Prominent Asymmetric Muscle Weakness and Atrophy in Seronegative Immune-Mediated Necrotizing Myopathy
Abstract
:1. Introduction
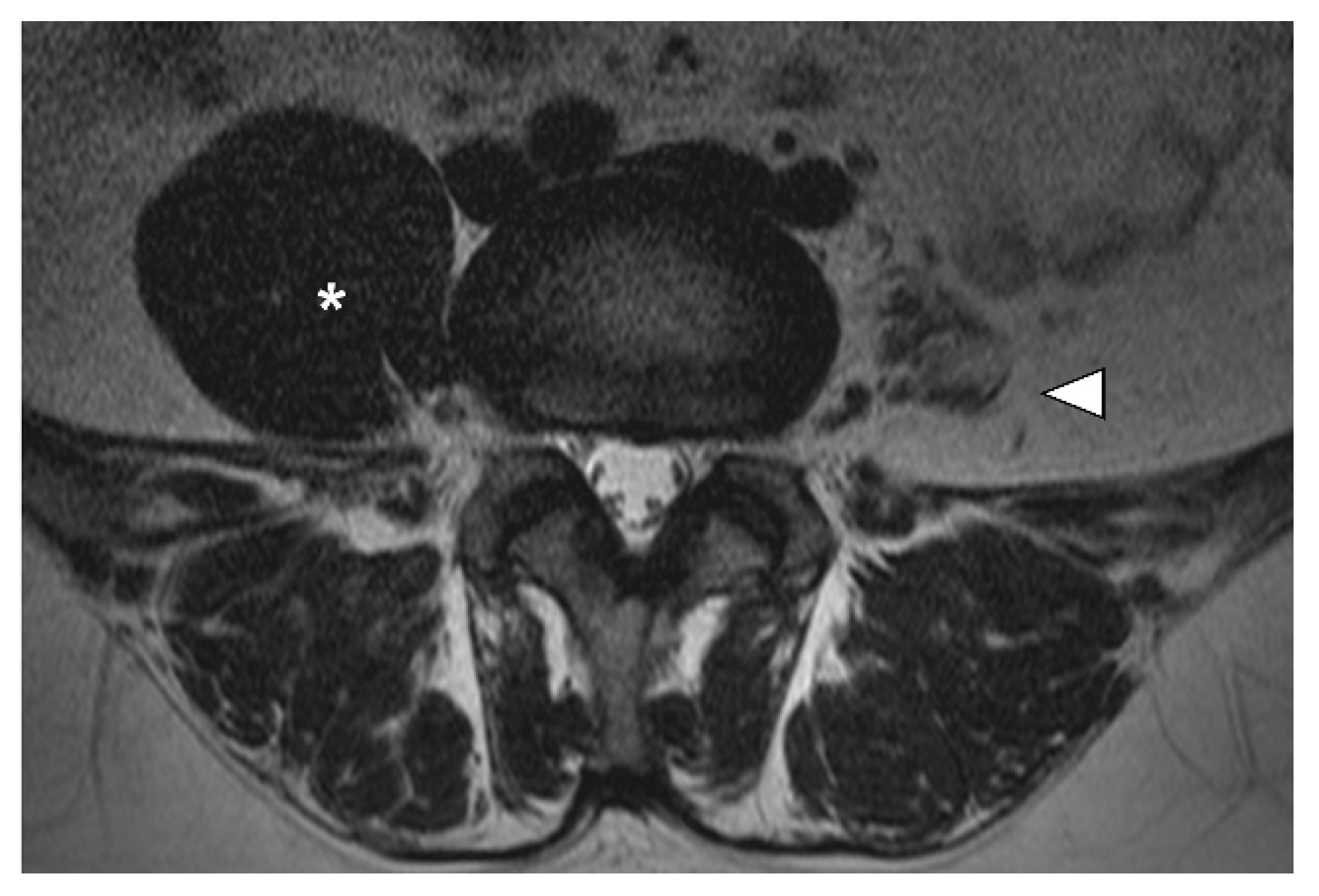
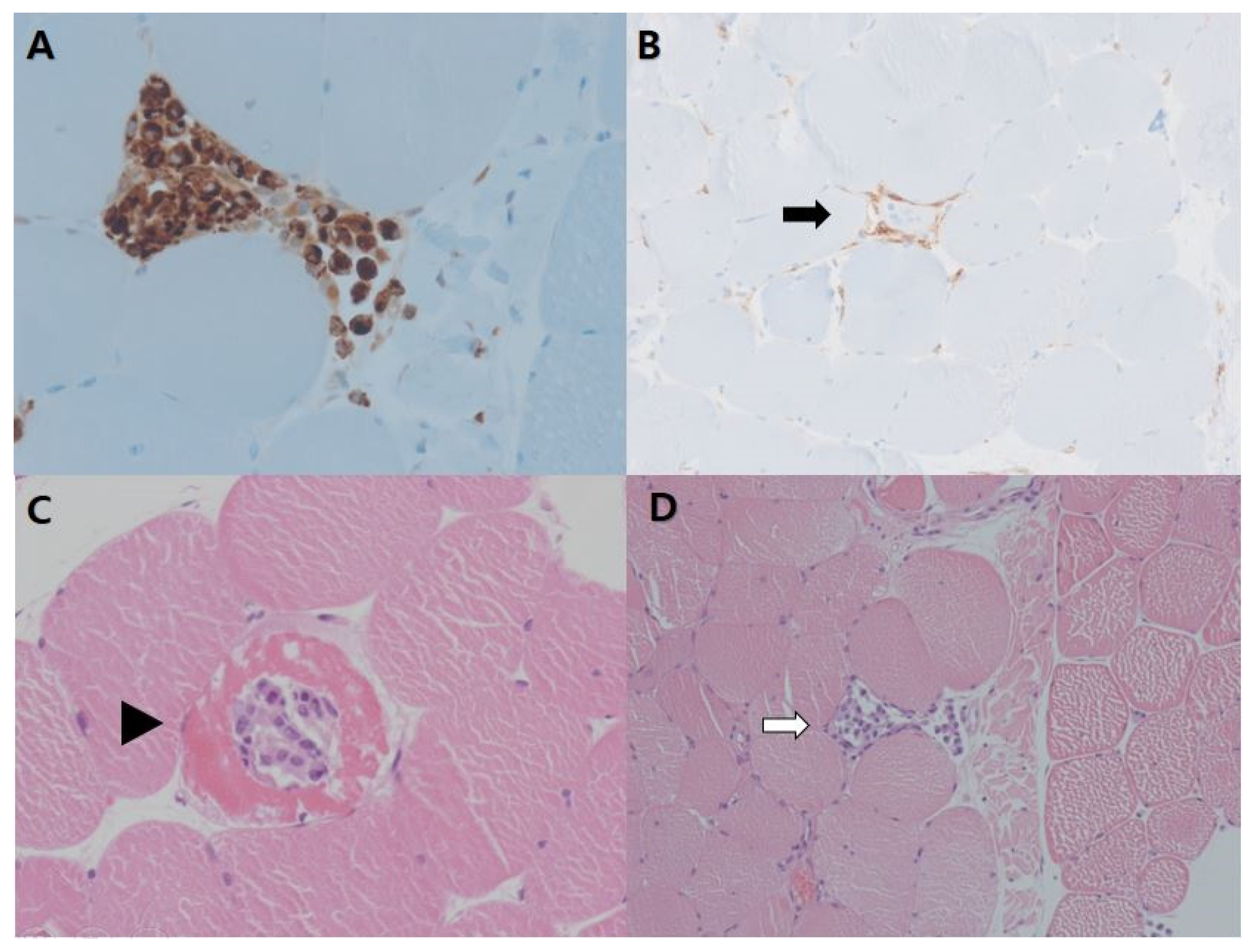
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allenbach, Y.; Benveniste, O.; Stenzel, W.; Boyer, O. Immune-mediated necrotizing myopathy: Clinical features and pathogenesis. Nat. Rev. Rheumatol. 2020, 16, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Allenbach, Y.; Mammen, A.L.; Benveniste, O.; Stenzel, W. 224th ENMC International Workshop: Clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14–16 October 2016. Neuromuscul. Disord. 2018, 28, 87–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selva-O’Callaghan, A.; Pinal-Fernandez, I.; Trallero-Araguás, E.; Milisenda, J.C.; Grau-Junyent, J.M.; Mammen, A.L. Classification and management of adult inflammatory myopathies. Lancet Neurol. 2018, 17, 816–828. [Google Scholar] [CrossRef]
- Lim, J.; Rietveld, A.; De Bleecker, J.L.; Badrising, U.A.; Saris, C.G.J.; van der Kooi, A.J.; de Visser, M. Seronegative patients form a distinctive subgroup of immune-mediated necrotizing myopathy. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Oh, H.M.; Park, H.J.; Cho, A.R.; Lee, D.W.; Jang, J.H.; Jang, D.H. Usefulness of comprehensive targeted multigene panel sequencing for neuromuscular disorders in Korean patients. Mol. Genet. Genom. Med. 2019, 7, e00947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Needham, M. Necrotizing autoimmune myopathy. Curr. Opin. Rheumatol. 2011, 23, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hou, Y.; Dai, T.J.; Lv, J.W.; Li, W.; Zhao, Y.Y.; Fang, Q.; Yan, C.Z. The clinical and histopathological features of idiopathic inflammatory myopathies with asymmetric involvement. J. Clin. Neurosci. 2019, 65, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Allenbach, Y.; Keraen, J.; Bouvier, A.M.; Jooste, V.; Champtiaux, N.; Hervier, B.; Schoindre, Y.; Rigolet, A.; Gilardin, L.; Musset, L.; et al. High risk of cancer in autoimmune necrotizing myopathies: Usefulness of myositis specific antibody. Brain 2016, 139, 2131–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, R.; Yeoh, S.A.; Berkeley, R.; Woods, A.; Stevens, M.; Marino, S.; Radunovic, A. Initial seronegative immune-mediated necrotising myopathy with subsequent anti-HMGCR antibody development and response to rituximab: Case report. BMC Rheumatol. 2020, 4, 29. [Google Scholar] [CrossRef] [PubMed]

| Spontaneous Activities | Motor Unit Action Potential | Recruitment | |||||
|---|---|---|---|---|---|---|---|
| IA | Fib | PSW | Polyphasic | Amplitude | Duration | ||
| R. L-PSP (L5) | N | 1+ | 1+ | ||||
| R. L-PSP (S1) | Decr | None | None | ||||
| L. L-PSP (L5) | N | 1+ | 1+ | ||||
| R. Biceps | N | None | None | N | N | N | N |
| R. FCR | N | None | None | N | N | N | N |
| R. FDI | N | None | None | N | N | N | N |
| R. G-max | N | None | None | N | N | N | N |
| R. Iliopsoas | N | None | None | N | Small | Short | N |
| R. V-med | N | None | None | N | N | N | N |
| R. TA | N | None | None | N | N | N | N |
| L. G-max | Decr | None | None | N | N | N | N |
| L. Iliopsoas | Decr | None | None | N | Small | Short | N |
| L. TFL | Decr | None | None | N | N | N | N |
| L. RF | Decr | 2+ | 2+ | N | Small | Short | Early |
| L. V-med | Decr | None | None | N | Small | Short | Early |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Jang, D.-H.; Kim, J.-M.; Yoon, N. Prominent Asymmetric Muscle Weakness and Atrophy in Seronegative Immune-Mediated Necrotizing Myopathy. Diagnostics 2021, 11, 2064. https://doi.org/10.3390/diagnostics11112064
Park S, Jang D-H, Kim J-M, Yoon N. Prominent Asymmetric Muscle Weakness and Atrophy in Seronegative Immune-Mediated Necrotizing Myopathy. Diagnostics. 2021; 11(11):2064. https://doi.org/10.3390/diagnostics11112064
Chicago/Turabian StylePark, Sunha, Dae-Hyun Jang, Jae-Min Kim, and Nara Yoon. 2021. "Prominent Asymmetric Muscle Weakness and Atrophy in Seronegative Immune-Mediated Necrotizing Myopathy" Diagnostics 11, no. 11: 2064. https://doi.org/10.3390/diagnostics11112064
APA StylePark, S., Jang, D.-H., Kim, J.-M., & Yoon, N. (2021). Prominent Asymmetric Muscle Weakness and Atrophy in Seronegative Immune-Mediated Necrotizing Myopathy. Diagnostics, 11(11), 2064. https://doi.org/10.3390/diagnostics11112064







