–2) | n/a 1.000 0.157 0.046 * 1.000 | 2.0 (2–2) 2.0 (2–2) 2.0 (1–2) 1.5 (1–2) 2.0 (2–2) | n/a 1.000 0.157 0.046 * 1.000 |
| Chorda tympani | RP LD1 LD2 LD3 CBCT | 3.0 (1–4) 3.0 (1–3) 2.0 (1–2) 1.0 (1–2) 3.0 (1–3) | n/a 0.083 0.024 * 0.026 * 1.000 | 3.0 (1–4) 3.0 (1–3) 2.0 (1–2) 1.0 (1–2) 3.0 (1–3) | n/a 0.083 0.024 * 0.026 * 1.000 | 3.0 (1–4) 3.0 (1–3) 2.0 (1–2) 1.0 (1–2) 3.0 (1–3) | n/a 0.083 0.024 * 0.026 * 1.000 |
| Modiolus | RP LD1 LD2 LD3 CBCT | 4.0 (3–5) 3.5 (3–5) 3.0 (2–4) 3.0 (2–3) 3.0 (3–4) | n/a 0.317 0.025 * 0.014 * 0.083 | 4.0 (3–5) 3.5 (3–5) 3.0 (2–4) 3.0 (2–3) 3.0 (3–4) | n/a 0.317 0.025 * 0.014 * 0.046 * | 4.0 (3–5) 3.5 (3–5) 3.0 (2–4) 3.0 (2–3) 3.0 (3–4) | n/a 0.317 0.025 * 0.014 * 0.046 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kofler, B.; Jenetten, L.; Runge, A.; Degenhart, G.; Fischer, N.; Hörmann, R.; Steurer, M.; Widmann, G. ALADA Dose Optimization in the Computed Tomography of the Temporal Bone: The Diagnostic Potential of Different Low-Dose CT Protocols. Diagnostics 2021, 11, 1894. https://doi.org/10.3390/diagnostics11101894
Kofler B, Jenetten L, Runge A, Degenhart G, Fischer N, Hörmann R, Steurer M, Widmann G. ALADA Dose Optimization in the Computed Tomography of the Temporal Bone: The Diagnostic Potential of Different Low-Dose CT Protocols. Diagnostics. 2021; 11(10):1894. https://doi.org/10.3390/diagnostics11101894
Chicago/Turabian StyleKofler, Barbara, Laura Jenetten, Annette Runge, Gerald Degenhart, Natalie Fischer, Romed Hörmann, Michael Steurer, and Gerlig Widmann. 2021. "ALADA Dose Optimization in the Computed Tomography of the Temporal Bone: The Diagnostic Potential of Different Low-Dose CT Protocols" Diagnostics 11, no. 10: 1894. https://doi.org/10.3390/diagnostics11101894







