Real-Time PCR Assay for Rapid and Simultaneous Detection of vanA and vanB Genes in Clinical Strains
Abstract
1. Introduction
2. Materials and Methods
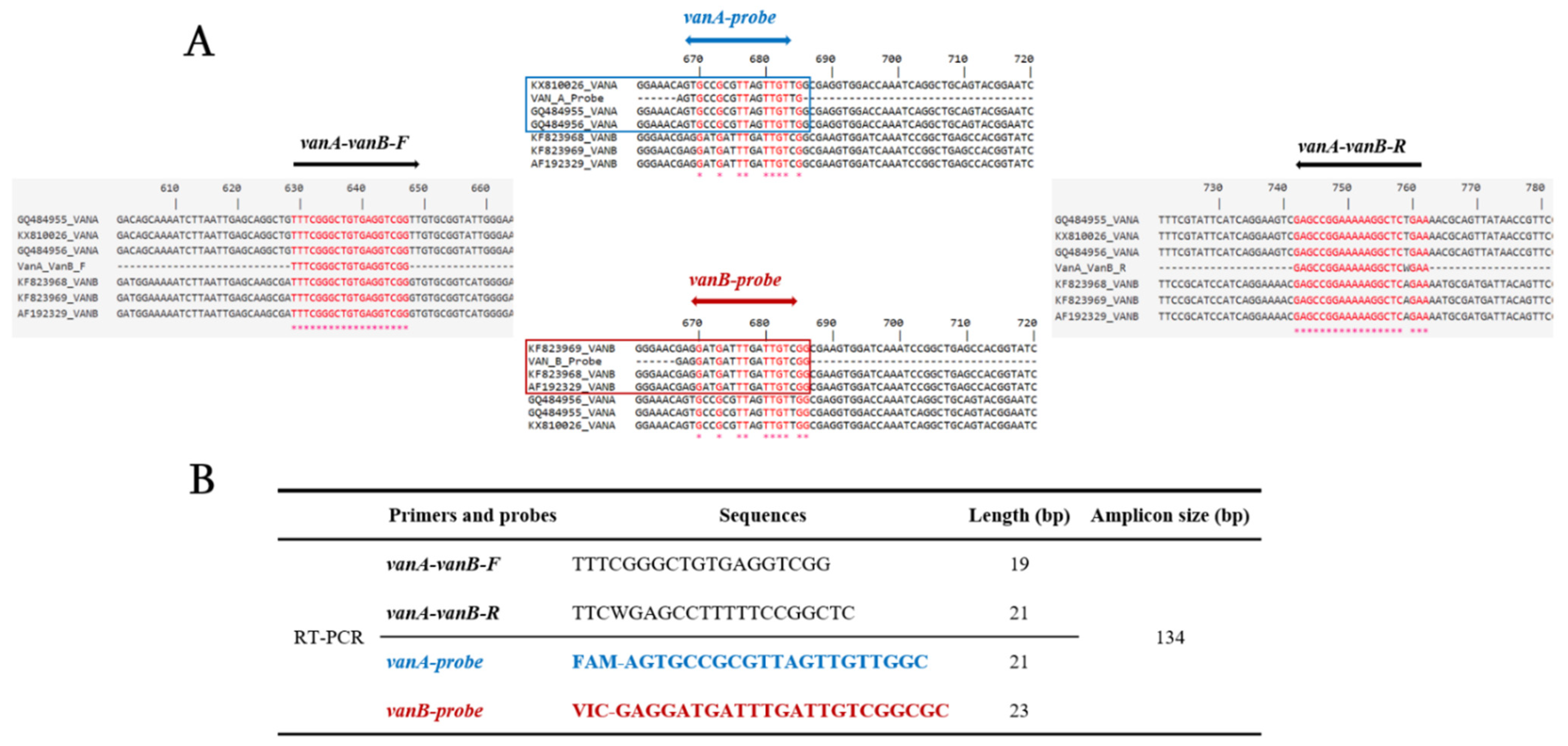
2.1. Design of Primers and Probes
2.2. DNA Extraction
2.3. RT-PCR Assay
3. Results
3.1. Design of Primers and Probes
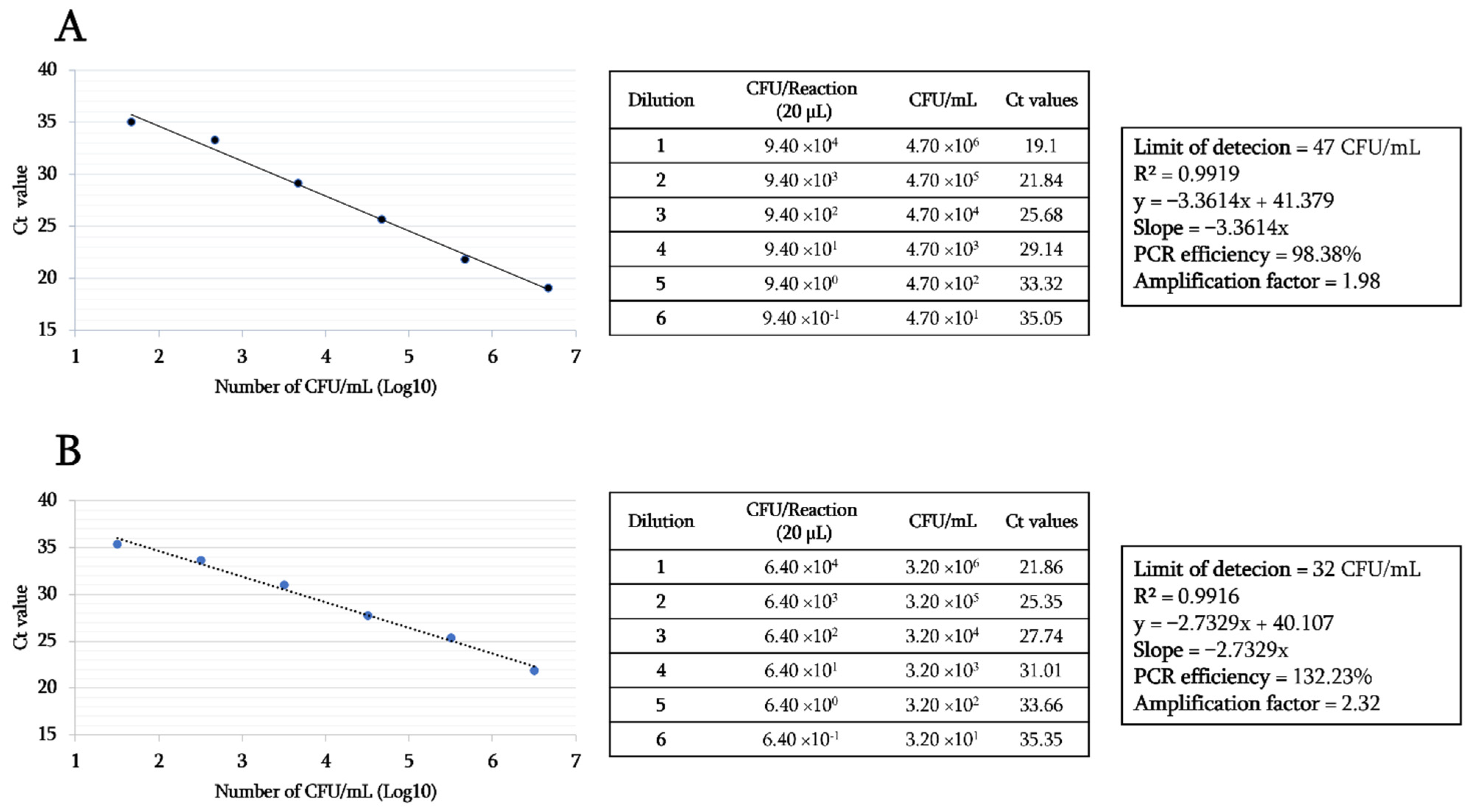
3.2. Specificity and Sensitivity Tests of the Designed RT-PCR Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faron, M.L.; Ledeboer, N.A.; Buchan, B.W. Resistance mechanisms, epidemiology, and approaches to screening for vancomycin-resistant Enterococcus in the health care setting. J. Clin. Microbiol. 2016, 54, 2436–2447. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, H. Controlling the spread of vancomycin-resistant enterococci. Is active screening worthwhile? J. Hosp. Infect. 2014, 88, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Reyes, K.; Bardossy, A.C.; Zervos, M. Vancomycin-Resistant Enterococci: Epidemiology, Infection Prevention, and Control. Infect. Dis. Clin. N. Am. 2016, 30, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.L.; Kos, V.N.; Gilmore, M.S. Horizontal gene transfer and the genomics of enterococcal antibiotic resistance. Curr. Opin. Microbiol. 2010, 13, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Guzman Prieto, A.M.; van Schaik, W.; Rogers, M.R.C.; Coque, T.M.; Baquero, F.; Corander, J.; Willems, R.J.L. Global emergence and dissemination of enterococci as nosocomial pathogens: Attack of the clones? Front. Microbiol. 2016, 7, 788. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.R.; Tedim, A.P.; Francia, M.V.; Jensen, L.B.; Novais, C.; Peixe, L.; Sánchez-Valenzuela, A.; Sundsfjord, A.; Hegstad, K.; Werner, G.; et al. Multilevel population genetic analysis of vanA and vanB Enterococcus faecium causing nosocomial outbreaks in 27 countries (1986–2012). J. Antimicrob. Chemother. 2016, 71, 3351–3366. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.; Ballard, S.; Sullivan, S.; Marshall, C. An outbreak of vanA vancomycin-resistant Enterococcus faecium in a hospital with endemic vanB VRE. Infect. Dis. Health 2019, 24, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Rangberg, A.; Larsen, A.L.; Kacelnik, O.; Sæther, H.S.; Bjørland, M.; Ringstad, J.; Jonassen, C.M. Molecular analysis and epidemiological typing of Vancomycin-resistant Enterococcus outbreak strains. Sci. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- He, Y.H.; Ruan, G.J.; Hao, H.; Xue, F.; Ma, Y.K.; Zhu, S.N.; Zheng, B. Real-time PCR for the rapid detection of vanA, vanB and vanM genes. J. Microbiol. Immunol. Infect. 2019, 9, 11917. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.F.; Soumakis, S.A.; Rendo, A.; Herrington, J.A.; Gianarkis, D.G.; Thurberg, B.E.; Painter, B.G. Epidemiologic analysis and genotypic characterization of a nosocomial outbreak of vancomycin-resistant enterococci. J. Clin. Microbiol. 1993, 31, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Palladino, S.; Kay, I.D.; Flexman, J.P.; Boehm, I.; Costa, A.M.G.; Lambert, E.J.; Christiansen, K.J. Rapid detection of vanA and vanB genes directly from clinical specimens and enrichment broths by real-time multiplex PCR assay. J. Clin. Microbiol. 2003, 41, 2483–2486. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frakking, F.N.J.; Bril, W.S.; Sinnige, J.C.; van’t Klooster, J.E.; de Jong, B.A.W.; van Hannen, E.J.; Tersmette, M. Recommendations for the successful control of a large outbreak of vancomycin-resistant Enterococcus faecium in a non-endemic hospital setting. J. Hosp. Infect. 2018, 100, e216–e225. [Google Scholar] [CrossRef] [PubMed]
- Sloan, L.M.; Uhl, J.R.; Vetter, E.A.; Schleck, C.D.; Harmsen, W.S.; Manahan, J.; Thompson, R.L.; Rosenblatt, J.E.; Cockerill, F.R. Comparison of the Roche LightCycler vanA/vanB detection assay and culture for detection of vancomycin-resistant enterococci from perianal swabs. J. Clin. Microbiol. 2004, 42, 2636–2643. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Domingo, M.C.; Huletsky, A.; Bernal, A.; Giroux, R.; Boudreau, D.K.; Picard, F.J.; Bergeron, M.G. Characterization of a Tn5382-like transposon containing the vanB2 gene cluster in a Clostridium strain isolated from human faeces. J. Antimicrob. Chemother. 2005, 55, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Cekin, Y.; Daloǧlu, A.E.; Öǧünç, D.; Baysan, B.Ö.; Daǧlar, D.; Inan, D.; Mutlu, D.; Öngüt, G.; Çolak, D. Evaluation of vancomycin resistance 3 multiplexed pcr assay for detection of vancomycin-resistant enterococci from rectal swabs. Ann. Lab. Med. 2013, 33, 326–330. [Google Scholar] [CrossRef]
- Shanmugakani, R.K.; Fujiya, Y.; Akeda, Y.; Hamaguchi, S.; Hamada, S.; Tomono, K. Rapid multiplex detection of the resistance genes mecA, vanA and vanB from Gram-positive cocci-positive blood cultures using a PCR-dipstick technique. J. Med. Microbiol. 2020, 69, 249–255. [Google Scholar] [CrossRef]
| Type of Bacteria | Bacterial Species | Number of Strains | Origin | Probe | |
|---|---|---|---|---|---|
| vanA | vanB | ||||
| Gram-positive bacteria | Staphylococcus aureus | 1 | Marseille | − | − |
| Staphylococcus epidermidis | 1 | Marseille | − | − | |
| Streptococcus agalactiae | 1 | Marseille | − | − | |
| Streptococcus pneumoniae | 1 | Marseille | − | − | |
| Staphylococcus haemolyticus | 1 | Marseille | − | − | |
| Staphylococcus capitis | 1 | Marseille | − | − | |
| Staphylococcus lugdunensis | 1 | Marseille | − | − | |
| Streptococcus mitis | 1 | Marseille | − | − | |
| Staphylococcus warneri | 1 | Marseille | − | − | |
| Corynebacterium striatum | 1 | Marseille | − | − | |
| Staphylococcus saprophyticus | 1 | Marseille | − | − | |
| Corynebacterium jeikeium | 1 | Marseille | − | − | |
| Staphylococcus simulans | 1 | Marseille | − | − | |
| Staphylococcus pasteuri | 1 | Marseille | − | − | |
| Corynebacterium amycolatum | 1 | Marseille | − | − | |
| Bacillus cereus | 1 | Marseille | − | − | |
| Staphylococcus cohnii | 1 | Marseille | − | − | |
| Streptococcus salivarius | 1 | Marseille | − | − | |
| Streptococcus equinus | 1 | Marseille | − | − | |
| Corynebacterium propinquum | 1 | Marseille | − | − | |
| Micrococcus luteus | 1 | Marseille | − | − | |
| Streptococcus dysgalactiae | 1 | Marseille | − | − | |
| Staphylococcus hominis | 1 | Marseille | − | − | |
| C. perfringens | 1 | * CSUR (P6929) | − | − | |
| C. butyricum | 1 | * CSUR (P0102) | − | − | |
| P. sordellii | 1 | * DSMZ 2141 | − | − | |
| C. sporogenes | 1 | * CSUR (P6393) | − | − | |
| C. septicum | 1 | * CSUR (P1044) | − | − | |
| C. difficile | 5 | Marseille | − | − | |
| Total of Strains = 33 | |||||
| Gram-negative bacteria | Proteus mirabilis | 1 | Marseille | − | − |
| Citrobacter freundii | 1 | Marseille | − | − | |
| Achromobacter xylosoxidans | 1 | Marseille | − | − | |
| Enterobacter cloacae | 1 | Marseille | − | − | |
| Bacteroides fragilis | 1 | Marseille | − | − | |
| Moraxella catarrhalis | 1 | Marseille | − | − | |
| Proteus vulgaris | 1 | Marseille | − | − | |
| Providencia stuartii | 1 | Marseille | − | − | |
| Haemophilus parainfluenzae | 1 | Marseille | − | − | |
| Klebsiella pneumonia | 1 | Marseille | − | − | |
| Pseudomonas aeruginosa | 1 | Marseille | − | − | |
| Enterobacter kobei | 1 | Marseille | − | − | |
| Enterobacter asburiae | 1 | Marseille | − | − | |
| Hafnia alvei | 1 | Marseille | − | − | |
| Raoultella ornithinolytica | 1 | Marseille | − | − | |
| Citrobacter braakii | 1 | Marseille | − | − | |
| Escherichia coli | 1 | Marseille | − | − | |
| Pasteurella multocida | 1 | Marseille | − | − | |
| Stenotrophomonas maltophilia | 1 | Marseille | − | − | |
| Morganella morganii | 1 | Marseille | − | − | |
| Citrobacter koseri | 1 | Marseille | − | − | |
| Enterobacter aerogenes | 1 | Marseille | − | − | |
| Haemophilus influenzae | 1 | Marseille | − | − | |
| Klebsiella oxytoca | 1 | Marseille | − | − | |
| Acinetobacter baumannii | 2 | Marseille | − | − | |
| Total of Strains = 26 | |||||
| Vancomycin-resistant Enterococci (VRE) | E. avium (vanA) | 2 | Tlemcen | + | − |
| E. casseliflavus (vanA/vanC2) | 1 | Tlemcen | + | − | |
| E. faecalis (vanA) | 5 | Tlemcen | + | − | |
| E. faecalis (vanB) | 5 | Tlemcen | − | + | |
| E. faecium (vanA) | 40 | Tlemcen | + | − | |
| E. faecium (vanA/vanC1) | 6 | Tlemcen | + | − | |
| E. gallinarum (vanA/vanC1) | 16 | Tlemcen | + | − | |
| Vancomycin-susceptible Enterococci (VSE) | E. avium | 2 | Tlemcen | − | − |
| E. casseliflavus | 1 | Tlemcen | − | − | |
| E. casseliflavus (vanC2) | 10 | Tlemcen | − | − | |
| E. faecalis (vanC1) | 5 | Tlemcen | − | − | |
| E. faecalis | 50 | Tlemcen | − | − | |
| E. faecium | 27 | Tlemcen | − | − | |
| E. otavius | 1 | Tlemcen | − | − | |
| E. gallinarum | 2 | Tlemcen | − | − | |
| E. gallinarum (vanC1) | 10 | Tlemcen | − | − | |
| E. hirae | 8 | Tlemcen | − | − | |
| Total of Strains = 191 | |||||
| Reference strains (van genes) | E. faecium (vanA) | 1 | DSM 17050 | + | − |
| E. faecium (vanA) | 1 | DSM 13590 | + | − | |
| E. faecium (vanA) | 1 | DSM 25698 | + | − | |
| E. faecium (vanA) | 1 | DSM 25697 | + | − | |
| E. faecalis (vanB) | 1 | DSM 12956 | − | + | |
| Total of Strains = 5 | |||||
| Human samples | Stools | 50 | Marseille | − | − |
| Rectal sawbs | 50 | Tlemcen | − | − | |
| Total of Samples = 100 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerrouki, H.; Rebiahi, S.-A.; Hadjadj, L.; Rolain, J.-M.; Diene, S.M. Real-Time PCR Assay for Rapid and Simultaneous Detection of vanA and vanB Genes in Clinical Strains. Diagnostics 2021, 11, 2081. https://doi.org/10.3390/diagnostics11112081
Zerrouki H, Rebiahi S-A, Hadjadj L, Rolain J-M, Diene SM. Real-Time PCR Assay for Rapid and Simultaneous Detection of vanA and vanB Genes in Clinical Strains. Diagnostics. 2021; 11(11):2081. https://doi.org/10.3390/diagnostics11112081
Chicago/Turabian StyleZerrouki, Hanane, Sid-Ahmed Rebiahi, Linda Hadjadj, Jean-Marc Rolain, and Seydina M. Diene. 2021. "Real-Time PCR Assay for Rapid and Simultaneous Detection of vanA and vanB Genes in Clinical Strains" Diagnostics 11, no. 11: 2081. https://doi.org/10.3390/diagnostics11112081
APA StyleZerrouki, H., Rebiahi, S.-A., Hadjadj, L., Rolain, J.-M., & Diene, S. M. (2021). Real-Time PCR Assay for Rapid and Simultaneous Detection of vanA and vanB Genes in Clinical Strains. Diagnostics, 11(11), 2081. https://doi.org/10.3390/diagnostics11112081






