Autonomic and Somatic Nerve Functions in Type 2 Diabetes Mellitus Patients: Electrophysiological Aspects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Electrophysiological Studies in Somatic Nerves
2.3. Cardiac Parasympathetic Autonomic Testing
2.4. Statistical Analysis
3. Results
3.1. General Characteristics of Study Patients
3.2. Correlations between Parasympathetic Function and Motor Nerve Function
3.3. Correlations between Parasympathetic Function and Sensory Nerve Function
3.4. Multivariate Analysis between Parasympathetic Function and Sensory–Motor Nerve Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 136–154. [Google Scholar] [CrossRef] [Green Version]
- Verrotti, A.; Prezioso, G.; Scattoni, R.; Chiarelli, F. Autonomic neuropathy in diabetes mellitus. Front. Endocrinol. (Laussane) 2014, 5, 205. [Google Scholar] [CrossRef] [Green Version]
- Shabeeb, D.; Najafi, M.; Hasanzadeh, G.; Hadian, M.R.; Musa, A.E.; Shirazi, A. Electrophysiological measurements of diabetic peripheral neuropathy: A systematic review. Diabetes Metab. Syndr. 2018, 12, 591–600. [Google Scholar] [CrossRef]
- Vinik, A.I.; Maser, R.E.; Mitchell, B.D.; Freeman, R. Diabetic autonomic neuropathy. Diabetes Care 2003, 26, 1553–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balcıoğlu, A.S.; Müderrisoğlu, H. Diabetes and cardiac autonomic neuropathy: Clinical manifestations, cardiovascular consequences, diagnosis and treatment. World J. Diabetes 2015, 6, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.I. The conductor of the autonomic orchestra. Front. Endocrinol. 2012, 3, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinik, A.I.; Erbas, T.; Casellini, C.M. Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. J. Diabetes Investig. 2013, 4, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Pop-Busui, R. Cardiac autonomic neuropathy in diabetes: A clinical perspective. Diabetes Care 2010, 33, 434–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinik, A.I.; Casellini, C.; Parson, H.K.; Colberg, S.R.; Nevoret, M.L. Cardiac autonomic neuropathy in diabetes: A predictor of cardiometabolic events. Front. Neurosci. 2018, 12, 591. [Google Scholar] [CrossRef] [PubMed]
- Azmi, S.; Petropoulos, I.N.; Ferdousi, M.; Ponirakis, G.; Alam, U.; Malik, R.A. An update on the diagnosis and treatment of somatic and autonomic neuropathy. F1000 Res. 2019, 8, F1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyck, P.J.; Overland, C.J.; Low, P.A.; Litchy, W.J.; Davies, J.L.; Dyck, P.J.B.; O’Brien, P.C. (Coordinating Committee) for the Cl vs. NPhys Trial Investigators. Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs. NPhys trial. Muscle Nerve 2010, 42, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardi, L.; Spallone, V.; Stevens, M.; Hilsted, J.; Frontoni, S.; Pop-Busui, R.; Ziegler, D.; Kempler, P.; Freeman, R.; Low, P.; et al. Methods of investigation for cardiac autonomic dysfunction in human research studies. Diabetes Metab. Res. Rev. 2011, 27, 654–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesfaye, S.; Boulton, A.J.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic Neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef] [Green Version]
- England, J.D.; Gronseth, G.S.; Franklin, G.; Miller, R.G.; Asbury, A.K.; Carter, G.T.; Cohen, J.A.; Fisher, M.A.; Howard, J.F.; Kinsella, L.J.; et al. Distal symmetrical polyneuropathy: Definition for clinical research. Muscle Nerve 2005, 3, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Kimura, J. (Ed.) Principles and variations of nerve conduction studies. In Electrodiagnosis in Diseases of the Nerve and Muscle: Principles and Practice; Oxford University Press: New York, NY, USA, 2013; pp. 91–129. [Google Scholar]
- Pease, W.S.; Lew, H.L.; Johnson, E.W. Johnson’s Practical Electromyography; Pease, W.S., Ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2007; pp. 221–244. [Google Scholar]
- Ewing, D.J.; Clarke, B.F. Diagnosis and management of diabetic autonomic neuropathy. Br. Med. J. (Clin. Res. Ed.) 1982, 285, 916–918. [Google Scholar] [CrossRef] [Green Version]
- Spallone, V.; Ziegler, D.; Freeman, R.; Bernardi, L.; Frontoni, S.; Pop-Busui, R.; Stevens, M.; Kempler, P.; Hilsted, J.; Tesfaye, S.; et al. Toronto Consensus Panel on Diabetic Neuropathy. Cardiovascular autonomic neuropathy in diabetes: Clinical impact, assessment, diagnosis, and management. Diabetes Metab. Res. Rev. 2011, 27, 639–653. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, D.; Laux, G.; Dannehl, K.; Spüler, M.; Mühlen, H.; Mayer, P.; Gries, F.A. Assessment of cardiovascular autonomic function: Age-related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabetes Med. 1992, 9, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Ewing, D.J.; Martyn, C.N.; Young, R.J.; Clarke, B.F. The Value of Cardiovascular Autonomic Function Tests: 10 Years’ Experience in Diabetes. Diabetes Care 1985, 8, 491–498. [Google Scholar] [CrossRef]
- Porges, S.W. The polyvagal theory: New insights into adaptive reactions of the autonomic nervous system. Cleve Clin. J. Med. 2009, 76, S86. [Google Scholar] [CrossRef]
- Rea, P. Chaper 10—Vagus Nerve. In Clinical Anatomy of the Cranial Nerves; Academic Press: San Diego, CA, USA, 2014; pp. 105–116. [Google Scholar]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Korei, A.E.; Istenes, I.; Papanas, N.; Kempler, P. Small-fiber neuropathy: A diabetic microvascular complication of special clinical, diagnostic and prognostic importance. Angiology 2016, 67, 49–57. [Google Scholar] [CrossRef]
- Myers, M.I.; Peltier, A.C. Uses of skin biopsy for sensory and autonomic nerve assessment. Curr. Neurol. Neurosci. Rep. 2013, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where we go? J. Diabetes Investig. 2011, 2, 18–32. [Google Scholar] [CrossRef] [Green Version]
- Vincent, A.M.; Callaghan, B.C.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Cellular mechanisms as therapeutic targets. Nat. Rev. Neurol. 2011, 7, 573–583. [Google Scholar] [CrossRef]
- Javed, S.; Petropoulos, I.N.; Tavakoli, M.; Malik, R.A. Clinical and diagnostic features of small fiber damage in diabetic polyneuropathy. Handb. Clin. Neurol. 2014, 126, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Said, G. Diabetic neuropathy. Handb. Clin. Neurol. 2013, 115, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Wang, T.; Wang, J. Amplitude of sensory nerve action potential in early stage diabetic peripheral neuropathy: An analysis of 500 cases. Neural. Regen. Res. 2014, 9, 1389–1394. [Google Scholar] [CrossRef]
- Bi, J.; Lu, Z.S.; Chu, H.; Dong, H.J. Diagnostic significance of sensory nerve action potential amplitude in early-stage diabetic neuropathy. Zhonghua Shenjing Ke Zazhi 2008, 41, 657–660. [Google Scholar]
- Burke, D.; Howells, J.; Kiernan, M.C. Sensory and motor axons are different: Implications for neurological disease. Ann. Clin. Neurophysiol. 2017, 19, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Gibbons, C.H.; Freeman, R.; Veves, A. Diabetic neuropathy. A cross-sectional study of the relationships among neuropahysiology. Diabetes Care 2010, 33, 2629–2634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pop-Busui, R.P.; Evans, G.E.; Gerstein, H.C.; Fonseca, V.; Fleg, J.L.; Hoogwerf, B.J.; Genuth, S.; Grimm, R.H.; Corson, M.A.; Prineas, R.; et al. Effects of cardiac autonomic dysfunction on mortality risk in the action to control cardiovascular risk in diabetes (ACCORD) trial. Diabetes Care 2010, 7, 1578–1884. [Google Scholar] [CrossRef] [Green Version]
- Adler, G.K.; Bonyhay, I.; Failing, H.; Waring, E.; Dotson, S.; Freeman, R. Antecedente hypoglycemia impairs autonomic cardiovascular function: Implications for rigorous glycemic control. Diabetes 2009, 58, 360–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finfer, S.; Chittock, D.R.; Su, S.Y.; Blair, D.; Foster, D.; Dhingra, V.; Bellomo, R.; Cook, D.; Dodek, P.; Henderson, W.R.; et al. Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar] [CrossRef] [Green Version]
- Orlov, S.; Bril, V.; Orszag, A.; Perkins, B.A. Heart rate variability and sensorimotor polyneuropathy in type 1 diabetes. Diabetes Care 2012, 35, 809–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sveen, K.A.; Karimé, B.; Jørum, E.; Mellgren, S.I.; Fagerland, M.W.; Monnier, V.M.; Dahl-Jørgensen, K.; Hanssen, K.F. Small- and large-fiber neuropathy after 40 years of type 1 Diabetes. Diabetes Care 2013, 36, 3712–3717. [Google Scholar] [CrossRef] [Green Version]
- Töyry, J.P.; Partanen, J.V.; Niskanen, L.K.; Länsimies, E.A.; Uusitupa, M.I. Divergent development of autonomic and peripheral somatic neuropathies in NIDDM. Diabetologia 1997, 40, 953–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Løseth, S.; Stålberg, E.V.; Lindal, S.; Olsen, E.; Jorde, R.; Mellgren, S.I. Small and large fiber neuropahy in those with type 1 and type 2 diabetes: A 5-year follow-up study. J. Peripher. Nerv. Syst. 2016, 21, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.A.; Veves, A.; Tesfaye, S.; Smith, G.; Cameron, N.; Zochodne, D.; Lauria, G. Toronto Consensus Panel on Diabetic Neuropathy. Small fibre neuropathy: Role in the diagnosis of diabetic sensorimotor polyneuropathy. Diabetes Metab. Res. Rev. 2011, 27, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.R.; Huang, C.C.; Chiu, W.C.; Liu, R.T.; Tsai, N.W.; Wang, H.C.; Lin, W.C.; Cheng, B.C.; Su, Y.J.; Su, C.M.; et al. Close relationship between cardiovagal function and sural sensory nerve action potential in type 2 diabetes. Clin. Neurophysiol. 2019, 130, 1160–1165. [Google Scholar] [CrossRef]
- Moţăţăianu, A.; Bălaşa, R.; Voidăzan, S.; Bajkó, Z. Cardiovascular autonomic neuropathy in context of other complications of type 2 diabetes mellitus. Biomed. Res. Int. 2013, 2013, 507216. [Google Scholar] [CrossRef]
- Tesfaye, S.; Selvarajah, D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab. Res. Rev. 2012, 28, 8–14. [Google Scholar] [CrossRef] [PubMed]
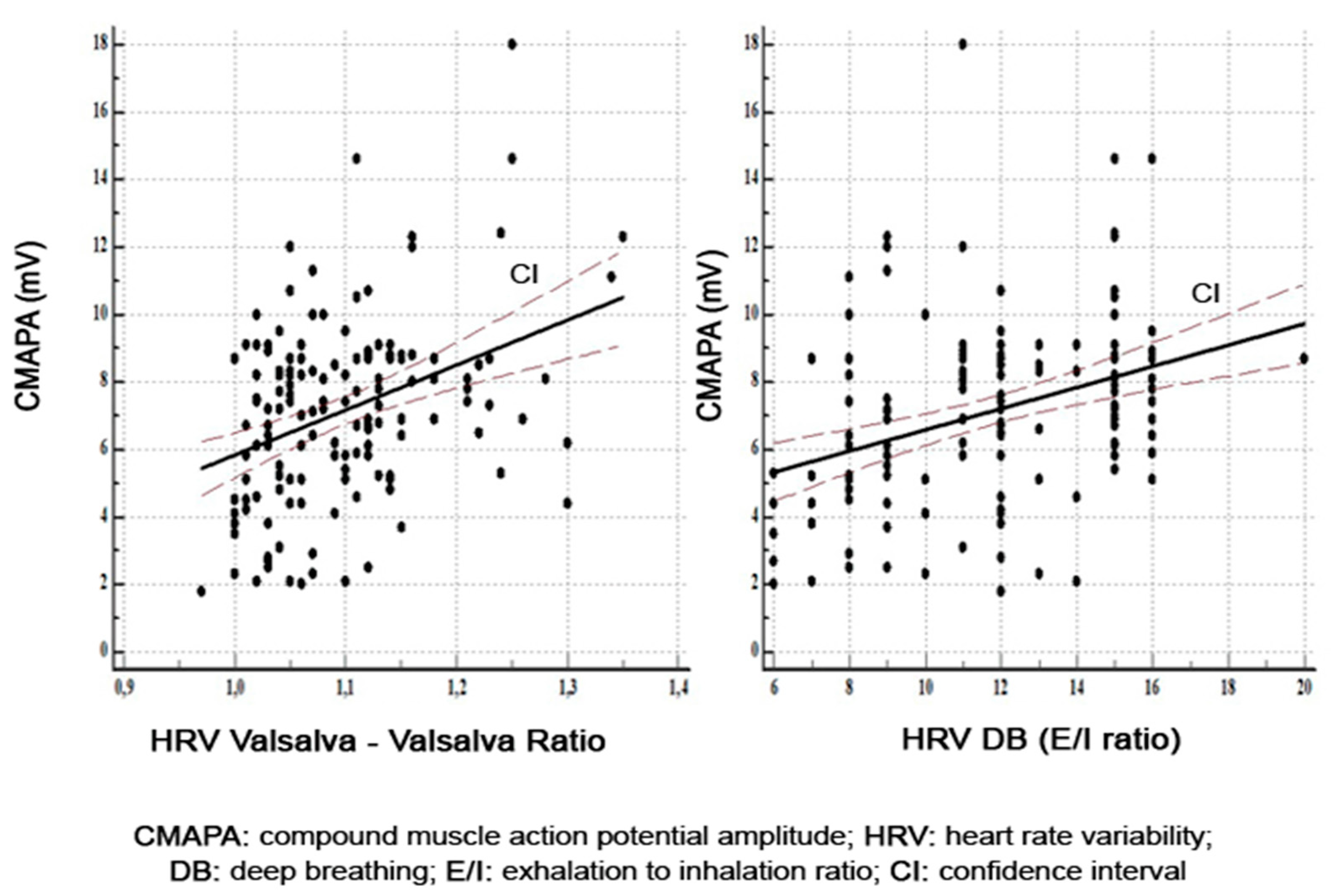

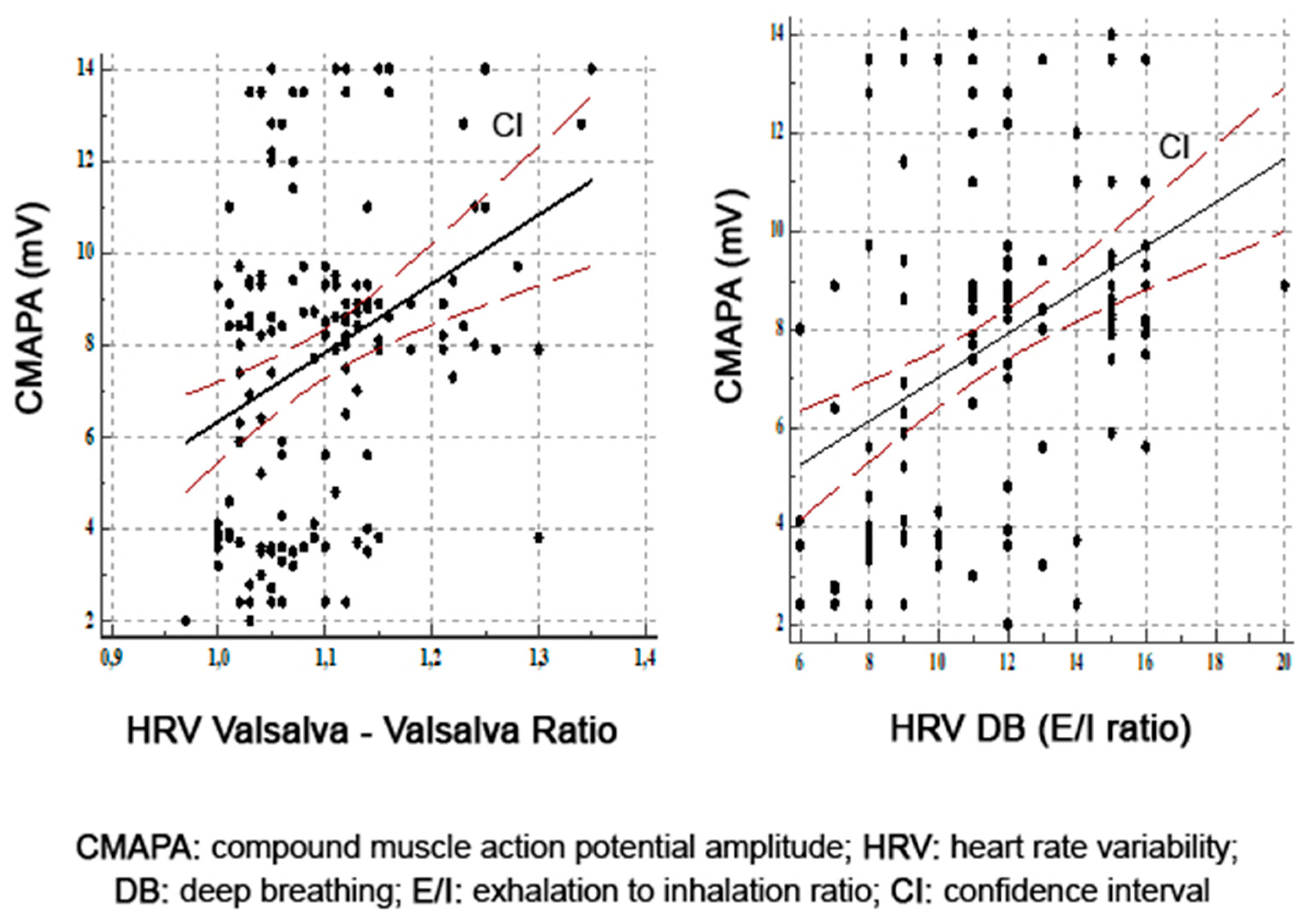
| Variable | T2DM Patients |
|---|---|
| Patients number | 161 |
| Male/Female, no. (%) | 76 (47.2)/85 (52.8) |
| Age (years) | 58.1 ± 8.2 |
| Minimum age | 33 |
| Maximum age | 77 |
| Age at diabetes diagnosis (years) | 49.8 ± 9.2 |
| Diabetes duration (years) | 6 (1–37) |
| <5 years, no. (%) | 61 (37.9) |
| 5–10 years, no. (%) | 28 (17.4) |
| 11–15 years, no. (%) | 54 (33.5) |
| >15 years, no. (%) | 18 (11.2) |
| Body mass index (kg/m2) | 30.8 ± 5.3 |
| Systolic BP (mmHg) | 143.4 ± 19.6 |
| Diastolic BP (mmHg) | 81.4 ± 9.9 |
| Heart rate (beats/minute) | 83 (51–102) |
| Hypertension (yes), no. (%) | 106 (65.8) |
| Ex-smokers, no. (%) | 92 (57.1) |
| Smokers (yes), no. (%) | 69 (42.9) |
| <20 cigarettes/day, no. (%) >20 cigarettes/day, no. (%) | 33 (47.8) 36 (52.2) |
| Triglycerides (mg) | 178 (60–1100) |
| Cholesterol (mg) | 228 ± 51.3 |
| HgbA1c (%) | 8.3 ± 1.4 |
| <6.5, no. (%) | 14 (8.7) |
| 6.5–6.9, no. (%) | 16 (9.9) |
| 7–8, no. (%) | 40 (24.8) |
| >8, no. (%) | 91 (56.5) |
| FPG (mg) | 183.9 ± 64.0 |
| Waist-to-hip ratio (cm) | 0.90 ± 0.08 |
| Abdominal circumference (cm) | 104.6 ± 12.3 |
| Nerve | Mean ± SD | Normal Values (Mean ± SD) | ||
|---|---|---|---|---|
| Motor | Median nerve | CMAPA | 6.98 ± 2.60 mV | 13.2 ± 5.0 mV |
| MNCV | 51.30 ± 3.86 m/s | 56.7 ± 3.8 m/s | ||
| Ulnar nerve | CMAPA | 7.64 ± 2.46 mV | 11.6 ± 2.1 mV | |
| MNCV | 53.38 ± 3.47 m/s | 61.5 ± 5 m/s | ||
| Peroneal nerve | CMAPA | 3.780 ± 1.44 mV | 5.9 ± 2.6 mV | |
| MNCV | 42.53 ± 2.48 m/s | 47 ± 4.0 m/s | ||
| Tibial nerve | CMAPA | 7.81 ± 3.44 mV | 11.6 ± 4.3 mV | |
| MNCV | 43.90 ± 3.27 m/s | 49.8 ± 6.0 m/s | ||
| Sensory | Median nerve | SNAPA | 16.34 ± 6.76 µV | 37 ± 19.0 µV |
| SNCV | 50.27 ± 5.37 m/s | 64 ± 8.0 m/s | ||
| Ulnar nerve | SNAPA | 13.79 ± 4.61 µV | 32 ± 20 µV | |
| SNCV | 52.42 ± 2.98 m/s | 55 ± 3.6 m/s | ||
| Sural nerve | SNAPA | 9.36 ± 4.52 µV | 23.7 ± 3.8 µV | |
| SNCV | 43.33 ± 2.60 m/s | 43.3 ± 4.3 m/s | ||
| NCS Parameters for Motor and Sensory Nerves | HRV OC | HRV Valsalva | HRV DB | ||||
|---|---|---|---|---|---|---|---|
| r-Values | p-Values | r-Values | p-Values | r-Values | p-Values | ||
| Median motor | DML | −0.14 | 0.07 | −0.12 | 0.11 | −0.23 | 0.004 |
| CMAPA | 0.13 | 0.0001 | 0.37 | 0.0001 | 0.35 | 0.0001 | |
| MNCV | 0.16 | 0.04 | 0.17 | 0.03 | 0.17 | 0.03 | |
| Ulnarmotor | DML | −0.22 | 0.005 | −0.11 | 0.18 | −0.05 | 0.55 |
| CMAPA | 0.29 | 0.0002 | 0.24 | 0.002 | 0.35 | 0.0001 | |
| MNCV | 0.15 | 0.06 | 0.31 | 0.0001 | 0.32 | 0.0001 | |
| Peroneal | DML | −0.17 | 0.03 | −0.23 | 0.003 | −0.25 | 0.001 |
| CMAPA | 0.20 | 0.01 | 0.33 | 0.0001 | 0.52 | 0.0001 | |
| MNCV | 0.10 | 0.22 | 0.22 | 0.007 | 0.38 | 0.0001 | |
| Tibial | DML | 0.05 | 0.55 | 0.11 | 0.15 | 0.08 | 0.33 |
| CMAPA | 0.27 | 0.0007 | 0.32 | 0.0001 | 0.39 | 0.0001 | |
| MNCV | 0.10 | 0.22 | 0.39 | 0.0001 | 0.34 | 0.0001 | |
| NCS Parameters for Motor and Sensory Nerves | HRV OC | HRV Valsalva | HRV DB | ||||
|---|---|---|---|---|---|---|---|
| r-Values | p-Values | r-Values | p-Values | r-Values | p-Values | ||
| Median sensory | DSL | −0.17 | 0.03 | −0.17 | 0.03 | −0.28 | 0.0005 |
| SNAPA | 0.28 | 0.0004 | 0.34 | 0.0001 | 0.46 | 0.0001 | |
| SNCV | 0.09 | 0.25 | 0.15 | 0.06 | 0.25 | 0.001 | |
| Ulnar sensory | DSL | −0.03 | 0.63 | −0.05 | 0.49 | −0.15 | 0.06 |
| SNAPA | 0.27 | 0.0007 | 0.39 | 0.0001 | 0.44 | 0.0001 | |
| SNCV | 0.17 | 0.03 | 0.29 | 0.0002 | 0.29 | 0.0002 | |
| Sural | DSL | −0.03 | 0.7 | −0.01 | 0.9 | −0.14 | 0.07 |
| SNAPA | 0.20 | 0.01 | 0.36 | 0.0001 | 0.57 | 0.0001 | |
| SNCV | 0.09 | 0.28 | 0.07 | 0.35 | 0.15 | 0.07 | |
| Nerve | Age | Diabetes Duration | HgbA1c | |||||
|---|---|---|---|---|---|---|---|---|
| r-Values | p-Values | r-Values | p-Values | r-Values | p-Values | |||
| Motor | Median | CMAPA | −0.12 | 0.13 | −0.51 | <0.0001 | −0.22 | 0.006 |
| MNCV | −0.11 | 0.15 | −0.25 | <0.0001 | −0.13 | 0.10 | ||
| Ulnar | CMAPA | −0.10 | 0.20 | −0.53 | <0.0001 | −0.19 | 0.01 | |
| MNCV | −0.04 | 0.55 | −0.44 | <0.0001 | −0.31 | <0.0001 | ||
| Peroneal | CMAPA | −0.19 | 0.01 | −0.61 | <0.0001 | −0.20 | 0.01 | |
| MNCV | −0.09 | 0.25 | −0.38 | <0.0001 | −0.22 | 0.006 | ||
| Tibial | CMAPA | −0.19 | 0.01 | −0.55 | <0.0001 | −0.24 | 0.003 | |
| MNCV | −0.03 | 0.63 | −0.48 | <0.0001 | −0.42 | <0.0001 | ||
| Sensory | Median | SNAPA | −0.10 | 0.19 | −0.59 | <0.0001 | −0.31 | <0.0001 |
| SNCV | −0.17 | 0.02 | −0.45 | <0.0001 | −0.18 | 0.020 | ||
| Ulnar | SNAPA | −0.09 | 0.25 | −0.60 | <0.0001 | −0.35 | <0.0001 | |
| SNCV | −0.16 | 0.04 | −0.45 | <0.0001 | −0.15 | 0.05 | ||
| Sural | SNAPA | −0.02 | 0.81 | −0.61 | <0.0001 | −0.37 | <0.0001 | |
| SNCV | −0.02 | 0.74 | −0.16 | 0.04 | −0.17 | 0.035 | ||
| Variable | Standardised Coefficient | p Value (CI = 95%) |
|---|---|---|
| Beta (β) | ||
| Median CMAPA | 0.38 | 0.01 |
| Median MNCV | −0.11 | 0.20 |
| Ulnar CMAPA | 0.30 | 0.02 |
| Ulnar MNCV | 0.11 | 0.23 |
| Peroneal DML | −0.16 | 0.07 |
| Peroneal CMAPA | −0.06 | 0.68 |
| Peroneal MNCV | −0.02 | 0.76 |
| Tibial CMAPA | 0.01 | 0.44 |
| Tibial MNCV | 0.29 | 0.01 |
| Tibial DML | −0.03 | 0.73 |
| Median SNAPA | −0.15 | 0.41 |
| Median DSL | 0.07 | 0.43 |
| Ulnar SNAPA | 0.33 | 0.09 |
| Sural SNAPA | 0.23 | 0.04 |
| Variable | Standardised Coefficient | p Value (CI = 95%) |
|---|---|---|
| Beta (β) | ||
| Median DML | 0.07 | 0.39 |
| Median CMAPA | 0.13 | 0.35 |
| Median MNCV | −0.002 | 0.97 |
| Ulnar CMAPA | −0.13 | 0.27 |
| Ulnar MNCV | 0.07 | 0.41 |
| Peroneal DML | 0.03 | 0.72 |
| Peroneal CMAPA | 0.62 | 0.01 |
| Peroneal MNCV | 0.29 | 0.01 |
| Tibial CMAPA | −0.06 | 0.62 |
| Tibial MNCV | −0.22 | 0.05 |
| Median SNAPA | 0.25 | 0.12 |
| Median SNCV | 0.16 | 0.03 |
| Ulnar SNAPA | −0.29 | 0.08 |
| Ulnar SNCV | −0.09 | 0.23 |
| Sural SNAPA | 0.66 | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motataianu, A.; Barcutean, L.; Bajko, Z.; Stoian, A.; Maier, S.; Voidazan, S.; Balasa, R. Autonomic and Somatic Nerve Functions in Type 2 Diabetes Mellitus Patients: Electrophysiological Aspects. Diagnostics 2021, 11, 2005. https://doi.org/10.3390/diagnostics11112005
Motataianu A, Barcutean L, Bajko Z, Stoian A, Maier S, Voidazan S, Balasa R. Autonomic and Somatic Nerve Functions in Type 2 Diabetes Mellitus Patients: Electrophysiological Aspects. Diagnostics. 2021; 11(11):2005. https://doi.org/10.3390/diagnostics11112005
Chicago/Turabian StyleMotataianu, Anca, Laura Barcutean, Zoltan Bajko, Adina Stoian, Smaranda Maier, Septimiu Voidazan, and Rodica Balasa. 2021. "Autonomic and Somatic Nerve Functions in Type 2 Diabetes Mellitus Patients: Electrophysiological Aspects" Diagnostics 11, no. 11: 2005. https://doi.org/10.3390/diagnostics11112005






