Reactive Hyperemia-Triggered Wrist Pulse Analysis for Early Monitoring of Young Men with High Atherosclerotic Risk
Abstract
:1. Introduction
2. Physiological Signal Acquisition and Medical Indexes
2.1. Study Population and Grouping
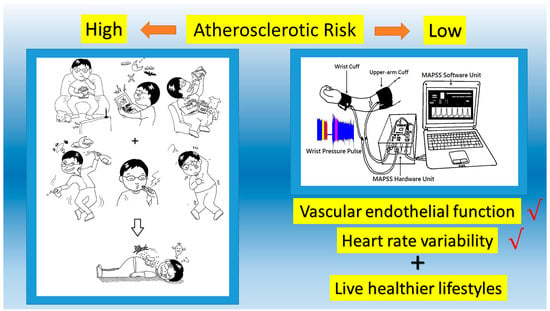
2.2. Signals Acquisition System Description
2.3. Medical Indexes from Physiological Signals
2.3.1. Amplitude Variation for Vascular Relaxation Assessment
- Endothelial Function Assessment
- Medical Index for Endothelial Function
2.3.2. Periods Variation for HRV Assessment
- LFP/HFP ratio (LHR)
- Percussion entropy index
2.4. Signal Analysis and Statistical Analysis
3. Results
3.1. Characteristics of the Study Subjects
3.2. Grouping of the Young Men by TC/HDL and Medical Indexes
3.3. Effects of Risk Factors
4. Discussion
5. Conclusions, Limitations, and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chung, R.J.; Touloumtzis, C.; Gooding, H. Staying young at heart: Cardiovascular disease prevention in adolescents and young adults. Curr. Treat. Options Cardiovasc. Med. 2015, 17, 61. [Google Scholar] [CrossRef] [Green Version]
- Fernberg, U.; Fernström, M.; Hurtig-Wennlöf, A. Higher total physical activity is associated with lower arterial stiffness in Swedish, young adults: The cross-sectional lifestyle, biomarkers, and atherosclerosis study. Vasc. Health Risk Manag. 2021, 17, 175–185. [Google Scholar] [CrossRef]
- Arena, R.; Guazzi, M.; Lianov, L.; Whitsel, L.; Berra, K.; Lavie, C.J.; Kaminsky, L.; Williams, M.; Hivert, M.-F.; Franklin, N.C.; et al. Healthy lifestyle interventions to combat noncommunicable disease-a novel nonhierarchical connectivity model for key stakeholders: A policy statement from the American Heart Association, European Society of Cardiology, European Association for Cardiovascular Prevention and Rehabilitation, and American College of Preventive Medicine. Eur. Heart J. 2015, 36, 2097–2109. [Google Scholar]
- The NHS Long Term Plan. Available online: https://www.longtermplan.nhs.uk/wp-content/uploads/2019/01/nhs-long-term-plan-june-2019.pdf (accessed on 28 June 2021).
- Hernández-Vásquez, A.; Chacón-Torrico, H.; Vargas-Fernández, R.; Bendezu-Quispe, G.; Santero, M. Metrics of Ideal Cardiovascular Health are Unequally Distributed between Peruvian Men and Women: Analysis of a National Population-Based Survey in 2017. Int. J. Prev. Med. 2020, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Candales, A.; Burgos PM, H.; Hernandez-Suarez, D.F.; Harris, D. Linking chronic inflammation with cardiovascular disease: From normal aging to the metabolic syndrome. J. Nat. Sci. 2017, 3, e341. Available online: https://pubmed.ncbi.nlm.nih.gov/33953561/ (accessed on 28 June 2021).
- Schmidt, M.; Johannesdottir, S.A.; Lemeshow, S.; Lash, T.L.; Ulrichsen, S.P.; Bøtker, H.E.; Sørensen, H.T. Obesity in young men, and individual and combined risks of type 2 diabetes, cardiovascular morbidity and death before 55 years of age: A Danish 33-year follow-up study. BMJ Open 2013, 3, e002698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oosterveen, E.; Tzelepis, F.; Ashton, L.; Hutchesson, M.J. A systematic review of eHealth behavioral interventions targeting smoking, nutrition, alcohol, physical activity and/or obesity for young adults. Prev. Med. 2017, 99, 197–206. [Google Scholar] [CrossRef]
- Lemieux, I.; Lamarche, B.; Couillard, C.; Pascot, A.; Cantin, B.; Bergeron, J.; Dagenais, G.R.; Després, J.-P. Total Cholesterol/HDL Cholesterol Ratio vs LDL Cholesterol/HDL Cholesterol Ratio as Indices of Ischemic Heart Disease Risk in Men: The Quebec Cardiovascular Study. Arch. Intern. Med. 2001, 161, 2685–2692. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M.; Miller, M.; Nasir, K.; McEvoy, J.W.; Herrington, D.; Blumenthal, R.S.; Blaha, M.J. Primary low level of high-density lipoprotein cholesterol and risks of coronary heart disease, cardiovascular disease, and death: Results from the multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 2016, 183, 875–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, J.; Huang, Y.; Lu, Y.; Yuan, H. Associations of non-high-density lipoprotein cholesterol, triglycerides and the total cholesterol/HDL-c ratio with arterial stiffness independent of low-density lipoprotein cholesterol in a Chinese population. Hypertens. Res. 2019, 42, 1223–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.Y.; Shi, D.; Ding, J.; Cheng, Z.Y.; Li, H.Y.; Li, J.S.; Pu, H.Q.; Yang, A.; He, C.L.; Zhang, J.P.; et al. Total cholesterol to high-density lipoprotein cholesterol ratio is a significant predictor of nonalcoholic fatty liver: Jinchang cohort study. Lipids Health Dis. 2019, 18, 47. [Google Scholar] [CrossRef]
- Kostenchak, S.O.; Klymuk, A.R.; Nemesh, M.I.; Palamarchuk, O.S.; Feketa, V.P. Endothelial function and heart-rate variability parameters before and after body correction program. Pak. J. Med. Health Sci. 2021, 15, 1097–1104. [Google Scholar]
- Singh, A.; Collins, B.L.; Gupta, A.; Fatima, A.; Qamar, A.; Biery, D.; Baez, J.; Cawley, M.; Klein, J.; Hainer, J.; et al. Cardiovascular risk and statin eligibility of young adults after an MI: Partners YOUNG-MI Registry. J. Am. Coll. Cardiol. 2018, 71, 292–302. [Google Scholar] [CrossRef]
- Mudau, M.; Genis, A.; Lochner, A.; Strijdom, H. Endothelial dysfunction: The early predictor of atherosclerosis. Cardiovasc. J. Afr. 2012, 23, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Miguelez, P.R.; Seigler, N.; Harris, R.A. Ultrasound assessment of endothelial function: A technical guideline of the flow-mediated dilation test. J. Vis. Exp. 2016, 110, 54011. [Google Scholar] [CrossRef] [Green Version]
- Petterson, J.L.; O’Brien, M.W.; Johns, J.A.; Chiasson, J.; Kimmerly, D.S. Influence of prostaglandins and endothelial-derived hyperpolarizing factors on brachial and popliteal endothelial-dependent function in young adults. J. Appl. Physiol. 2021, 130, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-C.; Kor, C.-T.; Lin, C.-H.; Kuo, J.; Tsai, J.-Z.; Ko, W.-J.; Kuo, C.-D. High-frequency power of heart rate variability can predict the outcome of thoracic surgical patients with acute respiratory distress syndrome on admission to the intensive care unit: A prospective, single-centric, case-controlled study. BMC Anesthesiol. 2018, 18, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.C.; Xiao, M.X.; Ta, N.; Wu, H.T.; Sun, C.K. Assessment of Diabetic Autonomic Nervous Dysfunction with a Novel Percussion Entropy Approach. Complexity 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Jamin, A.; Humeau-Heurtier, A. (Multiscale) cross-entropy methods: A review. Entropy 2020, 22, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.-C.; Hu, W.-R.; Ta, N.; Xiao, M.-X.; Tang, X.-J.; Wu, H.-T. Prognosis of Diabetic Peripheral Neuropathy via Decomposed Digital Volume Pulse from the Fingertip. Entropy 2020, 22, 754. [Google Scholar] [CrossRef]
- Wu, H.T.; Lee, C.H.; Sun, C.K.; Hsu, J.T.; Huang, R.M.; Tang, C.J. Arterial waveforms measured at the wrist as indicators of diabetic endothelial dysfunction in the elderly. IEEE Trans. Instrum. Meas. 2012, 61, 162–169. [Google Scholar] [CrossRef]
- Wu, H.T.; Hsu, P.C.; Sun, C.K.; Wang, H.J.; Liu, C.C.; Chen, H.R.; Liu, A.B.; Tang, C.J.; Lo, M.T. Assessment of autonomic dysfunction in patients with type 2 diabetes using reactive hyperemia. J. Theor. Biol. 2013, 330, 9–17. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Castaldo, R.; Montesinos, L.; Melillo, P.; James, C.; Pecchia, L. Ultra-short term HRV features as surrogates of short term HRV: A case study on mental stress detection in real life. BMC Med. Inform. Decis. Mak. 2019, 19, 12. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.K.; Liu, C.C.; Liu, W.M.; Wu, H.T.; Huang, R.M.; Liu, A.B. Compatibility of pulse-pulse intervals with R-R intervals in assessing cardiac autonomic function and its relation to risks of atherosclerosis. Tzu-Chi Med. J. 2019, 32, 41. Available online: http://www.tcmjmed.com/preprintarticle.asp?id=252625 (accessed on 28 June 2021).
- Duraisamy, R.; Krishnan, C.S.; Ramasubramanian, H.; Sampathkumar, J.; Mariappan, S.; Sivaprakasam, A.N. Compatibility of Nonoriginal Abutments With Implants. Implant. Dent. 2019, 28, 289–295. [Google Scholar] [CrossRef]
- Gooding, H.C.; Gidding, S.S.; Moran, A.E.; Redmond, N.; Allen, N.B.; Bacha, F.; Burns, T.L.; Catov, J.M.; Grandner, M.A.; Harris, K.M.; et al. Challenges and opportunities for the prevention and treatment of cardiovascular disease among young adults: Report from a national heart, lung, and blood institute working group. J. Am. Heart Assoc. 2020, 9, e016115. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.F.; Adji, A.; Safar, M.E. Structure and function of systemic arteries: Reflections on the arterial pulse. Am. J. Hypertens. 2018, 31, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.S.; Ouyang, A.; Krishnakumar, I.M.; Charnigo, R.J.; Westgate, P.M.; Fleenor, B.S. Influence of enhanced bioavailable curcumin on obesity-associated cardiovascular disease risk factors and arterial function: A double-blinded, randomized, controlled trial. Nutrition 2019, 62, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Fernberg, U.; Op ’t Roodt, J.; Fernström, M.; Hurtig-Wennlöf, A. Body composition is a strong predictor of local carotid stiffness in Swedish, young adults-the cross sectional Lifestyle, biomarkers, and atherosclerosis study. BMC Cardiovasc. Disord. 2019, 19, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, A.H.; Kimball, T.R.; Khoury, P.R.; Dolan, L.M.; Urbina, E.M. Obese and type 2 diabetic youth have increased forward and backward wave reflections. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 944–950. [Google Scholar] [CrossRef]
- Rosenberry, R.; Nelson, M.D. Reactive hyperemia: A review of methods, mechanisms, and considerations. Am. J. Physiol. Integr. Comp. Physiol. 2020, 318, R605–R618. [Google Scholar] [CrossRef]
- Xiao, M.-X.; Lu, C.-H.; Ta, N.; Wei, H.-C.; Haryadi, B.; Wu, H.-T. Machine learning prediction of future peripheral neuropathy in type 2 diabetics with percussion entropy and body mass indices. Biocybern. Biomed. Eng. 2021, 41, 1140–1149. [Google Scholar] [CrossRef]
- Zobel, E.H.; Ripa, R.S.; von Scholten, B.J.; Rotbain Curovic, V.; Kjaer, A.; Hansen, T.W.; Rossing, P.; Størling, J. Effect of liraglutide on expression of inflammatory genes in type 2 diabetes. Sci. Rep. 2021, 11, 18522. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, J.P.; Rong, J.; Larson, M.G.; Hamburg, N.M.; Benjamin, E.J.; Vasan, R.S.; Mitchell, G.F. Metabolic predictors of change in vascular function: Prospective associations from a community-based cohort. Hypertension 2018, 71, 237–242. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Ke, Y.; Vucic, S.; Ittner, L.; Seeley, W.; Hodges, J.R.; Piguet, O.; Halliday, G.; Kiernan, M.C. Physiological changes in neurodegeneration—mechanistic insights and clinical utility. Nat. Rev. Neurol. 2018, 14, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P. Correlating changes in metabolic status with arterial health: Modest associations but any clinical implications? Hypertension 2018, 71, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.J.; Jae, S.Y.; Kim, H.J.; Yoon, E.S.; Park, Y.R.; Bunsawat, K. Comparison of the effects of short-term stair climbing and walking exercise on vascular function in healthy young adults. Int. J. Appl. Sports Sci. 2018, 30, 125–133. [Google Scholar]
- Pereira, T.; Bergqvist, J.; Vieira, C.; Sveälv, B.G.; Castanheira, J.; Conde, J. Randomized study of the effects of cocoa-rich chocolate on the ventricle-arterial coupling and vascular function of young, healthy adults. Nutrition 2019, 63–64, 175–183. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Value | Normal Value | ||
|---|---|---|---|---|
| Age (year) | 22.36 | ± | 3.01 | - |
| Body Height (cm) | 172.51 | ± | 5.87 | - |
| Body Weight (kg) | 70.45 | ± | 14.42 | - |
| Waist Circumference (cm) | 84.87 | ± | 12.05 | <90, for man |
| Body mass index (kg/cm2) | 23.95 | ± | 4.05 | 18.5 ≤ BMI < 24 |
| Systolic Blood Pressure (mmHg) | 119.75 | ± | 12.38 | <135 |
| Diastolic Blood Pressure (mmHg) | 71.99 | ± | 7.87 | <85 |
| Pulse Pressure (mmHg) | 47.48 | ± | 9.01 | 20~60 |
| High-density lipoprotein (mg/dL) | 44.68 | ± | 7.65 | >40, for man |
| Low-density lipoprotein (mg/dL) | 107.74 | ± | 31.94 | <110 |
| Total serum cholesterol (mg/dL) | 175.33 | ± | 34.09 | <200 |
| Triglyceride (mg/dL) | 93.28 | ± | 63.86 | <150 |
| TC/HDL Ratio | 4.02 | ± | 0.99 | ≤4 |
| Fasting Blood Sugar (mg/dL) | 94.05 | ± | 6.79 | <120 |
| HbA1c (%) | 5.45 | ± | 0.31 | <6.5 |
| Parameters | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| Age (year) | 22.96 ± 2.46 | 22.72 ± 3.30 | 52.04 ± 9.98 †† |
| Body Height (cm) | 172.08 ± 5.57 | 170.89 ± 5.32 | 161.91 ± 8.19 † |
| Body Weight (kg) | 67.58 ± 9.62 | 74.67 ± 16.82 | 65.79 ± 15.82 |
| WC (cm) | 83.34 ± 8.53 | 89.32 ± 13.91 | 87.58 ± 10.38 |
| BMI (kg/cm2) | 22.76 ± 2.68 | 25.51 ± 5.27 | 24.81 ± 3.49 |
| SBP (mmHg) | 119.00 ± 13.14 | 119.83 ± 11.74 | 127.43 ± 16.59 |
| DBP (mmHg) | 72.16 ± 7.48 | 71.50 ± 8.21 | 74.59 ± 12.52 |
| HDL (mg/dL) | 47.52 ± 7.51 | 42.83 ± 6.91 | 51.97 ± 19.52 |
| LDL (mg/dL) | 86.68 ± 17.56 | 134.92 ± 25.46 ** | 112.98 ± 27.89 † |
| TC (mg/dL) | 154.44 ± 19.69 | 201.84 ± 30.86 ** | 188.88 ± 42.69 |
| TG (mg/dL) | 91.48 ± 26.79 | 121.17 ± 85.98 | 110.83 ± 96.79 |
| FPG (mg/dL) | 92.48 ± 4.95 | 94.56 ± 8.38 | 105.34 ± 21.65 |
| HbA1c (%) | 5.47 ± 0.28 | 5.51 ± 0.30 | 6.02 ± 0.58 † |
| TC/HDL Ratio | 3.48 ± 0.40 | 5.09 ± 0.86 ** | 3.98 ± 1.70 |
| DI | 1.75 ± 0.39 | 1.45 ± 0.27 * | 1.31 ± 0.48 |
| LHR | 0.92 ± 0.47 | 0.86 ± 0.62 | 0.89 ± 0.87 |
| PEIWPP (%) | 70.56 ± 8.96 | 56.06 ± 11.17 * | 68.37 ± 9.17 † |
| Risk Factors | Coefficient | p | OR | 95% CI for OR |
|---|---|---|---|---|
| WC | 0.138 | 0.015 | 1.148 | 1.027–1.283 |
| PEIWPP | −0.163 | 0.003 | 0.849 | 0.761–0.948 |
| Constant | −1.548 | 0.076 | 0.213 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-J.; Wu, H.-T.; Haryadi, B. Reactive Hyperemia-Triggered Wrist Pulse Analysis for Early Monitoring of Young Men with High Atherosclerotic Risk. Diagnostics 2021, 11, 1918. https://doi.org/10.3390/diagnostics11101918
Chen J-J, Wu H-T, Haryadi B. Reactive Hyperemia-Triggered Wrist Pulse Analysis for Early Monitoring of Young Men with High Atherosclerotic Risk. Diagnostics. 2021; 11(10):1918. https://doi.org/10.3390/diagnostics11101918
Chicago/Turabian StyleChen, Jian-Jung, Hsien-Tsai Wu, and Bagus Haryadi. 2021. "Reactive Hyperemia-Triggered Wrist Pulse Analysis for Early Monitoring of Young Men with High Atherosclerotic Risk" Diagnostics 11, no. 10: 1918. https://doi.org/10.3390/diagnostics11101918
APA StyleChen, J.-J., Wu, H.-T., & Haryadi, B. (2021). Reactive Hyperemia-Triggered Wrist Pulse Analysis for Early Monitoring of Young Men with High Atherosclerotic Risk. Diagnostics, 11(10), 1918. https://doi.org/10.3390/diagnostics11101918