Mesonephric-like Adenocarcinoma of the Uterine Corpus: Comprehensive Immunohistochemical Analyses Using Markers for Mesonephric, Endometrioid and Serous Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. IHC
2.3. IHC Interpretation
2.4. FISH
2.5. Statistical Analysis
2.6. NGS
3. Results
3.1. Clinicopathological Characteristics
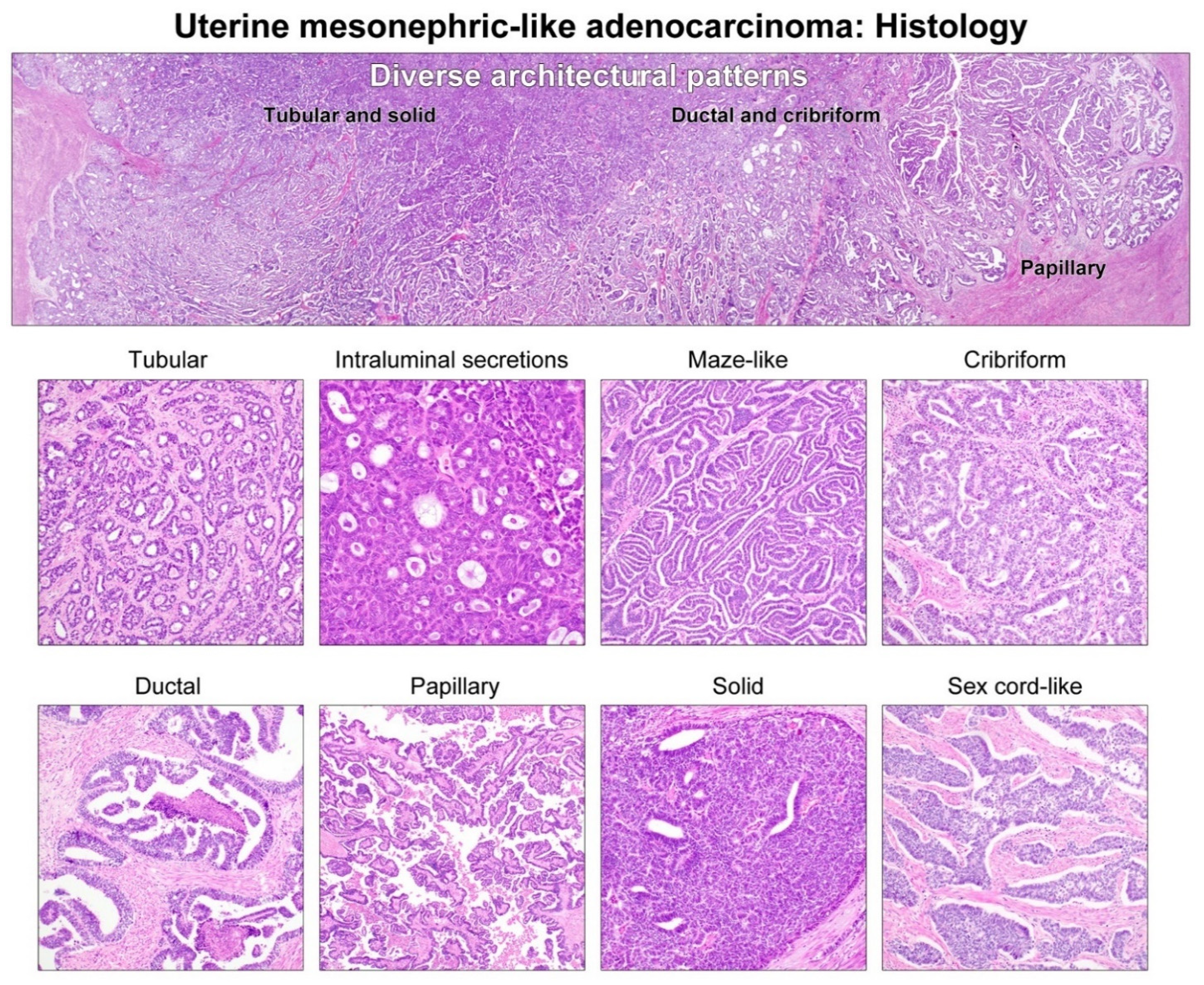
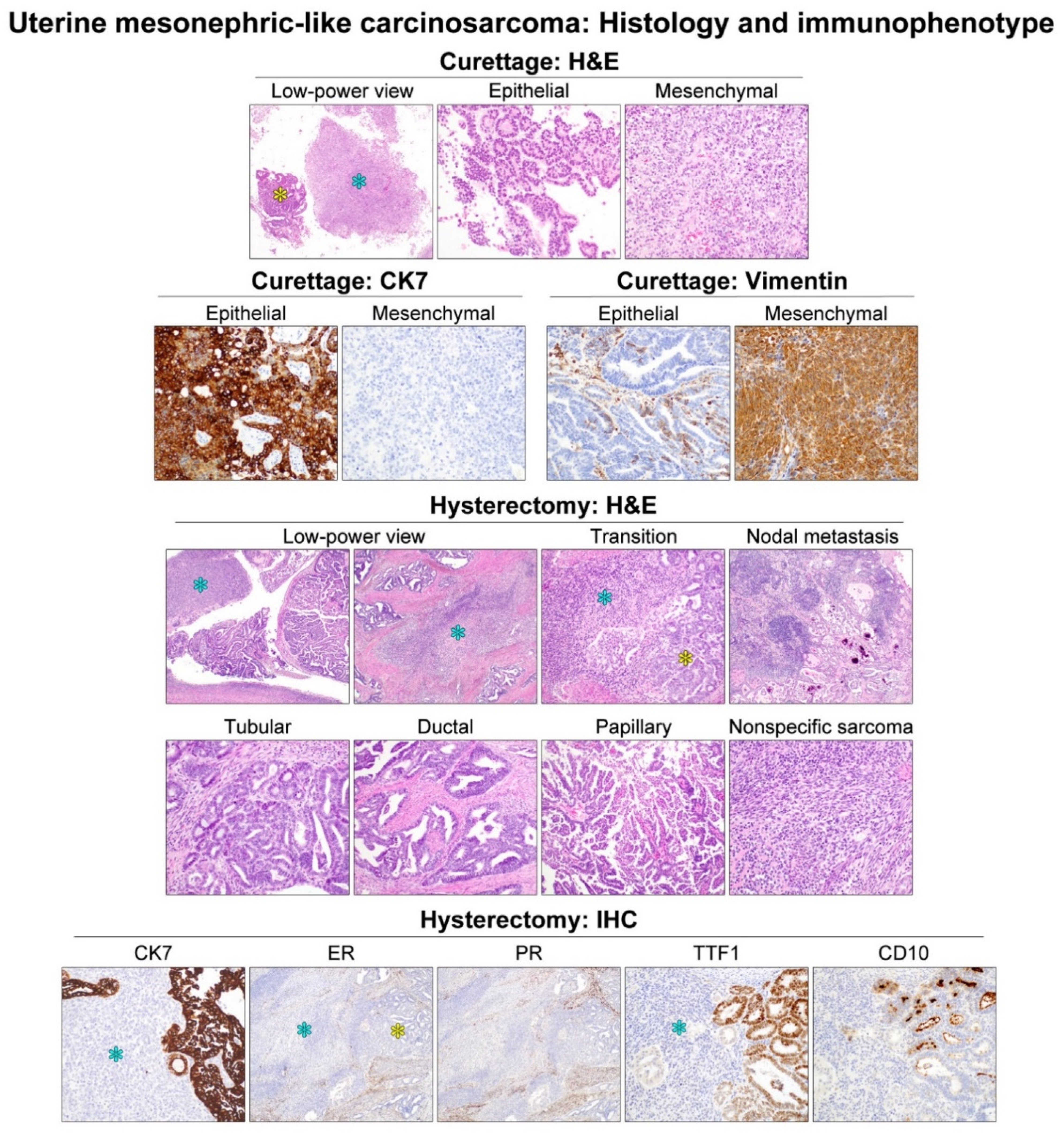
3.2. Histological Features and Immunophenotype of Mesonephric-like Carcinosarcoma (MLCS)
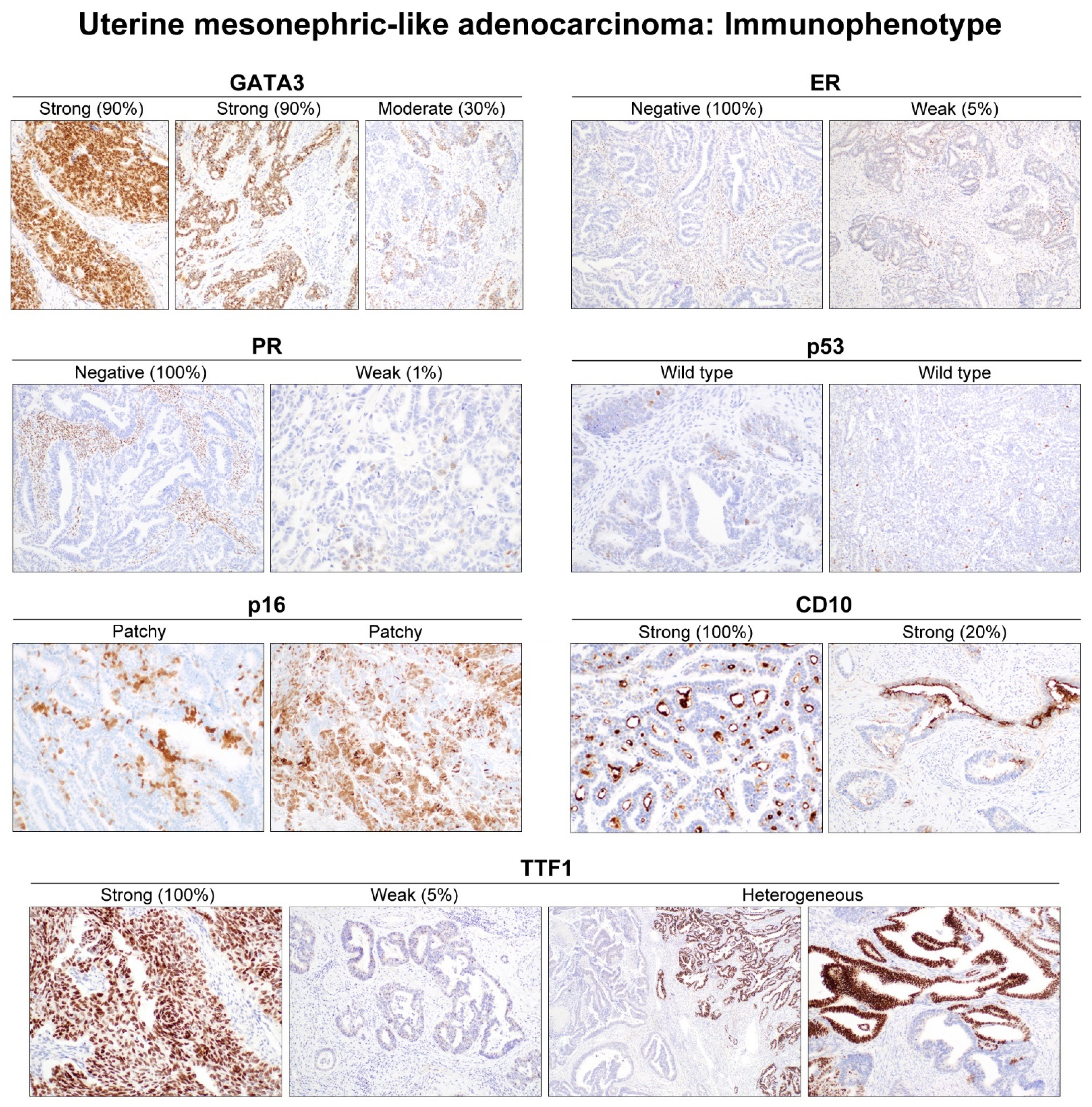
3.3. IHC Results
3.3.1. ER and PR
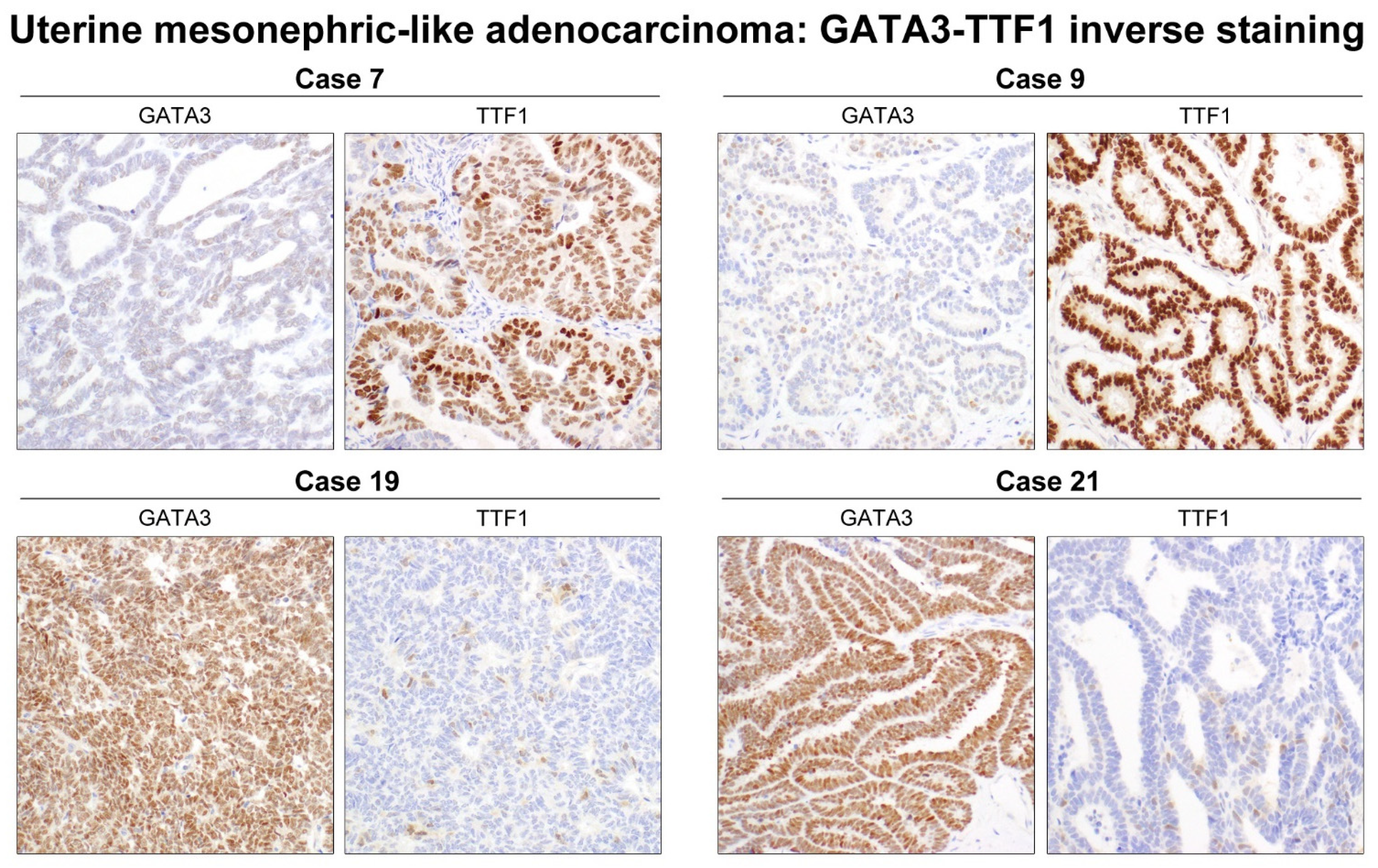
3.3.2. GATA3
3.3.3. p16 and p53
3.3.4. HER2
3.3.5. TTF1
3.3.6. CD10
3.4. NGS Results
3.5. Results of Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Na, K.; Kim, H.S. Clinicopathologic and molecular characteristics of mesonephric adenocarcinoma arising from the uterine body. Am. J. Surg. Pathol. 2019, 43, 12–25. [Google Scholar] [CrossRef]
- Pellegrino, B.; Mateo, J.; Serra, V.; Balmana, J. Controversies in oncology: Are genomic tests quantifying homologous recombination repair deficiency (HRD) useful for treatment decision making? ESMO Open 2019, 4, e000480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kezlarian, B.; Muller, S.; Werneck Krauss Silva, V.; Gonzalez, C.; Fix, D.J.; Park, K.J.; Murali, R. Cytologic features of upper gynecologic tract adenocarcinomas exhibiting mesonephric-like differentiation. Cancer Cytopathol. 2019, 127, 521–528. [Google Scholar] [CrossRef] [PubMed]
- McFarland, M.; Quick, C.M.; McCluggage, W.G. Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: Report of a series of mesonephric-like adenocarcinomas. Histopathology 2016, 68, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Euscher, E.D.; Bassett, R.; Duose, D.Y.; Lan, C.; Wistuba, I.; Ramondetta, L.; Ramalingam, P.; Malpica, A. Mesonephric-like carcinoma of the endometrium: A subset of endometrial carcinoma with an aggressive behavior. Am. J. Surg. Pathol. 2020, 44, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.C.; Hohn, A.K.; Krucken, I.; Stiller, M.; Obeck, U.; Brambs, C.E. Mesonephric-like adenocarcinomas of the uterine corpus: Report of a case series and review of the literature indicating poor prognosis for this subtype of endometrial adenocarcinoma. J. Cancer Res. Clin. Oncol. 2020, 146, 971–983. [Google Scholar] [CrossRef]
- Pors, J.; Segura, S.; Chiu, D.S.; Almadani, N.; Ren, H.; Fix, D.J.; Howitt, B.E.; Kolin, D.; McCluggage, W.G.; Mirkovic, J.; et al. Clinicopathologic Characteristics of Mesonephric Adenocarcinomas and Mesonephric-like Adenocarcinomas in the Gynecologic Tract. Am. J. Surg. Pathol. 2021, 45, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Jung, Y.Y.; Kim, H.S. Serous carcinoma of the endometrium with mesonephric-like differentiation initially misdiagnosed as uterine mesonephric-like adenocarcinoma: A case report with emphasis on the immunostaining and the identification of splice site TP53 mutation. Diagnostics 2021, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; NA, K.; Kim, S.-W.; Kim, H.-S. Dedifferentiated Mesonephric-like Adenocarcinoma of the Uterine Corpus. Anticancer Res. 2021, 41, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yoon, N.; Woo, H.Y.; Lee, E.-J.; Do, S.-I.; Na, K.; Kim, H.-S. Atypical Mesonephric Hyperplasia of the Uterus Harbors Pathogenic Mutation of Kirsten Rat Sarcoma 2 Viral Oncogene Homolog (KRAS) and Gain of Chromosome 1q. Cancer Genom. Proteom. 2020, 17, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Kolin, D.; Costigan, D.C.; Dong, F.; Nucci, M.R.; Howitt, B.E. A Combined Morphologic and Molecular Approach to Retrospectively Identify KRAS-Mutated Mesonephric-like Adenocarcinomas of the Endometrium. Am. J. Surg. Pathol. 2019, 43, 389–398. [Google Scholar] [CrossRef]
- Pors, J.; Cheng, A.; Leo, J.M.; Kinloch, M.; Gilks, B.; Hoang, L. A Comparison of GATA3, TTF1, CD10, and Calretinin in Identifying Mesonephric and Mesonephric-like Carcinomas of the Gynecologic Tract. Am. J. Surg. Pathol. 2018, 42, 1596–1606. [Google Scholar] [CrossRef]
- Mirkovic, J.; McFarland, M.; Garcia, E.; Sholl, L.M.; Lindeman, N.; MacConaill, L.; Dong, F.; Hirsch, M.; Nucci, M.R.; Quick, C.M.; et al. Targeted Genomic Profiling Reveals Recurrent KRAS Mutations in Mesonephric-like Adenocarcinomas of the Female Genital Tract. Am. J. Surg. Pathol. 2018, 42, 227–233. [Google Scholar] [CrossRef]
- Howitt, B.E.; Nucci, M.R. Mesonephric proliferations of the female genital tract. Pathology 2018, 50, 141–150. [Google Scholar] [CrossRef]
- Mirkovic, J.; Sholl, L.M.; Garcia, E.; I Lindeman, N.; E Macconaill, L.; Hirsch, M.S.; Cin, P.D.; Gorman, M.; A Barletta, J.; Nucci, M.R.; et al. Targeted genomic profiling reveals recurrent KRAS mutations and gain of chromosome 1q in mesonephric carcinomas of the female genital tract. Mod. Pathol. 2015, 28, 1504–1514. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, J.; Lee, S.K.; Cho, E.Y.; Cho, S.Y. TFE3-Expressing Perivascular Epithelioid Cell Tumor of the Breast. J. Pathol. Transl. Med. 2019, 53, 62–65. [Google Scholar] [CrossRef]
- Choi, S.; Park, S.; Chung, M.P.; Kim, T.S.; Cho, J.H.; Han, J. A Rare Case of Adenosquamous Carcinoma Arising in the Background of IgG4-Related Lung Disease. J. Pathol. Transl. Med. 2019, 53, 188–191. [Google Scholar] [CrossRef]
- Choi, S.; Cho, J.; Lee, S.E.; Baek, C.-H.; Kim, Y.-K.; Kim, H.-J.; Ko, Y.H. Adenocarcinoma of the minor salivary gland with concurrent MAML2 and EWSR1 alterations. J. Pathol. Transl. Med. 2021, 55, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Song, S.Y.; Kim, H.-S. Prominent Papillary Growth Pattern and Severe Nuclear Pleomorphism Induced by Neoadjuvant Chemotherapy in Ovarian Mucinous Carcinoma: Potential for Misdiagnosis as High-grade Serous Carcinoma. Anticancer Res. 2021, 41, 1579–1586. [Google Scholar] [CrossRef]
- Koh, H.H.; Jung, Y.Y.; Kim, H.-S. Clinicopathological Characteristics of Gastric-type Endocervical Adenocarcinoma Misdiagnosed as an Endometrial, Ovarian or Extragenital Malignancy, or Mistyped as Usual-type Endocervical Adenocarcinoma. Vivo 2021, 35, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S.; Woo, H.Y.; Kim, H.-S. Clinicopathological Characteristics of Microscopic Tubal Intraepithelial Metastases from Adenocarcinoma and Small Cell Neuroendocrine Carcinoma of the Uterine Cervix. Vivo 2021, 35, 2469–2481. [Google Scholar] [CrossRef]
- Kim, H.-S.; Do, S.-I.; Kim, D.-H.; Apple, S. Clinicopathological and Prognostic Significance of Programmed Death Ligand 1 Expression in Korean Patients With Triple-negative Breast Carcinoma. Anticancer Res. 2020, 40, 1487–1494. [Google Scholar] [CrossRef]
- Park, S.; Cho, E.Y.; Oh, Y.L.; Park, Y.H.; Kim, H.-S. Primary Peritoneal High-grade Serous Carcinoma Misinterpreted as Metastatic Breast Carcinoma: A Rare Encounter in Peritoneal Fluid Cytology. Anticancer Res. 2020, 40, 2933–2939. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Bae, G.; Kim, H.M.; Kim, H.-S. Clinicopathological and Molecular Differences Between Gastric-type Mucinous Carcinoma and Usual-type Endocervical Adenocarcinoma of the Uterine Cervix. Cancer Genom. Proteom. 2020, 17, 627–641. [Google Scholar] [CrossRef]
- Choi, S.; Joo, J.W.; Do, S.I.; Kim, H.S. Endometrium-limited metastasis of extragenital malignancies: A challenge in the diagnosis of endometrial curettage specimens. Diagnostics 2020, 10, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.K.; Kim, Y.-W.; Koh, H.H.; Yoon, N.; Bae, G.E.; Kim, H.-S. Clinicopathological Characteristics of Squamous Cell Carcinoma and High-grade Squamous Intraepithelial Lesions Involving Endocervical Polyps. Vivo 2020, 34, 2613–2621. [Google Scholar] [CrossRef]
- Jang, Y.; Jung, H.; Kim, H.-N.; Seo, Y.; Alsharif, E.; Nam, S.J.; Kim, S.W.; Lee, J.E.; Park, Y.H.; Cho, E.Y.; et al. Clinicopathologic characteristics of HER2-positive pure mucinous carcinoma of the breast. J. Pathol. Transl. Med. 2020, 54, 95–102. [Google Scholar] [CrossRef]
- Mccarty, K.S.; Szabo, E.; Flowers, J.L.; Cox, E.B.; Leight, G.S.; Miller, L.; Konrath, J.; Soper, J.T.; A Budwit, D.; Creasman, W.T. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res. 1986, 46, 4244s–4248s. [Google Scholar]
- Hirsch, F.R.; Varella-Garcia, M.; Bunn, P.A.; Di Maria, M.V.; Veve, R.; Bremnes, R.M.; Barón, A.E.; Zeng, C.; Franklin, W.A. Epidermal Growth Factor Receptor in Non–Small-Cell Lung Carcinomas: Correlation Between Gene Copy Number and Protein Expression and Impact on Prognosis. J. Clin. Oncol. 2003, 21, 3798–3807. [Google Scholar] [CrossRef]
- Kobel, M.; Ronnett, B.M.; Singh, N.; Soslow, R.A.; Gilks, C.B.; McCluggage, W.G. Interpretation of p53 immunohistochemistry in endometrial carcinomas: Toward increased reproducibility. Int. J. Gynecol. Pathol. 2019, 38, S123–S131. [Google Scholar] [CrossRef] [PubMed]
- Buza, N. HER2 Testing and Reporting in Endometrial Serous Carcinoma: Practical Recommendations for HER2 Immunohistochemistry and Fluorescent In Situ Hybridization: Proceedings of the ISGyP Companion Society Session at the 2020 USCAP Annual Meeting. Int. J. Gynecol. Pathol. 2021, 40, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Hong, M.; Van Vrancken, M.; Lyou, Y.J.; Kim, S.T.; Park, S.H.; Kang, W.K.; Park, Y.S.; Jung, S.-H.; Woo, M.; et al. A nCounter CNV Assay to Detect HER2 Amplification: A Correlation Study with Immunohistochemistry and In Situ Hybridization in Advanced Gastric Cancer. Mol. Diagn. Ther. 2016, 20, 375–383. [Google Scholar] [CrossRef]
- Hyeon, J.; Cho, S.Y.; Hong, M.E.; Kang, S.Y.; Do, I.; Im, Y.H.; Cho, E.Y. NanoString nCounter® Approach in Breast Cancer: A Comparative Analysis with Quantitative Real-Time Polymerase Chain Reaction, In Situ Hybridization, and Immunohistochemistry. J. Breast Cancer 2017, 20, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-W.; Park, B.J.; Kim, H.-S.; NA, K. Diagnostic Utility of Oncomine Comprehensive Assay v3 in Differentiating Between Isocitrate Dehydrogenase (IDH)-mutated Grade II-III Astrocytoma and Oligodendroglioma. Vivo 2021, 35, 921–927. [Google Scholar] [CrossRef]
- Da Silva, E.M.; Fix, D.J.; Sebastiao, A.P.M.; Selenica, P.; Ferrando, L.; Kim, S.H.; Stylianou, A.; Paula, A.D.C.; Pareja, F.; Smith, E.S.; et al. Mesonephric and mesonephric-like carcinomas of the female genital tract: Molecular characterization including cases with mixed histology and matched metastases. Mod. Pathol. 2021, 34, 1570–1587. [Google Scholar] [CrossRef] [PubMed]
- Deolet, E.; Van Dorpe, J.; Van de Vijver, K. Mesonephric-like Adenocarcinoma of the Endometrium: Diagnostic Advances to Spot This Wolf in Sheep’s Clothing. A Review of the Literature. J. Clin. Med. 2021, 10, 698. [Google Scholar] [CrossRef]
- Reid-Nicholson, M.; Iyengar, P.; Hummer, A.J.; Linkov, I.; Asher, M.; Soslow, R. Immunophenotypic diversity of endometrial adenocarcinomas: Implications for differential diagnosis. Mod. Pathol. 2006, 19, 1091–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, F.; Gao, Y.; Ding, J.; Chen, Q. Is the positivity of estrogen receptor or progesterone receptor different between type 1 and type 2 endometrial cancer? Oncotarget 2016, 8, 506–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenny, S.L.; McBride, H.A.; Jamison, J.; McCluggage, W.G. Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-beta. Am. J. Surg. Pathol. 2012, 36, 799–807. [Google Scholar] [CrossRef]
- Yamamoto, S.; Sakai, Y. Pulmonary metastasis of mesonephric-like adenocarcinoma arising from the uterine body: A striking mimic of follicular thyroid carcinoma. Histopathology 2019, 74, 651–653. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, Z.; Ambelil, M.; Conyers, J.; Zhu, H. Mesonephric adenocarcinoma of the uterine corpus: Report of 2 cases and review of the literature. Int. J. Gynecol. Pathol. 2019, 38, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Bae, G.E.; Kim, J.; Kim, H.S. Mesonephric-like differentiation of endometrial endometrioid carcinoma: Clinicopathological and molecular characteristics distinct from those of uterine mesonephric-like adenocarcinoma. Diagnostics 2021, 11, 1450. [Google Scholar] [CrossRef]
- Yano, M.; Shintani, D.; Katoh, T.; Hamada, M.; Ito, K.; Kozawa, E.; Hasegawa, K.; Yasuda, M. Coexistence of endometrial mesonephric-like adenocarcinoma and endometrioid carcinoma suggests a Mullerian duct lineage: A case report. Diagn. Pathol. 2019, 14, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yemelyanova, A.; Ji, H.; Shih Ie, M.; Wang, T.L.; Wu, L.S.; Ronnett, B.M. Utility of p16 expression for distinction of uterine serous carcinomas from endometrial endometrioid and endocervical adenocarcinomas: Immunohistochemical analysis of 201 cases. Am. J. Surg. Pathol. 2009, 33, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Buza, N. HER2 Testing in Endometrial Serous Carcinoma: Time for Standardized Pathology Practice to Meet the Clinical Demand. Arch. Pathol. Lab. Med. 2021, 145, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; McCue, P.A.; Sarlomo-Rikala, M.; Rys, J.; Czapiewski, P.; Wazny, K.; Langfort, R.; Waloszczyk, P.; Biernat, W.; Lasota, J.; et al. GATA3: A multispecific but potentially useful marker in surgical pathology: A systematic analysis of 2500 epithelial and nonepithelial tumors. Am. J. Surg. Pathol. 2014, 38, 13–22. [Google Scholar] [CrossRef]
- Vermij, L.; Smit, V.; Nout, R.; Bosse, T. Incorporation of molecular characteristics into endometrial cancer management. Histopathology 2020, 76, 52–63. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N.; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Santoro, A.; Angelico, G.; Travaglino, A.; Inzani, F.; Arciuolo, D.; Valente, M.; D’Alessandris, N.; Scaglione, G.; Fiorentino, V.; Raffone, A.; et al. New pathological and clinical insights in endometrial cancer in view of the updated ESGO/ESTRO/ESP Guidelines. Cancers 2021, 13, 2623. [Google Scholar] [CrossRef] [PubMed]
- D’Amati, A.; Pezzuto, F.; Serio, G.; Marzullo, A.; Fortarezza, F.; Lettini, T.; Cazzato, G.; Cormio, G.; Resta, L. Mesonephric-like carcinosarcoma of the ovary associated with low-grade serous carcinoma: A case report. Diagnostics 2021, 11, 827. [Google Scholar] [CrossRef]
- Ribeiro, B.; Silva, R.; Dias, R.; Patrício, V. Carcinosarcoma of the uterine cervix: A rare pathological finding originating from mesonephric remnants. BMJ Case Rep. 2019, 12, e227050. [Google Scholar] [CrossRef]
- Roma, A.A. Mesonephric carcinosarcoma involving uterine cervix and vagina: Report of 2 cases with immunohistochemical positivity For PAX2, PAX8, and GATA-3. Int. J. Gynecol. Pathol. 2014, 33, 624–629. [Google Scholar] [CrossRef]
- Meguro, S.; Yasuda, M.; Shimizu, M.; Kurosaki, A.; Fujiwara, K. Mesonephric adenocarcinoma with a sarcomatous component, a notable subtype of cervical carcinosarcoma: A case report and review of the literature. Diagn. Pathol. 2013, 8, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, Y.; Akagi, A.; Izumi, K.; Kishi, Y. Carcinosarcoma of the uterine body of mesonephric origin. Pathol. Int. 1995, 45, 303–309. [Google Scholar] [CrossRef] [PubMed]
- De Jong, R.A.; Nijman, H.W.; Wijbrandi, T.F.; Reyners, A.K.; Boezen, H.M.; Hollema, H. Molecular markers and clinical behavior of uterine carcinosarcomas: Focus on the epithelial tumor component. Mod. Pathol. 2011, 24, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, T.; Durieux, E.; Morel, A.P.; de Saint Hilaire, P.; Ray-Coquard, I.; Puisieux, A.; Devouassoux-Shisheboran, M. Role of epithelial-mesenchymal transition factors in the histogenesis of uterine carcinomas. Virchows Arch 2019, 475, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, M.; Fukagawa, D.; Sato, C.; Sugimoto, R.; Uesugi, N.; Ishida, K.; Itamochi, H.; Sugiyama, T.; Sugai, T. Immunohistochemical analysis of the epithelial to mesenchymal transition in uterine carcinosarcoma. Int. J. Gynecol. Cancer 2019, 29, 277–281. [Google Scholar] [CrossRef] [PubMed]

| Antibody | Clone | Company | Dilution |
|---|---|---|---|
| GATA3 | L50-823 | Ventana Medical Systems (Roche, Oro Valley, AZ, USA) | Prediluted |
| TTF1 | 8G7G3/1 | Dako (Agilent Technologies, Santa Clara, CA, USA) | 1:100 |
| CD10 | 56C6 | Novocastra (Leica Biosystems, Buffalo Grove, IL, USA) | 1:100 |
| ER | 6F11 | Novocastra (Leica Biosystems, Buffalo Grove, IL, USA) | 1:200 |
| PR | 16 | Novocastra (Leica Biosystems, Buffalo Grove, IL, USA) | 1:800 |
| p53 | DO-7 | Novocastra (Leica Biosystems, Buffalo Grove, IL, USA) | 1:200 |
| p16 | E6H4 | Ventana Medical Systems (Roche, Oro Valley, AZ, USA) | Prediluted |
| HER2 | 4B5 | Ventana Medical Systems (Roche, Oro Valley, AZ, USA) | Prediluted |
| Case No. | Age (Years) | Initial FIGO Stage | Recurrence | Lung Metastasis | DFS (Months) |
|---|---|---|---|---|---|
| 1 | 50 | IA | Yes | Yes | 53 |
| 2 | 62 | IA | Yes | Yes | 60 |
| 3 | 62 | IIIC2 | Yes | Yes | 33 |
| 4 | 53 | IIIA | Yes | No | 55 |
| 5 | 67 | IIIC2 | Yes | No | 12 |
| 6 | 47 | IVB | Yes | No | 5 |
| 7 | 59 | IIIC1 | NA (recent) | NA (recent) | NA (recent) |
| 8 | 48 | IIIC1 | Yes | No | 20 |
| 9 | 72 | IA | Yes | Yes | 22 |
| 10 | 58 | IIIB | No | No | 19 |
| 11 | 59 | IIIC1 | Yes | Yes | 15 |
| 12 | 52 | IIIB | No | No | 15 |
| 13 | 56 | IIIB | No | No | 12 |
| 14 | 66 | IB | No | No | 11 |
| 15 | 68 | IVB | Yes | Yes | 8 |
| 16 | 57 | IVB | Yes | No | 4 |
| 17 | 77 | IA | NA (recent) | NA (recent) | NA (recent) |
| 18 | 55 | II | Yes | Yes | 26 |
| 19 | 69 | IVB | No | Yes | 36 |
| 20 | 61 | IB | Yes | Yes | 5 |
| 21 | 62 | IVB | Yes | Yes | 16 |
| 22 | 43 | II | Yes | Yes | 39 |
| 23 | 46 | IIIC1 | Yes | Yes | 6 |
| 24 | 60 | IB | Yes | Yes | 15 |
| 25 | 61 | IVB | Yes | Yes | 13 |
| Case No. | GATA3 | ER | PR | p16 | p53 | TTF1 | CD10 | HER2 IHC | HER2 FISH | KRAS | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S (%) | M (%) | W (%) | N (%) | H | S (%) | M (%) | W (%) | N (%) | H | S (%) | M (%) | W (%) | N (%) | H | S (%) | M (%) | W (%) | N (%) | H | S (%) | M (%) | W (%) | N (%) | H | ||||||
| 1 | 0 | 40 | 30 | 30 | 110 | 0 | 0 | 0 | 100 | 0 | 10 | 30 | 0 | 60 | 90 | WT | P | 0 | 5 | 0 | 95 | 10 | 5 | 5 | 0 | 90 | 25 | 1+ | p.G12D | |
| 2 | 0 | 5 | 5 | 90 | 15 | 0 | 0 | 1 | 99 | 1 | 0 | 0 | 1 | 99 | 1 | WT | P | 0 | 5 | 0 | 95 | 10 | 0 | 0 | 0 | 100 | 0 | 0 | WT | |
| 3 | 5 | 5 | 0 | 90 | 25 | 10 | 20 | 10 | 60 | 80 | 0 | 0 | 0 | 100 | 0 | WT | P | 80 | 0 | 0 | 20 | 240 | 10 | 0 | 0 | 90 | 30 | 0 | p.G12D | |
| 4 | 80 | 10 | 0 | 10 | 260 | 10 | 20 | 20 | 50 | 90 | 0 | 0 | 0 | 100 | 0 | WT | P | 0 | 40 | 30 | 30 | 110 | 20 | 0 | 0 | 80 | 60 | 1+ | WT | |
| 5 | 80 | 10 | 0 | 10 | 260 | 0 | 10 | 10 | 80 | 30 | 0 | 0 | 0 | 100 | 0 | WT | N | 80 | 0 | 0 | 20 | 240 | 50 | 0 | 0 | 50 | 150 | 1+ | p.G12D | |
| 6 | 10 | 20 | 20 | 50 | 90 | 0 | 0 | 5 | 95 | 5 | 0 | 0 | 0 | 100 | 0 | WT | P | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0 | NA | |
| 7 | 0 | 5 | 5 | 90 | 15 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | WT | P | 30 | 20 | 0 | 50 | 130 | 5 | 0 | 0 | 95 | 15 | 0 | p.G12V | |
| 8 | 10 | 30 | 0 | 60 | 90 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | WT | N | 60 | 0 | 0 | 40 | 180 | 30 | 20 | 0 | 50 | 130 | 0 | p.G12D | |
| 9 | 0 | 5 | 0 | 95 | 10 | 0 | 1 | 4 | 95 | 6 | 0 | 0 | 0 | 100 | 0 | WT | P | 100 | 0 | 0 | 0 | 300 | 50 | 0 | 0 | 50 | 150 | 2+ | N | p.G12V |
| 10 | 15 | 60 | 10 | 15 | 175 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | WT | P | 50 | 0 | 0 | 50 | 150 | 30 | 20 | 0 | 50 | 130 | 0 | p.G12D | |
| 11 | 20 | 20 | 0 | 60 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | WT | P | 40 | 0 | 0 | 60 | 120 | 20 | 20 | 0 | 60 | 100 | 1+ | p.G12C | |
| 12 | 20 | 60 | 10 | 10 | 190 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | WT | P | 0 | 30 | 0 | 70 | 60 | 0 | 0 | 0 | 100 | 0 | 1+ | WT | |
| 13 | 1 | 2 | 2 | 95 | 9 | 0 | 0 | 1 | 99 | 1 | 0 | 0 | 0 | 100 | 0 | WT | P | 0 | 0 | 10 | 90 | 10 | 50 | 10 | 0 | 40 | 170 | 0 | WT | |
| 14 | 90 | 5 | 0 | 5 | 280 | 15 | 10 | 0 | 75 | 65 | 4 | 1 | 0 | 95 | 14 | WT | P | 5 | 0 | 0 | 95 | 15 | 0 | 0 | 0 | 100 | 0 | 2+ | N | WT |
| 15 | 10 | 20 | 50 | 20 | 120 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | WT | P | 100 | 0 | 0 | 0 | 300 | 100 | 0 | 0 | 0 | 300 | 1+ | p.G12D | |
| 16 | 80 | 5 | 0 | 15 | 250 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | WT | P | 0 | 25 | 0 | 75 | 40 | 60 | 10 | 0 | 50 | 200 | 1+ | p.G12V | |
| 17 | 10 | 15 | 15 | 60 | 75 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | WT | P | 100 | 0 | 0 | 0 | 300 | 50 | 20 | 0 | 30 | 190 | 1+ | p.G12V | |
| 18 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | WT | P | 0 | 0 | 10 | 90 | 10 | 90 | 0 | 0 | 10 | 270 | 2+ | N | p.G12V |
| 19 | 100 | 0 | 0 | 0 | 300 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | WT | P | 0 | 0 | 5 | 95 | 5 | 10 | 10 | 0 | 80 | 50 | 0 | p.G12D | |
| 20 | 0 | 5 | 5 | 90 | 15 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | WT | P | 20 | 20 | 0 | 60 | 100 | 5 | 0 | 0 | 95 | 15 | 2+ | N | p.G12V |
| 21 | 90 | 5 | 5 | 0 | 285 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | WT | P | 0 | 0 | 10 | 90 | 10 | 0 | 0 | 0 | 100 | 0 | 2+ | N | p.G13D |
| 22 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | WT | P | 0 | 5 | 0 | 95 | 10 | 20 | 10 | 0 | 70 | 80 | 0 | p.G12D | |
| 23 | 10 | 20 | 10 | 60 | 80 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | WT | P | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0 | NA | |
| 24 | 10 | 20 | 0 | 70 | 70 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | WT | P | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 1+ | NA | |
| 25 | 15 | 10 | 15 | 60 | 80 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | WT | P | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0 | NA | |
| Antibody | Intensity | Proportion (%) | Patients (%) |
|---|---|---|---|
| GATA3 | Moderate-to-strong | ≥75 | 8 (32.0) |
| Moderate-to-strong | 25–49 | 9 (36.0) | |
| Negative | ≥90 | 8 (32.0) | |
| ER | Moderate-to-strong | 25–49 | 3 (12.0) |
| Weak-to-moderate | 5–24 | 3 (12.0) | |
| Negative | ≥99 | 19 (76.0) | |
| PR | Moderate-to-strong | 5–49 | 2 (8.0) |
| Negative | ≥99 | 23 (88.0) | |
| p53 | Wild-type | 25 (100.0) | |
| p16 | Patchy | 23 (92.0) | |
| Negative | 2 (8.0) | ||
| TTF1 | Moderate-to-strong | ≥50 | 8 (32.0) |
| Moderate-to-strong | 25–49 | 5 (20.0) | |
| Negative | ≥90 | 8 (32.0) | |
| Not applicable | 4 (16.0) | ||
| CD10 | Moderate-to-strong | ≥50 | 9 (36.0) |
| Moderate-to-strong | 5–49 | 8 (32.0) | |
| Negative | ≥99 | 4 (20.0) | |
| Not applicable | 4 (16.0) | ||
| HER2 | Negative (0–1+) | 20 (80.0) | |
| Equivocal (2+) | 5 (20.0) | ||
| Parameter | Total (%) | GATA3 | ER | PR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H < 50 | H ≥50 | p Value | H < 5 | H ≥ 5 | p Value | H < 5 | H ≥ 5 | p Value | |||
| Initial FIGO stage | I | 7 (28.0) | 3 (42.9) | 4 (57.1) | 0.640 | 5 (71.4) | 2 (28.6) | 1.000 | 5 (71.4) | 2 (28.6) | 0.070 |
| II–IV | 18 (72.0) | 5 (27.8) | 13 (72.2) | 14 (77.8) | 4 (22.2) | 18 (100.0) | 0 (0.0) | ||||
| Recurrence | No | 5 (20.0) | 1 (20.0) | 4 (80.0) | 1.000 | 4 (80.0) | 1 (20.0) | 1.000 | 4 (80.0) | 1 (20.0) | 0.395 |
| Yes | 18 (72.0) | 6 (33.3) | 12 (66.7) | 13 (72.2) | 5 (27.8) | 17 (94.4) | 1 (5.6) | ||||
| NA | 2 (8.0) | ||||||||||
| Lung metastasis | No | 9 (36.0) | 1 (11.1) | 8 (88.9) | 0.176 | 5 (55.6) | 4 (44.4) | 0.162 | 8 (88.9) | 1 (11.1) | 1.000 |
| Yes | 14 (56.0) | 6 (42.9) | 8 (57.1) | 12 (85.7) | 2 (14.3) | 13 (92.9) | 1 (7.1) | ||||
| NA | 2 (8.0) | ||||||||||
| Mean DFS (months) | 31.6 | 22.8 | 0.237 | 24.8 | 26.3 | 0.972 | 23.4 | 53.0 | 0.384 | ||
| Parameter | Total (%) | TTF1 | CD10 | |||||
|---|---|---|---|---|---|---|---|---|
| H < 50 | H ≥ 50 | p Value | H < 50 | H ≥ 50 | p Value | |||
| Initial FIGO stage | I | 6 (28.6) | 3 (50.0) | 3 (50.0) | 1.000 | 4 (66.7) | 2 (33.3) | 0.146 |
| II–IV | 15 (71.4) | 6 (40.0) | 9 (60.0) | 4 (26.7) | 11 (73.3) | |||
| Recurrence | No | 5 (23.8) | 3 (60.0) | 2 (40.0) | 0.628 | 2 (40.0) | 3 (60.0) | 1.000 |
| Yes | 14 (66.7) | 6 (42.9) | 8 (57.1) | 5 (35.7) | 9 (64.3) | |||
| NA | 2 (9.5) | |||||||
| Lung metastasis | No | 8 (38.1) | 3 (37.5) | 5 (62.5) | 0.650 | 2 (25.0) | 6 (75.0) | 0.633 |
| Yes | 11 (52.4) | 6 (54.5) | 5 (45.5) | 5 (45.5) | 6 (54.5) | |||
| NA | 2 (9.5) | |||||||
| Mean DFS (months) | 34.5 | 23.5 | 0.233 | 35.4 | 25.1 | 0.297 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Na, K.; Bae, G.E.; Kim, H.-S. Mesonephric-like Adenocarcinoma of the Uterine Corpus: Comprehensive Immunohistochemical Analyses Using Markers for Mesonephric, Endometrioid and Serous Tumors. Diagnostics 2021, 11, 2042. https://doi.org/10.3390/diagnostics11112042
Kim H, Na K, Bae GE, Kim H-S. Mesonephric-like Adenocarcinoma of the Uterine Corpus: Comprehensive Immunohistochemical Analyses Using Markers for Mesonephric, Endometrioid and Serous Tumors. Diagnostics. 2021; 11(11):2042. https://doi.org/10.3390/diagnostics11112042
Chicago/Turabian StyleKim, Hyunjin, Kiyong Na, Go Eun Bae, and Hyun-Soo Kim. 2021. "Mesonephric-like Adenocarcinoma of the Uterine Corpus: Comprehensive Immunohistochemical Analyses Using Markers for Mesonephric, Endometrioid and Serous Tumors" Diagnostics 11, no. 11: 2042. https://doi.org/10.3390/diagnostics11112042
APA StyleKim, H., Na, K., Bae, G. E., & Kim, H.-S. (2021). Mesonephric-like Adenocarcinoma of the Uterine Corpus: Comprehensive Immunohistochemical Analyses Using Markers for Mesonephric, Endometrioid and Serous Tumors. Diagnostics, 11(11), 2042. https://doi.org/10.3390/diagnostics11112042






