From Zygote to Blastocyst: Application of Ultrashort Lasers in the Field of Assisted Reproduction and Developmental Biology
Abstract
:1. Introduction
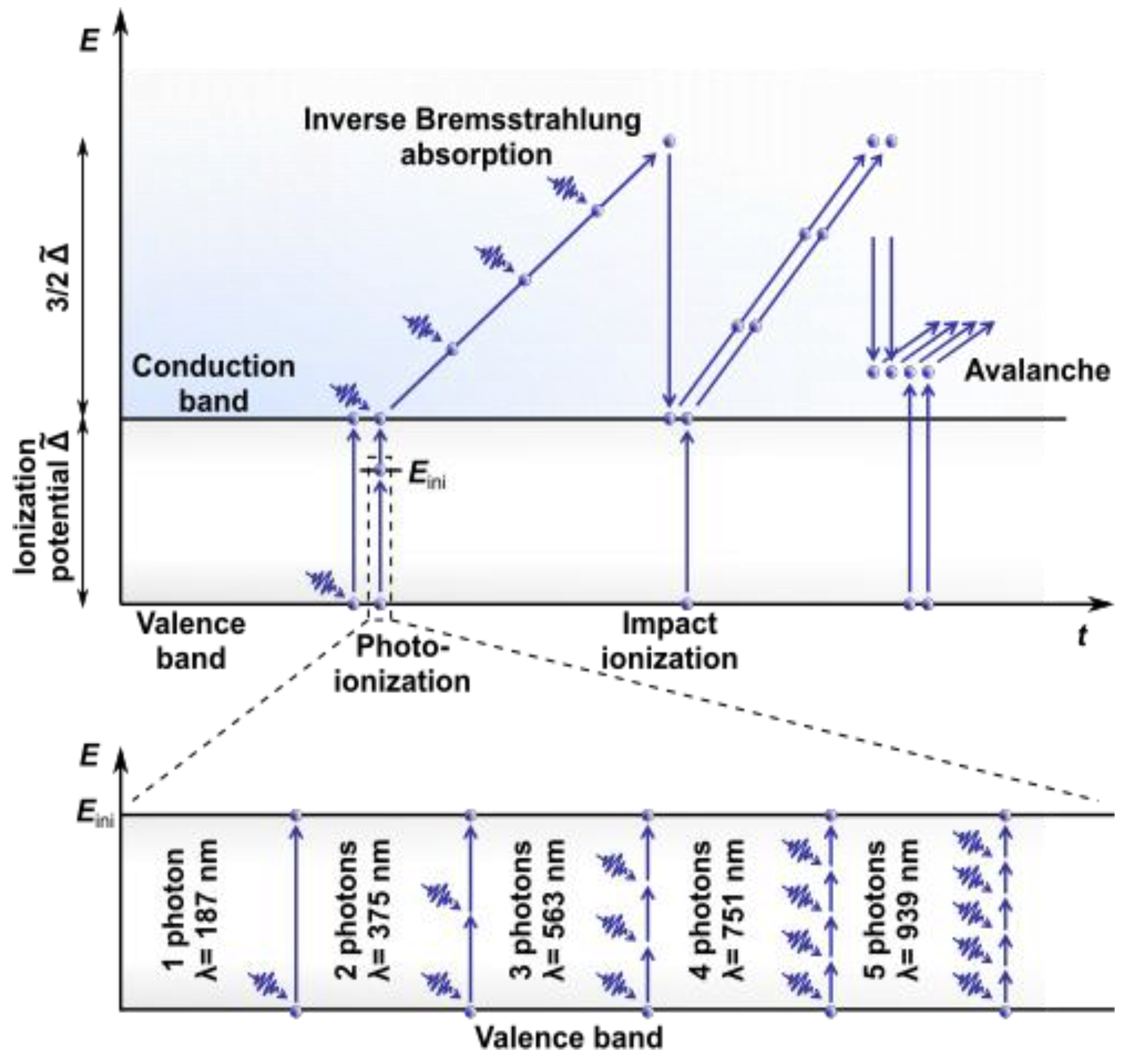
2. Interaction of Femtosecond Laser Pulses with Matter
2.1. Nonlinear Absorption
2.2. Effects Induced
2.3. Advantages of Nonlinear Absorption
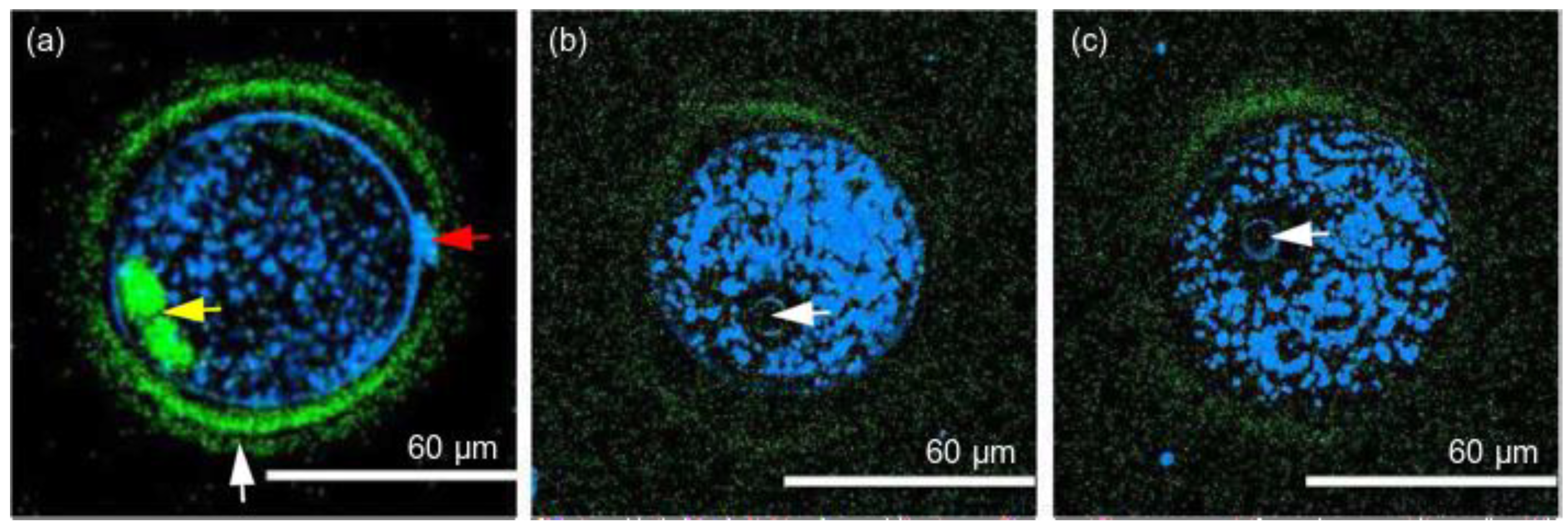
3. Application of Ultrashort Laser Pulses for Oocyte/Blastomere Enucleation and Embryonic Cell Fusion
4. Ultrashort Laser Microsurgery of Preimplantation Embryos for Application in ART
5. Application of Ultrashort Laser Pulses for Nonlinear Microscopy
5.1. TPEF Microscopy
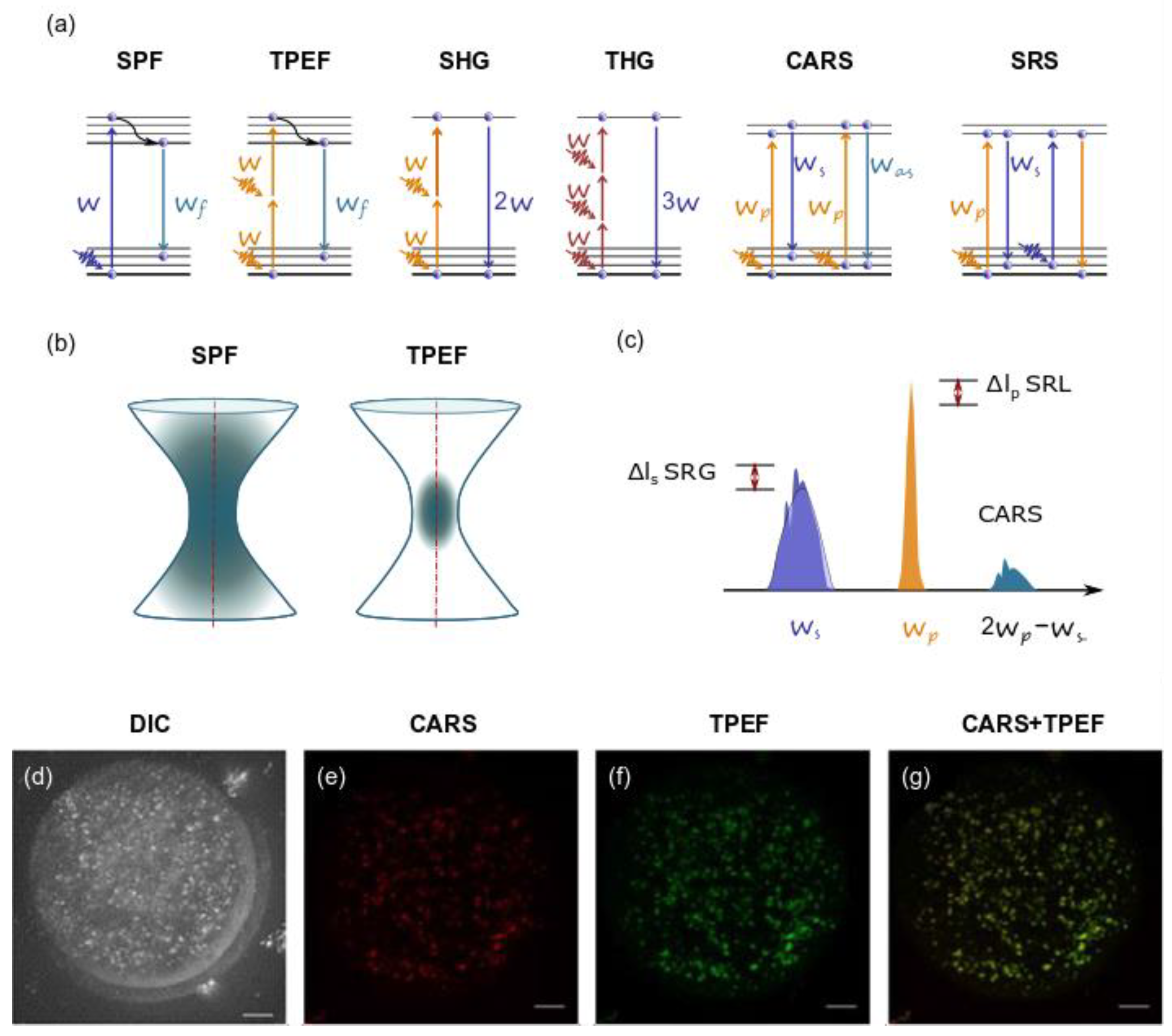
5.2. SHG
5.3. THG
5.4. CRS Microscopy
5.5. Studies
6. Ultrashort Laser Microsurgery of Embryos
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Palanker, D.; Ohad, S.; Lewis, A.; Simon, A.; Shenkar, J.; Penchas, S.; Laufer, N. Technique for cellular microsurgery using the 193-nm excimer laser. Lasers Surg. Med. 1991, 11, 580–586. [Google Scholar] [CrossRef]
- Tadir, Y.; Wright, W.H.; Vafa, O.; Liaw, L.H.; Asch, R.; Berns, M.W. Micromanipulation of gametes using laser microbeams. Hum. Reprod. 1991, 6, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Rink, K.; Delacrétaz, G.; Salathé, R.P.; Senn, A.; Nocera, D.; Germond, M.; Grandi, P.; De Fakan, S. Non-contact microdrilling of mouse zona pellucida with an objective-delivered 1.48-μm diode laser. Lasers Surg. Med. 1996, 18, 52–62. [Google Scholar] [CrossRef]
- Rienzi, L.; Greco, E.; Ubaldi, F.; Iacobelli, M.; Martinez, F.; Tesarik, J. Laser-assisted intracytoplasmic sperm injection. Fertil. Steril. 2001, 76, 1045–1047. [Google Scholar] [CrossRef]
- Choi, K.H.; Lee, J.H.; Yang, Y.H.; Yoon, T.K.; Lee, D.R.; Lee, W.S. Efficiency of laser-assisted intracytoplasmic sperm injection in a human assisted reproductive techniques program. Clin. Exp. Reprod. Med. 2011, 38, 148. [Google Scholar] [CrossRef]
- McArthur, S.J.; Leigh, D.; Marshall, J.T.; de Boer, K.A.; Jansen, R.P.S. Pregnancies and live births after trophectoderm biopsy and preimplantation genetic testing of human blastocysts. Fertil. Steril. 2005, 84, 1628–1636. [Google Scholar] [CrossRef]
- Bosshard, C.; Bösch, M.; Liakatas, I.; Jäger, M.; Günter, P. Nonlinear Optical Effects and Materials; Springer Series in Optical Sciences; Günter, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; Volume 72, ISBN 978-3-642-53694-6. [Google Scholar]
- Phillips, K.C.; Gandhi, H.H.; Mazur, E.; Sundaram, S.K. Ultrafast laser processing of materials: A review. Adv. Opt. Photonics 2015, 7, 684. [Google Scholar] [CrossRef]
- Docchio, F.; Sacchi, C.; Marshall, J. Experimental investigation of optical breakdown thresholds in ocular media under single pulse irradiation with different pulse durations. Lasers Ophthalmol. 1986, 1, 83–93. [Google Scholar]
- Tadir, Y.; Douglas-Hamilton, D.H.; Douglas-Hamilton, D.H.; Douglas-Hamilton, D.H.; Douglas-Hamilton, D.H. Laser effects in the manipulation of human eggs and embryos for in vitro fertilization. Methods Cell Biol. 2007, 82, 409–431. [Google Scholar] [CrossRef]
- Williams, F.; Varma, S.P.; Hillenius, S. Liquid water as a lone-pair amorphous semiconductor. J. Chem. Phys. 1976, 64, 1549–1554. [Google Scholar] [CrossRef]
- Sacchi, C.A. Laser-induced electric breakdown in water. J. Opt. Soc. Am. B 1991, 8, 337. [Google Scholar] [CrossRef]
- Sander, M.U.; Gudiksen, M.S.; Luther, K.; Troe, J. Liquid water ionization: Mechanistic implications of the H/D isotope effect in the geminate recombination of hydrated electrons. Chem. Phys. 2000, 258, 257–265. [Google Scholar] [CrossRef]
- Elles, C.G.; Jailaubekov, A.E.; Crowell, R.A.; Bradforth, S.E. Excitation-energy dependence of the mechanism for two-photon ionization of liquid H2O and D2O from 8.3 to 12.4 eV. J. Chem. Phys. 2006, 125, 044515. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.-X.; Zhang, Z.; Vogel, A. Multi-rate-equation modeling of the energy spectrum of laser-induced conduction band electrons in water. Opt. Express 2019, 27, 4672. [Google Scholar] [CrossRef]
- Göppert-Mayer, M. Über elementarakte mit zwei quantensprüngen. Ann. Phys. 1931, 401, 273–294. [Google Scholar] [CrossRef]
- Keldysh, L.V. Ionization in the field of a strong electromagnetic wave. Sov. Phys. JETP 1965, 20, 1307–1314. [Google Scholar]
- Linz, N.; Freidank, S.; Liang, X.X.; Vogel, A. Wavelength dependence of femtosecond laser-induced breakdown in water and implications for laser surgery. Phys. Rev. B 2016, 94, 024113. [Google Scholar] [CrossRef] [Green Version]
- Vogel, A.; Noack, J.; Hüttman, G.; Paltauf, G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl. Phys. B 2005, 81, 1015–1047. [Google Scholar] [CrossRef]
- Berns, M.W.; Cheng, W.K.; Floyd, A.D.; Ohnuki, Y. Chromosome lesions produced with an argon laser microbeam without dye sensitization. Science 1971, 171, 903–905. [Google Scholar] [CrossRef] [Green Version]
- König, K.; Riemann, I.; Fischer, P.; Halbhuber, K.J. Intracellular nanosurgery with near infrared femtosecond laser pulses. Cell Mol. Biol. 1999, 45, 195–201. [Google Scholar]
- König, K.; Riemann, I.; Fritzsche, W. Nanodissection of human chromosomes with near-infrared femtosecond laser pulses. Opt. Lett. 2001, 26, 819. [Google Scholar] [CrossRef]
- Vogel, A.; Venugopalan, V. Mechanisms of pulsed laser ablation of biological tissues. Chem. Rev. 2003, 103, 577–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrett, B.C.; Dixon, D.A.; Camaioni, D.M.; Chipman, D.M.; Johnson, M.A.; Jonah, C.D.; Kimmel, G.A.; Miller, J.H.; Rescigno, T.N.; Rossky, P.J.; et al. Role of water in electron-initiated processes and radical chemistry: Issues and scientific advances. Chem. Rev. 2005, 105, 355–390. [Google Scholar] [CrossRef]
- Boudaiffa, B. Resonant formation of DNA strand breaks by low-energy (3 to 20eV) electrons. Science 2000, 287, 1658–1660. [Google Scholar] [CrossRef]
- Quinto-Su, P.A.; Venugopalan, V. Mechanisms of laser cellular microsurgery. Methods Cell Biol. 2007, 82, 111–151. [Google Scholar]
- Paltauf, G.; Dyer, P.E. Photomechanical processes and effects in ablation. Chem. Rev. 2003, 103, 487–518. [Google Scholar] [CrossRef]
- Grill, S.; Stelzer, E.H.K. Method to calculate lateral and axial gain factors of optical setups with a large solid angle. J. Opt. Soc. Am. A 1999, 16, 2658. [Google Scholar] [CrossRef]
- Gouveia, C.; Huyser, C.; Egli, D.; Pepper, M.S. Lessons learned from somatic cell nuclear transfer. Int. J. Mol. Sci. 2020, 21, 2314. [Google Scholar] [CrossRef]
- Karmenyan, A.V.; Shakhbazyan, A.K.; Sviridova-Chailakhyan, T.A.; Krivokharchenko, A.S.; Chiou, A.E.; Chailakhyan, L.M. Use of picosecond infrared laser for micromanipulation of early mammalian embryos. Mol. Reprod. Dev. 2009, 76, 975–983. [Google Scholar] [CrossRef]
- Kuetemeyer, K.; Lucas-Hahn, A.; Petersen, B.; Lemme, E.; Hassel, P.; Niemann, H.; Heisterkamp, A. Combined multiphoton imaging and automated functional enucleation of porcine oocytes using femtosecond laser pulses. J. Biomed. Opt. 2010, 15, 046006. [Google Scholar] [CrossRef]
- Ilina, I.V.; Chefonov, O.V.; Agranat, M.B.; Khramova, Y.V.; Ovchinnikov, A.V.; Sitnikov, D.S.; Semenova, M.L.; Chefonov, O.V.; Agranat, M.B.; Yu, V.; et al. Microsurgery of cell membrane with femtosecond laser pulses for cell fusion and optical injection. In Proceedings of the ALT Proceedings, Lyon, France, 29–31 October 2012; Volume 1. [Google Scholar]
- Ilina, I.V.; Ovchinnikov, A.V.; Sitnikov, D.S.; Rakityanskiy, M.M.; Agranat, M.B.; Khramova, Y.V.; Semenova, M.L. Application of femtosecond laser pulses in biomedical cell technologies. High Temp. 2013, 51, 173–178. [Google Scholar] [CrossRef]
- Kuetemeyer, K.; Lucas-Hahn, A.; Petersen, B.; Niemann, H.; Heisterkamp, A. Femtosecond laser-induced fusion of nonadherent cells and two-cell porcine embryos. J. Biomed. Opt. 2011, 16, 088001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krivokharchenko, A.; Karmenyan, A.; Sarkisov, O.; Bader, M.; Chiou, A.; Shakhbazyan, A. Laser fusion of mouse embryonic cells and intra-embryonic fusion of blastomeres without affecting the embryo integrity. PLoS ONE 2012, 7, e50029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osychenko, A.; Zalessky, A.; Astafiev, A.; Shakhov, A.; Kostrov, A.; Krivokharchenko, A.; Nadtochenko, V. Femtosecond laser-induced blastomere fusion results in embryo tetraploidy by common metaphase plate formation. Exp. Cell Res. 2020, 389, 111887. [Google Scholar] [CrossRef] [PubMed]
- Schöpper, B.; Ludwig, M.; Edenfeld, J.; Al-Hasani, S.; Diedrich, K. Possible applications of lasers in assisted reproductive technologies. Hum. Reprod. 1999, 14, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Montag, M.H.M.; Klose, R.; Köster, M.; Rösing, B.; van der Ven, K.; Rink, K.; van der Ven, H. Application of non-contact laser technology in assisted reproduction. Med. Laser Appl. 2009, 24, 57–64. [Google Scholar] [CrossRef]
- Davidson, L.M.; Liu, Y.; Griffiths, T.; Jones, C.; Coward, K. Laser technology in the ART laboratory: A narrative review. Reprod. Biomed. Online 2019, 38, 725–739. [Google Scholar] [CrossRef] [Green Version]
- Rink, K.; Delacretaz, G.P.; Salathe, R.-P.; Senn, A.; Nocera, D.; Germond, M.; Fakan, S. 1.48-um diode laser microdissection of the zona pellucida of mouse zygotes. In Laser-Tissue Interaction V; and Ultraviolet Radiation Hazards, Proceedings of the OE/LASE’94, Los Angeles, CA, USA, 17 August 1994; Jacques, S.L., Sliney, D.H., Belkin, M., Eds.; SPIE: Bellingham, WA, USA, 1994; Volume 2134, pp. 412–422. [Google Scholar]
- Germond, M.; Nocera, D.; Senn, A.; Rink, K.; Delacrétaz, G.; Fakan, S. Microdissection of mouse and human zona pellucida using a 1.48-microns diode laser beam: Efficacy and safety of the procedure. Fertil. Steril. 1995, 64, 604–611. [Google Scholar] [CrossRef]
- Kaneko, T.; Yanagi, M.; Nakashima, T.; Nakagata, N. The improvement in fertilizing ability of cryopreserved mouse spermatozoa using laser-microdissected oocytes. Reprod. Med. Biol. 2006, 5, 249–253. [Google Scholar] [CrossRef] [Green Version]
- Li, M.-W.; Kinchen, K.L.; Vallelunga, J.M.; Young, D.L.; Wright, K.D.K.; Gorano, L.N.; Wasson, K.; Lloyd, K.C.K. Safety, efficacy and efficiency of laser-assisted IVF in subfertile mutant mouse strains. Reproduction 2013, 145, 245–254. [Google Scholar] [CrossRef]
- Woods, S.E.; Qi, P.; Rosalia, E.; Chavarria, T.; Discua, A.; Mkandawire, J.; Fox, J.G.; García, A. Laser-assisted in vitro fertilization facilitates fertilization of vitrified-warmed C57BL/6 mouse oocytes with fresh and frozen-thawed spermatozoa, producing live pups. PLoS ONE 2014, 9, e91892. [Google Scholar] [CrossRef] [Green Version]
- Abdelmassih, S.; Cardoso, J.; Abdelmassih, V.; Dias, J.A.; Abdelmassih, R.; Nagy, Z.P. Laser-assisted ICSI: A novel approach to obtain higher oocyte survival and embryo quality rates. Hum. Reprod. 2002, 17, 2694–2699. [Google Scholar] [CrossRef]
- Verza, S., Jr.; Schneider, D.T.; Siqueira, S.; Esteves, S. Laser-assisted intracytoplasmic sperm injection (LA-ICSI) is safe and less traumatic than conventional ICSI. Fertil. Steril. 2013, 100, S236. [Google Scholar] [CrossRef]
- Montag, M.; van der Ven, K.; Delacrétaz, G.; Rink, K.; van der Ven, H. Laser-assisted microdissection of the zona Pellucida facilitates polar body biopsy. Fertil. Steril. 1998, 69, 539–542. [Google Scholar] [CrossRef]
- Boada, M.; Carrera, M.; De La Iglesia, C.; Sandalinas, M.; Barri, P.N.; Veiga, A. Successful use of a laser for human embryo biopsy in preimplantation genetic diagnosis: Report of two cases. J. Assist. Reprod. Genet. 1998, 15, 302–307. [Google Scholar] [CrossRef]
- Alteri, A.; Viganò, P.; Maizar, A.A.; Jovine, L.; Giacomini, E.; Rubino, P. Revisiting embryo assisted hatching approaches: A systematic review of the current protocols. J. Assist. Reprod. Genet 2018, 35, 367–391. [Google Scholar] [CrossRef]
- Wan, C.Y.; Song, C.; Diao, L.H.; Li, G.G.; Bao, Z.J.; Hu, X.D.; Zhang, H.Z.; Zeng, Y. Laser-assisted hatching improves clinical outcomes of vitrified-warmed blastocysts developed from low-grade cleavage-stage embryos: A prospective randomized study. Reprod. Biomed. Online 2014, 28, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Miyata, H.; Matsubayashi, H.; Fukutomi, N.; Matsuba, J.; Koizumi, A.; Tomiyama, T. Relevance of the site of assisted hatching in thawed human blastocysts: A preliminary report. Fertil. Steril. 2010, 94, 2444–2447. [Google Scholar] [CrossRef]
- Debrock, S.; Peeraer, K.; Spiessens, C.; Willemen, D.; De Loecker, P.; Dhooghe, T.M. The effect of modified quarter laser-assisted zona thinning on the implantation rate per embryo in frozen/vitrified-thawed/warmed embryo transfer cycles: A prospective randomized controlled trial. Hum. Reprod. 2011, 26, 1997–2007. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Q.; Chen, W.; Zhu, G.; Zhang, H. Effect of the site of assisted hatching on vitrified-warmed blastocyst transfer cycles: A prospective randomized study. J. Assist. Reprod. Genet. 2013, 30, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Ryu, M.J.; Kim, H.S.; Lee, J.H.; Kim, M.H.; Jeong, H.J.; Chung, M.K. Assisted hatching close to inner cell mass (ICM) increases clinical outcomes of frozen-thawed blastocyst transfer cycles in unexplained or refractory repeated implantation failure patients. Fertil. Steril. 2014, 102, e324. [Google Scholar] [CrossRef]
- Hartshorn, C.; Anshelevich, A.; Wangh, L.J. Laser zona drilling does not induce hsp70i transcription in blastomeres of eight-cell mouse embryos. Fertil. Steril. 2005, 84, 1547–1550. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.H.; Gilchrist, J.W.; Hallowell, S.V.; Hanshew, K.K.; Orris, J.J.; Glassner, M.J.; Wininger, J.D. The effects of different laser pulse lengths on the embryo biopsy procedure and embryo development to the blastocyst stage. J. Assist. Reprod. Genet. 2010, 27, 663–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malter, H.E.; Schimmel, T.; Cohen, J. Zona dissection by infrared laser: Developmental consequences in the mouse, technical considerations, and controlled clinical trial. Reprod. Biomed. Online 2001, 3, 117–123. [Google Scholar] [CrossRef]
- Douglas-Hamilton, D.H.; Conia, J. Thermal effects in laser-assisted pre-embryo zona drilling. J. Biomed. Opt. 2001, 6, 205. [Google Scholar] [CrossRef]
- Tucker, M.J.; Ball, G.D. Assisted hatching as a technique for use in human in vitro fertilization and embryo transfer is long overdue for careful and appropriate study. J. Clin. Embryol. 2009, 12, 9–14. [Google Scholar]
- Chatzimeletiou, K.; Morrison, E.E.; Panagiotidis, Y.; Prapas, N.; Prapas, Y.; Rutherford, A.J.; Grudzinskas, G.; Handyside, A.H. Comparison of effects of zona drilling by non-contact infrared laser or acid Tyrode′s on the development of human biopsied embryos as revealed by blastomere viability, cytoskeletal analysis and molecular cytogenetics. Reprod. Biomed. Online 2005, 11, 697–710. [Google Scholar] [CrossRef]
- Il′ina, I.V.; Sitnikov, D.S.; Ovchinnikov, A.V.; Agranat, M.B.; Khramova, Y.V.; Semenova, M.L. Noncontact microsurgery and micromanipulation of living cells with combined system femtosecond laser scalpel-optical tweezers. In Biophotonics: Photonic Solutions for Better Health Care III, Proceedings of the SPIE Photonics Europe, Brussels, Belgium, 8 May 2012; Popp, J., Drexler, W., Tuchin, V.V., Matthews, D.L., Eds.; SPIE: Bellingham, WA, USA, 2012; Volume 8427, p. 84270S. [Google Scholar]
- Ilina, I.V.; Khramova, Y.V.; Filatov, M.A.; Semenova, M.L.; Sitnikov, D.S. Application of femtosecond laser scalpel and optical tweezers for noncontact biopsy of late preimplantation embryos. High Temp. 2015, 53, 804–809. [Google Scholar] [CrossRef]
- Sitnikov, D.S.; Ovchinnikov, A.V.; Ilina, I.V.; Chefonov, O.V.; Agranat, M.B. Laser microsurgery of cells by femtosecond laser scalpel and optical tweezers. High Temp. 2014, 52, 803–808. [Google Scholar] [CrossRef]
- Ilina, I.V.; Khramova, Y.V.; Filatov, M.A.; Sitnikov, D.S. Femtosecond laser is effective tool for zona pellucida engraving and tagging of preimplantation mammalian embryos. J. Assist. Reprod. Genet. 2019, 36, 1251–1261. [Google Scholar] [CrossRef]
- Ilina, I.V.; Khramova, Y.V.; Filatov, M.A.; Sitnikov, D.S. Application of femtosecond laser microsurgery in assisted reproductive technologies for preimplantation embryo tagging. Biomed. Opt. Express 2019, 10, 2985–2995. [Google Scholar] [CrossRef]
- Ilina, I.V.; Khramova, Y.V.; Ivanova, A.D.; Filatov, M.A.; Silaeva, Y.Y.; Deykin, A.V.; Sitnikov, D.S. Controlled hatching at the prescribed site using femtosecond laser for zona pellucida drilling at the early blastocyst stage. J. Assist. Reprod. Genet. 2021, 38, 517–529. [Google Scholar] [CrossRef]
- Spriggs, M. IVF mixup: White couple have black babies. J. Med. Ethics 2003, 29, 65. [Google Scholar] [CrossRef] [Green Version]
- Bender, L. To err is human′. ART mix-ups: A labor-based, relational. proposal. J. Race Gend. Justice 2006, 9, 443–508. [Google Scholar]
- Chailert, C.; Sanmee, U.; Piromlertamorn, W.; Samchimchom, S.; Vutyavanich, T. Effects of partial or complete laser-assisted hatching on the hatching of mouse blastocysts and their cell numbers. Reprod. Biol. Endocrinol. 2013, 11, 21. [Google Scholar] [CrossRef] [Green Version]
- Montag, M.; Koll, B.; Holmes, P.; van der Ven, H. Significance of the number of embryonic cells and the state of the zona pellucida for hatching of mouse blastocysts in vitro versus in vivo. Biol. Reprod. 2000, 62, 1738–1744. [Google Scholar] [CrossRef]
- Ebner, T.; Gruber, I.; Moser, M. Location of herniation predicts implantation behaviour of hatching blastocysts. J. Turk. Ger. Gynecol. Assoc. 2007, 8, 184–189. [Google Scholar]
- Webb, R.H. Handbook of Biological Confocal Microscopy; Pawley, J.B., Ed.; Springer: Boston, MA, USA, 1995; ISBN 978-1-4757-5350-9. [Google Scholar]
- Daniel, J.C. Cleavage of mammalian ova inhibited by visible light. Nature 1964, 201, 316–317. [Google Scholar] [CrossRef]
- Hirao, Y.; Yanagimachi, R. Detrimental effect of visible light on meiosis of mammalian eggs in vitro. J. Exp. Zool. 1978, 206, 365–369. [Google Scholar] [CrossRef]
- Hegele-Hartung, C.; Schumacher, A.; Fischer, B. Effects of visible light and room temperature on the ultrastructure of preimplantation rabbit embryos: A time course study. Anat. Embryol. 1991, 183, 559–571. [Google Scholar] [CrossRef]
- Squirrell, J.M.; Wokosin, D.L.; White, J.G.; Bavister, B.D. Long-term two-photon fluorescence imaging of mammalian embryos without compromising viability. Nat. Biotechnol. 1999, 17, 763–767. [Google Scholar] [CrossRef]
- Parodi, V.; Jacchetti, E.; Osellame, R.; Cerullo, G.; Polli, D.; Raimondi, M.T. Nonlinear optical microscopy: From fundamentals to applications in live bioimaging. Front. Bioeng. Biotechnol. 2020, 8, 1174. [Google Scholar] [CrossRef]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef]
- Hoover, E.E.; Squier, J.A. Advances in multiphoton microscopy technology. Nat. Photonics 2013, 7, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Denk, W.; Strickler, J.; Webb, W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Barad, Y.; Eisenberg, H.; Horowitz, M.; Silberberg, Y. Nonlinear scanning laser microscopy by third harmonic generation. Appl. Phys. Lett. 1997, 70, 922–924. [Google Scholar] [CrossRef] [Green Version]
- Campagnola, P.J.; Clark, H.A.; Mohler, W.A.; Lewis, A.; Loew, L.M. Second-harmonic imaging microscopy of living cells. J. Biomed. Opt. 2001, 6, 277. [Google Scholar] [CrossRef] [Green Version]
- Evans, C.L.; Xie, X.S. Coherent anti-stokes raman scattering microscopy: Chemical imaging for biology and medicine. Annu. Rev. Anal. Chem. 2008, 1, 883–909. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Zipfel, W.; Shear, J.B.; Williams, R.M.; Webb, W.W. Multiphoton fluorescence excitation: New spectral windows for biological nonlinear microscopy. Proc. Natl. Acad. Sci. USA 1996, 93, 10763–10768. [Google Scholar] [CrossRef] [Green Version]
- Drobizhev, M.; Makarov, N.S.; Tillo, S.E.; Hughes, T.E.; Rebane, A. Two-photon absorption properties of fluorescent proteins. Nat. Methods 2011, 8, 393–399. [Google Scholar] [CrossRef]
- Bradley, J.; Pope, I.; Masia, F.; Sanusi, R.; Langbein, W.; Swann, K.; Borri, P. Quantitative imaging of lipids in live mouse oocytes and early embryos using CARS microscopy. Development 2016, 143, 2238–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campagnola, P. Second harmonic generation imaging microscopy: Applications to diseases diagnostics. Anal. Chem. 2011, 83, 3224–3231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mertz, J.; Moreaux, L. Second-harmonic generation by focused excitation of inhomogeneously distributed scatterers. Opt. Commun. 2001, 196, 325–330. [Google Scholar] [CrossRef]
- Yelin, D.; Silberberg, Y. Laser scanning third-harmonic-generation microscopy in biology. Opt. Express 1999, 5, 169. [Google Scholar] [CrossRef] [PubMed]
- Rehberg, M.; Krombach, F.; Pohl, U.; Dietzel, S. Label-free 3D visualization of cellular and tissue structures in intact muscle with second and third harmonic generation microscopy. PLoS ONE 2011, 6, e28237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supatto, W.; Truong, T.V.; Débarre, D.; Beaurepaire, E. Advances in multiphoton microscopy for imaging embryos. Curr. Opin. Genet. Dev. 2011, 21, 538–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, C.-S.; Chen, S.-U.; Lee, Y.-W.; Yang, Y.-S.; Sun, C.-K. Higher harmonic generation microscopy of in vitro cultured mammal oocytes and embryos. Opt. Express 2008, 16, 11574. [Google Scholar] [CrossRef]
- Débarre, D.; Supatto, W.; Pena, A.-M.; Fabre, A.; Tordjmann, T.; Combettes, L.; Schanne-Klein, M.-C.; Beaurepaire, E. Imaging lipid bodies in cells and tissues using third-harmonic generation microscopy. Nat. Methods 2006, 3, 47–53. [Google Scholar] [CrossRef]
- Watanabe, T.; Thayil, A.; Jesacher, A.; Grieve, K.; Debarre, D.; Wilson, T.; Booth, M.; Srinivas, S. Characterisation of the dynamic behaviour of lipid droplets in the early mouse embryo using adaptive harmonic generation microscopy. BMC Cell Biol. 2010, 11, 38. [Google Scholar] [CrossRef] [Green Version]
- Meshulach, D.; Barad, Y.; Silberberg, Y. Measurement of ultrashort optical pulses by third-harmonic generation. J. Opt. Soc. Am. B 1997, 14, 2122. [Google Scholar] [CrossRef]
- Müller, M.; Squier, K.R.W.; Brakenhoff, G.J. 3D microscopy of transparent objects using third-harmonic generation. J. Microsc. 1998, 191, 266–274. [Google Scholar] [CrossRef]
- Tserevelakis, G.J.; Filippidis, G.; Megalou, E.V.; Fotakis, C.; Tavernarakis, N. Cell tracking in live Caenorhabditis elegans embryos via third harmonic generation imaging microscopy measurements. J. Biomed. Opt. 2011, 16, 046019. [Google Scholar] [CrossRef] [Green Version]
- Pomeroy, K.O.; Reed, M.L. The effect of light on embryos and embryo culture. J. Reprod. Stem Cell Biotechnol. 2012, 3, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Nandakumar, P.; Kovalev, A.; Volkmer, A. Vibrational imaging based on stimulated Raman scattering microscopy. New J. Phys. 2009, 11, 033026. [Google Scholar] [CrossRef]
- Cheng, J.-X.; Xie, X.S. Coherent anti-stokes Raman scattering microscopy: Instrumentation, theory, and applications. J. Phys. Chem. B 2004, 108, 827–840. [Google Scholar] [CrossRef]
- Thayil, A.; Watanabe, T.; Jesacher, A.; Wilson, T.; Srinivas, S.; Booth, M. Long-term imaging of mouse embryos using adaptive harmonic generation microscopy. J. Biomed. Opt. 2011, 16, 046018. [Google Scholar] [CrossRef] [Green Version]
- Débarre, D.; Supatto, W.; Farge, E.; Moulia, B.; Schanne-Klein, M.-C.; Beaurepaire, E. Velocimetric third-harmonic generation microscopy: Micrometer-scale quantification of morphogenetic movements in unstained embryos. Opt. Lett. 2004, 29, 2881. [Google Scholar] [CrossRef] [Green Version]
- Chu, S.-W.; Chen, S.-Y.; Tsai, T.-H.; Liu, T.-M.; Lin, C.-Y.; Tsai, H.-J.; Sun, C.-K. In vivo developmental biology study using noninvasive multi-harmonic generation microscopy. Opt. Express 2003, 11, 3093. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, T.; Venturas, M.; Aghvami, S.A.; Yang, X.; Fraden, S.; Sakkas, D.; Needleman, D.J. Combined noninvasive metabolic and spindle imaging as potential tools for embryo and oocyte assessment. Hum. Reprod. 2019, 34, 2349–2361. [Google Scholar] [CrossRef]
- Kyvelidou, C.; Tserevelakis, G.J.; Filippidis, G.; Ranella, A.; Kleovoulou, A.; Fotakis, C.; Athanassakis, I. Following the course of pre-implantation embryo patterning by non-linear microscopy. J. Struct. Biol. 2011, 176, 379–386. [Google Scholar] [CrossRef]
- Ma, N.; de Mochel, N.R.; Pham, P.D.; Yoo, T.Y.; Cho, K.W.Y.Y.; Digman, M.A. Label-free assessment of pre-implantation embryo quality by the Fluorescence Lifetime Imaging Microscopy (FLIM)-phasor approach. Sci. Rep. 2019, 9, 13206. [Google Scholar] [CrossRef] [Green Version]
- Jasensky, J.; Boughton, A.P.; Khmaladze, A.; Ding, J.; Zhang, C.; Swain, J.E.; Smith, G.W.; Chen, Z.; Smith, G.D. Live-cell quantification and comparison of mammalian oocyte cytosolic lipid content between species, during development, and in relation to body composition using nonlinear vibrational microscopy. Analyst 2016, 141, 4694–4706. [Google Scholar] [CrossRef]
- Borri, P.; Bradley, J.; Pope, I.; Langbein, W.; Swann, K. Imaging lipids in living mammalian oocytes and early embryos by coherent Raman scattering microscopy. In Label-Free Biomedical Imaging and Sensing (LBIS), Proceedings of the SPIE BiOS, San Francisco, CA, USA, 4 March 2019; Shaked, N.T., Hayden, O., Eds.; SPIE: Bellingham, WA, USA, 2019; Volume 10890, p. 1089004. [Google Scholar]
- Korkmaz, C.; Tekin, Y.B.; Sakinci, M.; Ercan, C.M. Effects of maternal ageing on ICSI outcomes and embryo development in relation to oocytes morphological characteristics of birefringent structures. Zygote 2015, 23, 550–555. [Google Scholar] [CrossRef]
- García-Oro, S.; Rey, M.I.; Rodríguez, M.; Durán, Á.; Devesa, R.; Valverde, D. Predictive value of spindle retardance in embryo implantation rate. J. Assist. Reprod. Genet. 2017, 34, 617–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomari, H.; Honjo, K.; Kunitake, K.; Aramaki, N.; Kuhara, S.; Hidaka, N.; Nishimura, K.; Nagata, Y.; Horiuchi, T. Meiotic spindle size is a strong indicator of human oocyte quality. Reprod. Med. Biol. 2018, 17, 268–274. [Google Scholar] [CrossRef]
- Hara, K.; Abe, Y.; Kumada, N.; Aono, N.; Kobayashi, J.; Matsumoto, H.; Sasada, H.; Sato, E. Extrusion and removal of lipid from the cytoplasm of porcine oocytes at the germinal vesicle stage: Centrifugation under hypertonic conditions influences vitrification. Cryobiology 2005, 50, 216–222. [Google Scholar] [CrossRef]
- Supatto, W.; Débarre, D.; Farge, E.; Beaurepaire, E. Femtosecond pulse-induced microprocessing of live Drosophila embryos. Med. Laser Appl. 2005, 20, 207–216. [Google Scholar] [CrossRef]
- Engelbrecht, C.J.; Greger, K.; Reynaud, E.G.; Kržic, U.; Colombelli, J.; Stelzer, E.H.K. Three-dimensional laser microsurgery in light-sheet based microscopy (SPIM). Opt. Express 2007, 15, 6420. [Google Scholar] [CrossRef]
- Rauzi, M.; Krzic, U.; Saunders, T.E.; Krajnc, M.; Ziherl, P.; Hufnagel, L.; Leptin, M. Embryo-scale tissue mechanics during Drosophila gastrulation movements. Nat. Commun. 2015, 6, 8677. [Google Scholar] [CrossRef] [Green Version]
- Galbraith, J.A.; Terasaki, M. Controlled damage in thick specimens by multiphoton excitation. Mol. Biol. Cell 2003, 14, 1808–1817. [Google Scholar] [CrossRef] [Green Version]
- Tsai, P.S.; Friedman, B.; Ifarraguerri, A.I.; Thompson, B.D.; Lev-Ram, V.; Schaffer, C.B.; Xiong, Q.; Tsien, R.Y.; Squier, J.A.; Kleinfeld, D. All-optical histology using ultrashort laser pulses. Neuron 2003, 39, 27–41. [Google Scholar] [CrossRef] [Green Version]
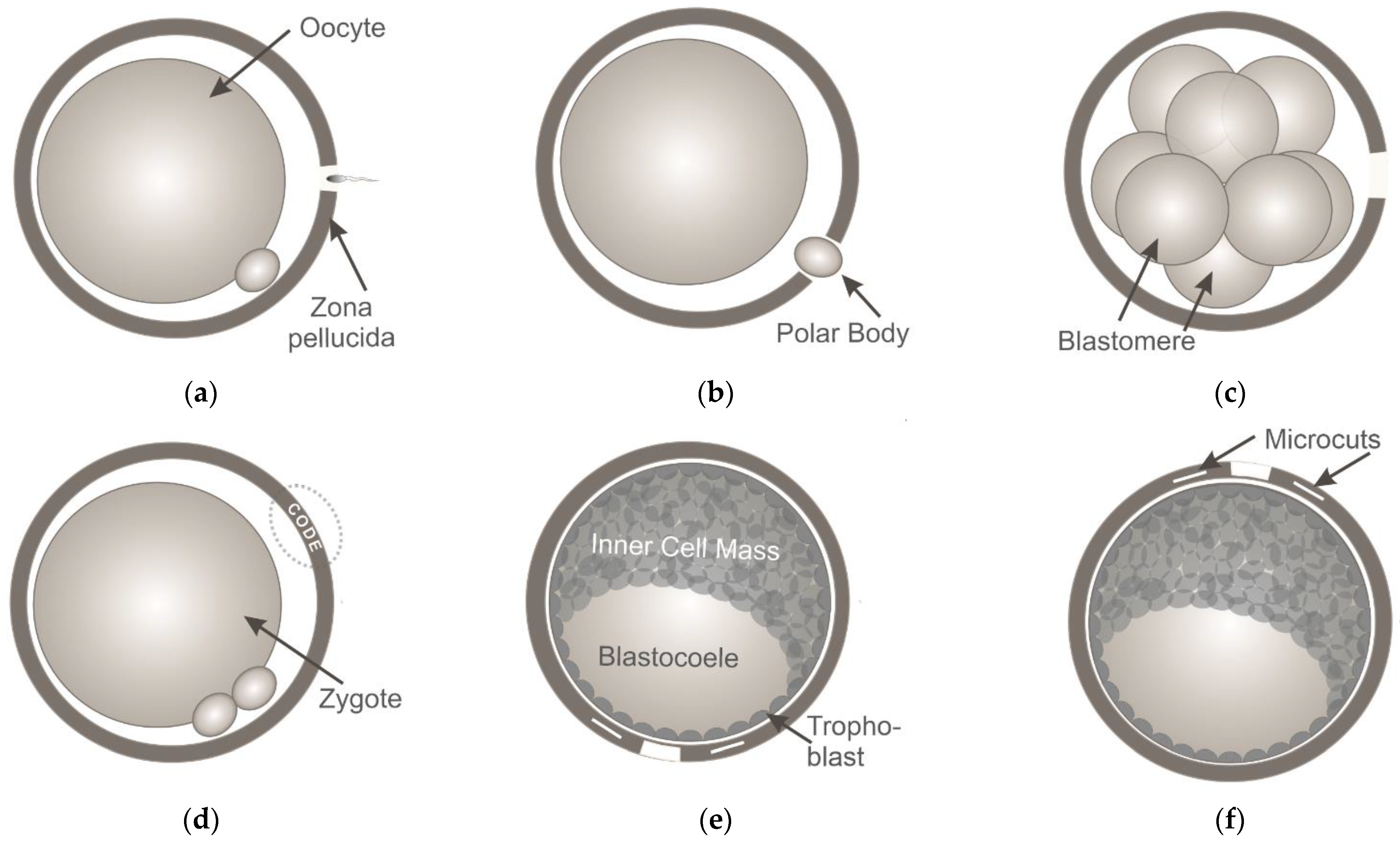
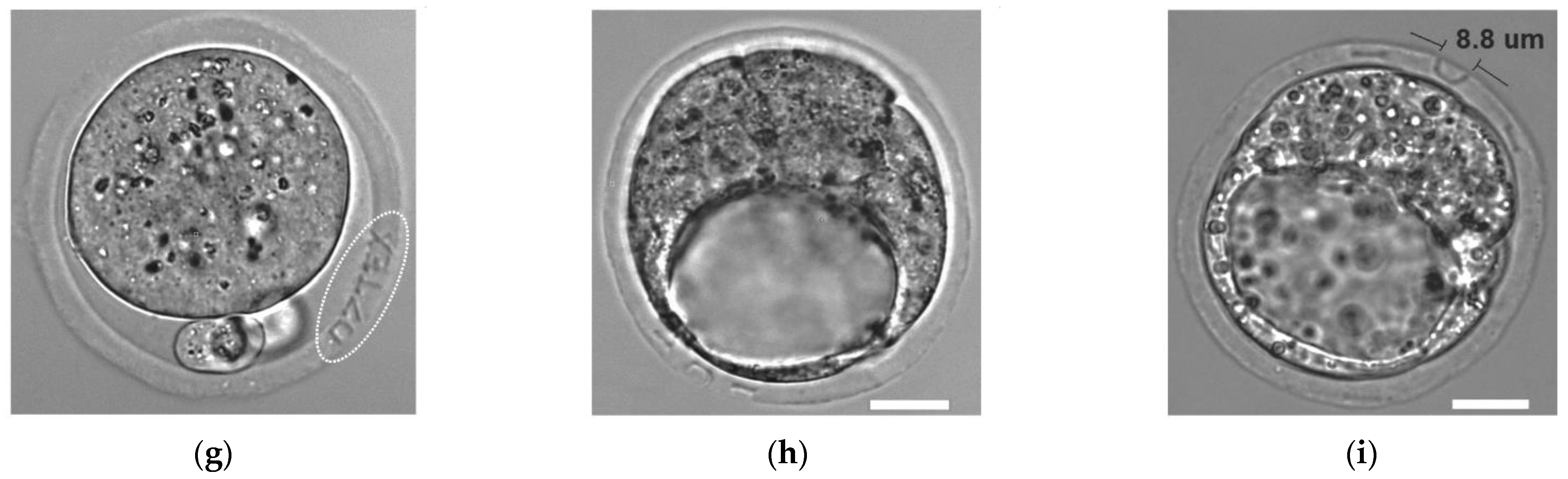
| Object | Method | Parameters | Aim of Study | Results | Source |
|---|---|---|---|---|---|
| Two-cell embryos (hamster) | LSCM | Argon laser: λ = 514 nm, Pav = 12 μW, Nd:YAG laser: λ = 532 nm, Pav = 10 μW, Krypton/argon laser: λ = 568 nm, Pav = 28 μW, t = 8 μs (dwell time), Etotal ~280 μJ per embryo Iav = 9∙103 W/cm2 | Influence on embryonic development | Confocal imaged embryos never reach the morula stage. Formation of ROS is suggested | [76] |
| Two-cell embryos (hamster) | Two-photon laser scanning microscopy | Nd:YLF laser τ = 175 fs, λ = 1047 nm, Iav = 6∙106 W/cm2, t = 8 μs (dwell time), Etotal ~2 J per embryo | Influence on embryonic development | Number of embryos developed to morulae and blastocysts did not significantly differ from control ones | [76] |
| Zygotes-blastocysts (mouse) | SHG, THG | Cr:forsterite laser τ = 65 fs, λ = 1230 nm, = 76 MHz, Pav = 35 mW | Central spindles during cytokinesis (SHG), lipid droplets, nucleoli, and plasma membranes (THG) | Embryos demonstrated normal development during 1-day-long imaging | [101] |
| Oocytes, embryos (mouse) | SHG, THG | Cr:forsterite laser τ = 140 fs, λ = 1230 nm, = 110 MHz Pav = 150 mW, Etotal = 29 J (per embryo over a total imaging time of 10 min) | Spindle fibers, thickness of the three layers of the zona pellucida (SHG), cell membranes (THG) | 67% of embryos have fully developed | [92] |
| Cryopreserved oocytes and one-cell embryos (mouse) | SHG | Ti:sapphire τ = 150 fs, = 80 MHz, λ = 750 nm, Pav = 3–60 mW; λ = 845/880 nm, Pav = 12–80 mW | Influence of FLIM-based metabolic imaging and SHG spindle imaging on embryonic development | The method does not significantly impair embryo viability | [104] |
| Zygotes-blastocysts (mouse) | THG | τ = 200 fs, λ = 1028 nm, = 50 MHz, Pav = 20 mW, E = 0.4 nJ | Pre-implantation embryo patterning and polarity, blastomere equivalence | THG revealed an energy divergence of blastomeres from 12% to 18% | [105] |
| Blastocysts (mouse) | FLIM+ THG | Ti:sapphire λ = 740 and 1040 nm, = 80 MHz, Pav = 3.5–15 mW | Developmental states, metabolic changes | FLIM does not disrupt embryonic development under 10 mW. Method for embryo quality estimation and viability prediction is proposed. | [106] |
| Oocytes (mouse) | CARS | Ti:sapphire τ = 150 fs, λ = 800 nm, = 80 MHz, Pav ~6 mW, t ~2 min (CARS-PMT), t ~20 min (CARS-CCD) | Lipid content in mammalian oocytes during development and in relation to body composition | Quantifiable difference is found in percent lipid composition across oocytes of different species, developmental stages, and in relation to body composition | [107] |
| Embryos (mouse) | CARS, TPEF | τ = 5 fs, λ = 660–730 nm (pump), λ = 730–900 nm (Stokes), λ = 930 nm (TPEF), t = 10 µs (dwell time), = 14 mW, = 9 mW | The number, size, and 3D spatial distribution of lipid droplets | The differences in the chemical composition of lipid droplets in living oocytes matured in media supplemented with different saturated and unsaturated fatty acids were observed | [86] |
| Oocytes, embryos (mouse, bovine) | CARS | τ = 5 fs, λ = 660–730 nm (pump), λ = 730–900 nm (Stokes), t = 10 µs (dwell time), = 27 mW, = 13 mW | The number, size, and 3D spatial distribution of lipid droplets | Specific aspects of the metabolic profile of living mammalian eggs and early embryos can be assessed | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilina, I.; Sitnikov, D. From Zygote to Blastocyst: Application of Ultrashort Lasers in the Field of Assisted Reproduction and Developmental Biology. Diagnostics 2021, 11, 1897. https://doi.org/10.3390/diagnostics11101897
Ilina I, Sitnikov D. From Zygote to Blastocyst: Application of Ultrashort Lasers in the Field of Assisted Reproduction and Developmental Biology. Diagnostics. 2021; 11(10):1897. https://doi.org/10.3390/diagnostics11101897
Chicago/Turabian StyleIlina, Inna, and Dmitry Sitnikov. 2021. "From Zygote to Blastocyst: Application of Ultrashort Lasers in the Field of Assisted Reproduction and Developmental Biology" Diagnostics 11, no. 10: 1897. https://doi.org/10.3390/diagnostics11101897
APA StyleIlina, I., & Sitnikov, D. (2021). From Zygote to Blastocyst: Application of Ultrashort Lasers in the Field of Assisted Reproduction and Developmental Biology. Diagnostics, 11(10), 1897. https://doi.org/10.3390/diagnostics11101897






