Current Insights: The Impact of Gut Microbiota on Postoperative Complications in Visceral Surgery—A Narrative Review
Abstract
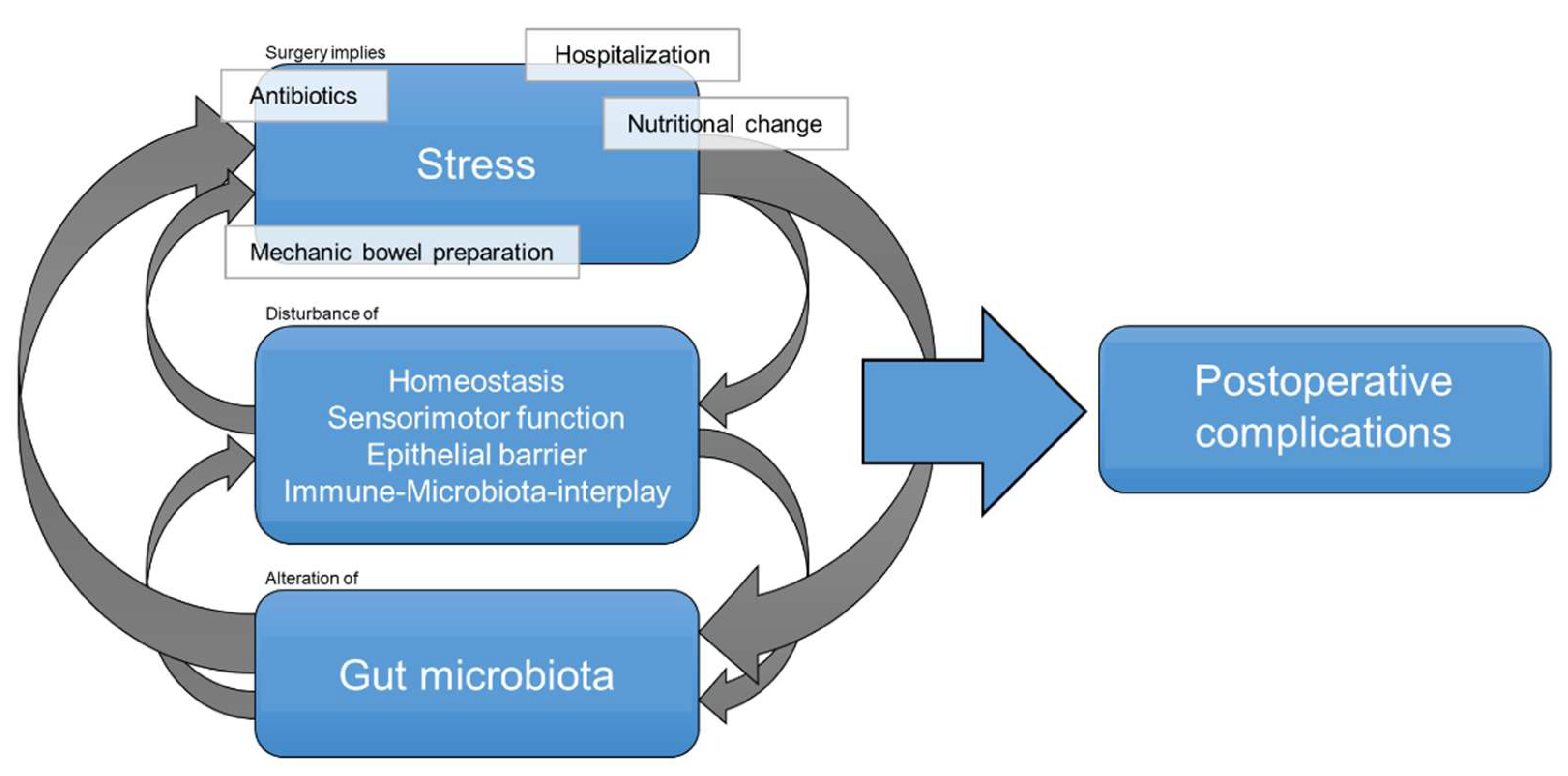
:1. Introduction
2. Methods
Research Questions
- (1)
- Does surgery alter the gut microbiota composition of a patient and if so, in which kind of extent?
- (2)
- Is the occurrence of an anastomotic leakage related to the composition of a patient’s gut microbiota?
- (3)
- Is the occurrence of a surgical site infection related to the composition of a patient’s gut microbiota?
3. Results
3.1. Research Question 1: Changes of Gut Microbiota in Surgical Patients
3.2. Research Question 2: The Relation between the Gut Microbiota and the Development of Anastomotic Leakage
3.2.1. Decades Ago: Oral Decontamination to Prevent Anastomotic Leakage
3.2.2. Bacteria Being Potentially Responsible for Development of Anastomotic Leakage
3.3. Research Question 3: The Relation between the Gut Microbiota and the Development of Surgical Site Infections
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Trencheva, K.; Morrissey, K.; Wells, M.; Mancuso, C.A.; Lee, S.W.; Sonoda, T.; Michelassi, F.; Charlson, M.E.; Milsom, J.W. Identifying Important Predictors for Anastomotic Leak After Colon and Rectal Resection Prospective Study on 616 Patients. Ann. Surg. 2013, 257, 108–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, P.Y.; Khadaroo, R.G. Surgical Site Infections. Surg. Clin. N. Am. 2014, 94, 1245–1264. [Google Scholar] [CrossRef] [PubMed]
- Boev, C.; Kiss, E. Hospital-Acquired Infections. Crit. Care Nurs. Clin. N. Am. 2017, 29, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, H.; Lv, P.; Peng, X.; Wu, C.; Ren, J.; Wang, P. Prospective multicenter study on the incidence of surgical site infection after emergency abdominal surgery in China. Sci. Rep. 2021, 11, 7794. [Google Scholar] [CrossRef] [PubMed]
- Jenks, P.J.; Laurent, M.; McQuarry, S.; Watkins, R. Clinical and economic burden of surgical site infection (SSI) and predicted financial consequences of elimination of SSI from an English hospital. J. Hosp. Infect. 2014, 86, 24–33. [Google Scholar] [CrossRef]
- Tanner, J.; Khan, D.; Aplin, C.; Ball, J.; Thomas, M.; Bankart, J. Post-discharge surveillance to identify colorectal surgical site infection rates and related costs. J. Hosp. Infect. 2009, 72, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barutcu, A.G.; Klein, D.; Kilian, M.; Biebl, M.; Raakow, R.; Pratschke, J.; Raakow, J. Long-term follow-up after single-incision laparoscopic surgery. Surg. Endosc. 2020, 34, 126–132. [Google Scholar] [CrossRef]
- Miserez, M.; Lefering, R.; Famiglietti, F.; Mathes, T.; Seidel, D.; Sauerland, S.; Korolija, D.; Heiss, M.; Weber, G.; Agresta, F.; et al. Synthetic Versus Biological Mesh in Laparoscopic and Open Ventral Hernia Repair (LAPSIS). Ann. Surg. 2021, 273, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Pedroso-Fernandez, Y.; Aguirre-Jaime, A.; Ramos, M.J.; Hernández, M.; Cuervo, M.; Bravo, A.; Carrillo, A. Prediction of surgical site infection after colorectal surgery. Am. J. Infect. Control 2016, 44, 450–454. [Google Scholar] [CrossRef]
- Owens, C.D.; Stoessel, K. Surgical site infections: Epidemiology, microbiology and prevention. J. Hosp. Infect. 2008, 70, 3–10. [Google Scholar] [CrossRef]
- Geffers, C.; Gastmeier, P.; Daschner, F.; Rüden, H. Prävention postoperativer Wundinfektionen. Zentralblatt Chir. Zeitschrift Allg. Visz. Thorax Gefäßchirurgie 2001, 126, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for Prevention of Surgical Site Infection, 1999. Am. J. Infect. Control 1999, 27, 97–134. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Ren, J. Microbiota-Immune Interaction in the Pathogenesis of Gut-Derived Infection. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Johnson, A.J.; Zheng, J.J.; Kang, J.W.; Saboe, A.; Knights, D.; Zivkovic, A.M. A Guide to Diet-Microbiome Study Design. Front. Nutr. 2020, 7, 1–16. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Morowitz, M.J.; Babrowski, T.; Carlisle, E.M.; Olivas, A.; Romanowski, K.S.; Seal, J.B.; Liu, D.C.; Alverdy, J.C. The Human Microbiome and Surgical Disease. Ann. Surg. 2011, 253, 1094–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirth, U.; Rogers, S.; Haubensak, K.; Schopf, S.; von Ahnen, T.; Schardey, H.M. Local antibiotic decontamination to prevent anastomotic leakage short-term outcome in rectal cancer surgery. Int. J. Colorectal Dis. 2018, 33, 53–60. [Google Scholar] [CrossRef]
- Bassetti, M.; Eckmann, C.; Giacobbe, D.R.; Sartelli, M.; Montravers, P. Post-operative abdominal infections: Epidemiology, operational definitions, and outcomes. Intensive Care Med. 2020, 46, 163–172. [Google Scholar] [CrossRef]
- Buffie, C.G.; Pamer, E.G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lederberg, J. Infectious History. Science 2000, 288, 287–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbara, G.; Stanghellini, V.; Brandi, G.; Cremon, C.; di Nardo, G.; de Giorgio, R.; Corinaldesi, R. Interactions Between Commensal Bacteria and Gut Sensorimotor Function in Health and Disease. Am. J. Gastroenterol. 2005, 100, 2560–2568. [Google Scholar] [CrossRef] [PubMed]
- Venara, A.; Neunlist, M.; Slim, K.; Barbieux, J.; Colas, P.A.; Hamy, A.; Meurette, G. Postoperative ileus: Pathophysiology, incidence, and prevention. J. Visc. Surg. 2016, 153, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Guyton, K.; Alverdy, J.C. The gut microbiota and gastrointestinal surgery. Nat. Rev. Gastroenterol. Hepatol. 2016, 14, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.-H.; Kim, M.; Yun, C.-H. Regulation of Gastrointestinal Immunity by Metabolites. Nutrients 2021, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Kanangat, S. Modulation of alloimmune response by commensal gut microbiota and potential new avenues to influence the outcome of allogeneic transplantation by modification of the ‘gut culture’. Int. J. Immunogenet. 2017, 44, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lederer, A.-K.; Pisarski, P.; Kousoulas, L.; Fichtner-Feigl, S.; Hess, C.; Huber, R. Postoperative changes of the microbiome: Are surgical complications related to the gut flora? A systematic review. BMC Surg. 2017, 17, 125. [Google Scholar] [CrossRef] [Green Version]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [Green Version]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Codella, R.; Luzi, L.; Terruzzi, I. Exercise has the guts: How physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig. Liver Dis. 2018, 50, 331–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voigt, R.M.; Forsyth, C.B.; Green, S.J.; Engen, P.A.; Keshavarzian, A. Circadian Rhythm and the Gut Microbiome. Int. Rev. Neurobiol. 2016, 131, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Agnes, A.; Puccioni, C.; D’Ugo, D.; Gasbarrini, A.; Biondi, A.; Persiani, R. The gut microbiota and colorectal surgery outcomes: Facts or hype? A narrative review. BMC Surg. 2021, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Hartl, W.H.; Jauch, K.W. Post-aggression metabolism: Attempt at a status determination. Infusionsther. Transfusionsmed. 1994, 21, 30–40. [Google Scholar]
- Tabasi, M.; Ashrafian, F.; Khezerloo, J.K.; Eshghjoo, S.; Behrouzi, A.; Javadinia, S.A.; Poursadegh, F.; Eybpoosh, S.; Ahmadi, S.; Radmanesh, A.; et al. Changes in Gut Microbiota and Hormones After Bariatric Surgery: A Bench-to-Bedside Review. Obes. Surg. 2019, 29, 1663–1674. [Google Scholar] [CrossRef]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of Antibiotics on Gut Microbiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef]
- Branch-Elliman, W.; O’Brien, W.; Strymish, J.; Itani, K.; Wyatt, C.; Gupta, K. Association of Duration and Type of Surgical Prophylaxis with Antimicrobial-Associated Adverse Events. JAMA Surg. 2019, 154, 590. [Google Scholar] [CrossRef]
- Bath, M.; McKelvie, M.; Canna, K. Rare postoperative complication: Clostridium perfringens septic shock following elective abdominal surgery. BMJ Case Rep. 2017, 2017, bcr-2017. [Google Scholar] [CrossRef]
- Southern, W.N.; Rahmani, R.; Aroniadis, O.; Khorshidi, I.; Thanjan, A.; Ibrahim, C.; Brandt, L.J. Postoperative Clostridium difficile-associated diarrhea. Surgery 2010, 148, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Ohigashi, S.; Sudo, K.; Kobayashi, D.; Takahashi, T.; Nomoto, K.; Onodera, H. Significant Changes in the Intestinal Environment After Surgery in Patients with Colorectal Cancer. J. Gastrointest. Surg. 2013, 17, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Lin, Y.-M.; Golovko, G.; Khanipov, K.; Cong, Y.; Savidge, T.; Fofanov, Y.; Shi, X.-Z. Microbiota dysbiosis and its pathophysiological significance in bowel obstruction. Sci. Rep. 2018, 8, 13044. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Geng, R.; Liu, Y.; Liu, L.; Jin, X.; Zhao, F.; Feng, J.; Wei, Y. Prediction of Postoperative Ileus in Patients with Colorectal Cancer by Preoperative Gut Microbiota. Front. Oncol. 2020, 10, 526009. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Halder, C.V.; de Faria, A.V.S.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Hussain, Z.; Lee, Y.J.; Park, H. An altered composition of fecal microbiota, organic acids, and the effect of probiotics in the guinea pig model of postoperative ileus. Neurogastroenterol. Motil. 2021, 33, e13966. [Google Scholar] [CrossRef]
- Kim, H.J.; Li, H.; Collins, J.J.; Ingber, D.E. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Natl. Acad. Sci. USA 2016, 113, E7–E15. [Google Scholar] [CrossRef] [Green Version]
- De Ruiter, J.; Weel, J.; Manusama, E.; Kingma, W.P.; van der Voort, P.H.J. The Epidemiology of Intra-Abdominal Flora in Critically Ill Patients with Secondary and Tertiary Abdominal Sepsis. Infection 2009, 37, 522–527. [Google Scholar] [CrossRef]
- Schmitt, F.C.F.; Schneider, M.; Mathejczyk, W.; Weigand, M.A.; Figueiredo, J.C.; Li, C.I.; Shibata, D.; Siegel, E.M.; Toriola, A.T.; Ulrich, C.M.; et al. Postoperative Complications Are Associated with Long-Term Changes in the Gut Microbiota Following Colorectal Cancer Surgery. Life 2021, 11, 246. [Google Scholar] [CrossRef]
- Shimizu, K.; Ogura, H.; Hamasaki, T.; Goto, M.; Tasaki, O.; Asahara, T.; Nomoto, K.; Morotomi, M.; Matsushima, A.; Kuwagata, Y.; et al. Altered Gut Flora Are Associated with Septic Complications and Death in Critically Ill Patients with Systemic Inflammatory Response Syndrome. Dig. Dis. Sci. 2011, 56, 1171–1177. [Google Scholar] [CrossRef] [Green Version]
- Yeh, A.; Rogers, M.B.; Firek, B.; Neal, M.D.; Zuckerbraun, B.S.; Morowitz, M.J. Dysbiosis Across Multiple Body Sites in Critically Ill Adult Surgical Patients. Shock 2016, 46, 649–654. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Macpherson, A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar] [CrossRef]
- Mohandas, S.; Soma, V.L.; Tran, T.D.B.; Sodergren, E.; Ambooken, T.; Goldman, D.L.; Weinstock, G.; Herold, B.C. Differences in Gut Microbiome in Hospitalized Immunocompetent vs. Immunocompromised Children, Including Those with Sickle Cell Disease. Front. Pediatr. 2020, 8, 583446. [Google Scholar] [CrossRef]
- Vindigni, S.M.; Surawicz, C.M. Fecal Microbiota Transplantation. Gastroenterol. Clin. N. Am. 2017, 46, 171–185. [Google Scholar] [CrossRef]
- Clancy, J.; Mcvicar, A. Homeostasis—The Key Concept to Physiological Control. Br. J. Theatr. Nurs. 1998, 8, 12–18. [Google Scholar] [CrossRef]
- Kehlet, H.; Dahl, J.B. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet 2003, 362, 1921–1928. [Google Scholar] [CrossRef]
- Wolf, A.-M. Anastomoseninsuffizienz im Gastrointestinaltrakt. Der. Chir. 2002, 73, 394–407. [Google Scholar] [CrossRef]
- McDermott, F.D.; Heeney, A.; Kelly, M.E.; Steele, R.J.; Carlson, G.L.; Winter, D.C. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br. J. Surg. 2015, 102, 462–479. [Google Scholar] [CrossRef]
- Slieker, J.C.; Daams, F.; Mulder, I.M.; Jeekel, J.; Lange, J.F. Systematic Review of the Technique of Colorectal Anastomosis. JAMA Surg. 2013, 148, 190. [Google Scholar] [CrossRef] [Green Version]
- Marjanovic, G.; Hopt, U.T. Physiologie der Anastomosenheilung. Der. Chir. 2011, 82, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Boesen, A.K.; Maeda, Y.; Madsen, M.R. Perioperative fluid infusion and its influence on anastomotic leakage after rectal cancer surgery: Implications for prevention strategies. Color. Dis. 2013, 15, e522–e527. [Google Scholar] [CrossRef]
- Tian, W.; Xu, X.; Yao, Z.; Yang, F.; Huang, M.; Zhao, R.; Zhao, Y. Early Enteral Nutrition Could Reduce Risk of Recurrent Leakage After Definitive Resection of Anastomotic Leakage After Colorectal Cancer Surgery. World J. Surg. 2021, 45, 320–330. [Google Scholar] [CrossRef]
- Sciuto, A.; Merola, G.; de Palma, G.D.; Sodo, M.; Pirozzi, F.; Bracale, U.M.; Bracale, U. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J. Gastroenterol. 2018, 24, 2247–2260. [Google Scholar] [CrossRef]
- Meyer, J.; Naiken, S.; Christou, N.; Liot, E.; Toso, C.; Buchs, N.C.; Ris, F. Reducing anastomotic leak in colorectal surgery: The old dogmas and the new challenges. World J. Gastroenterol. 2019, 25, 5017–5025. [Google Scholar] [CrossRef]
- Cohen, S.R.; Cornell, C.N.; Collins, M.H.; Sell, J.E.; Blanc, W.A.; Altman, R.P. Healing of ischemic colonic anastomoses in the rat: Role of antibiotic preparation. Surgery 1985, 97, 443–446. [Google Scholar]
- Schardey, H.M.; Joosten, U.; Finke, U.; Staubach, K.H.; Schauer, R.; Heiss, A.; Kooistra, A.; Rau, H.G.; Nibler, R.; Lüdeling, S.; et al. The Prevention of Anastomotic Leakage After Total Gastrectomy with Local Decontamination. Ann. Surg. 1997, 225, 172–180. [Google Scholar] [CrossRef]
- Abis, G.S.A.; Stockmann, H.B.A.C.; Bonjer, H.J.; van Veenendaal, N.; van Doorn-Schepens, M.L.M.; Budding, A.E.; Wilschut, J.A.; van Egmond, M.; Oosterling, S.J.; Abis, G.S.A.; et al. Randomized clinical trial of selective decontamination of the digestive tract in elective colorectal cancer surgery (SELECT trial). Br. J. Surg. 2019, 106, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Roos, D.; Dijksman, L.M.; Tijssen, J.G.; Gouma, D.J.; Gerhards, M.F.; Oudemans-van Straaten, H.M. Systematic review of perioperative selective decontamination of the digestive tract in elective gastrointestinal surgery. Br. J. Surg. 2013, 100, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Rollins, K.E.; Javanmard-Emamghissi, H.; Acheson, A.G.; Lobo, D.N. The Role of Oral Antibiotic Preparation in Elective Colorectal Surgery. Ann. Surg. 2019, 270, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Huttner, B.D.; de Lastours, V.; Wassenberg, M.; Maharshak, N.; Mauris, A.; Galperine, T.; Zanichelli, V.; Kapel, N.; Bellanger, A.; Olearo, F.; et al. A 5-day course of oral antibiotics followed by faecal transplantation to eradicate carriage of multidrug-resistant Enterobacteriaceae: A randomized clinical trial. Clin. Microbiol. Infect. 2019, 25, 830–838. [Google Scholar] [CrossRef] [Green Version]
- Huttner, B.; Haustein, T.; Uckay, I.; Renzi, G.; Stewardson, A.; Schaerrer, D.; Agostinho, A.; Andremont, A.; Schrenzel, J.; Pittet, D.; et al. Decolonization of intestinal carriage of extended-spectrum-lactamase-producing Enterobacteriaceae with oral colistin and neomycin: A randomized, double-blind, placebo-controlled trial. J. Antimicrob. Chemother. 2013, 68, 2375–2382. [Google Scholar] [CrossRef] [Green Version]
- Leo, S.; Lazarevic, V.; Girard, M.; Gaïa, N.; Schrenzel, J.; de Lastours, V.; Fantin, B.; Bonten, M.; Carmeli, Y.; Rondinaud, E.; et al. Metagenomic Characterization of Gut Microbiota of Carriers of Extended-Spectrum Beta-Lactamase or Carbapenemase-Producing Enterobacteriaceae Following Treatment with Oral Antibiotics and Fecal Microbiota Transplantation: Results from a Multicenter Randomi. Microorganisms 2020, 8, 941. [Google Scholar] [CrossRef] [PubMed]
- Flemming, S.; Germer, C.-T. Orale Antibiotikaprophylaxe zur Darmdekontamination vor elektiver kolorektaler Chirurgie. Der. Chir. 2020, 91, 128–133. [Google Scholar] [CrossRef]
- Manson, J.M.; Rauch, M.; Gilmore, M.S. The commensal microbiology of the gastrointestinal tract. In GI Microbiota and Regulation of the Immune System; Springer: New York, NY, USA, 2008; pp. 15–28. [Google Scholar]
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The Genus Enterococcus: Between Probiotic Potential and Safety Concerns—An Update. Front. Microbiol. 2018, 9, 1791. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Phillips, C. The ecology, epidemiology and virulence of Enterococcus. Microbiology 2009, 155, 1749–1757. [Google Scholar] [CrossRef] [Green Version]
- Alverdy, J.C.; Hyoju, S.K.; Weigerinck, M.; Gilbert, J.A. The gut microbiome and the mechanism of surgical infection. Br. J. Surg. 2017, 104, e14–e23. [Google Scholar] [CrossRef] [Green Version]
- Shogan, B.D.; Belogortseva, N.; Luong, P.M.; Zaborin, A.; Lax, S.; Bethel, C.; Muldoon, J.P.; Singer, M.; An, G.; Umanskiy, K.; et al. Collagen degradation and MMP9 activation by Enterococcus faecalis contributes to intestinal anastomotic leak. Sci. Transl. Med. 2016, 7, 286ra68. [Google Scholar] [CrossRef] [Green Version]
- Shogan, B.D.; Smith, D.P.; Christley, S.; Gilbert, J.A.; Zaborina, O.; Alverdy, J.C. Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome 2014, 2, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belmouhand, M.; Krohn, P.S.; Svendsen, L.B.; Henriksen, A.; Hansen, C.P.; Achiam, M.P. The occurrence of Enterococcus faecium and faecalis is significantly associated with anastomotic leakage after pancreaticoduodenectomy. Scand. J. Surg. 2018, 107, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, F.C.F.; Brenner, T.; Uhle, F.; Loesch, S.; Hackert, T.; Ulrich, A.; Hofer, S.; Dalpke, A.H.; Weigand, M.A.; Boutin, S. Gut microbiome patterns correlate with higher postoperative complication rates after pancreatic surgery. BMC Microbiol. 2019, 19, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mima, K.; Sakamoto, Y.; Kosumi, K.; Ogata, Y.; Miyake, K.; Hiyoshi, Y.; Ishimoto, T.; Iwatsuki, M.; Baba, Y.; Iwagami, S.; et al. Mucosal cancer-associated microbes and anastomotic leakage after resection of colorectal carcinoma. Surg. Oncol. 2020, 32, 63–68. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and Their Health-Promoting Effects. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Longhi, G.; van Sinderen, D.; Ventura, M.; Turroni, F. Microbiota and Cancer: The Emerging Beneficial Role of Bifidobacteria in Cancer Immunotherapy. Front. Microbiol. 2020, 11, 2188. [Google Scholar] [CrossRef]
- Praagh, J.B.; Goffau, M.C.; Bakker, I.S.; Harmsen, H.J.M.; Olinga, P.; Havenga, K. Intestinal microbiota and anastomotic leakage of stapled colorectal anastomoses: A pilot study. Surg. Endosc. 2016, 30, 2259–2265. [Google Scholar] [CrossRef]
- Van Praagh, J.B.; de Goffau, M.C.; Bakker, I.S.; van Goor, H.; Harmsen, H.J.M.; Olinga, P.; Havenga, K. Mucus Microbiome of Anastomotic Tissue During Surgery Has Predictive Value for Colorectal Anastomotic Leakage. Ann. Surg. 2018, 269, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; de Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Palmisano, S.; Campisciano, G.; Iacuzzo, C.; Bonadio, L.; Zucca, A.; Cosola, D.; Comar, M.; de Manzini, N. Role of preoperative gut microbiota on colorectal anastomotic leakage: Preliminary results. Updates Surg. 2020, 72, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Price, L.S.; Weinstein, R.A. Acinetobacter Infection. N. Engl. J. Med. 2008, 358, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Abbott, S.L. The Genus Hafnia: From Soup to Nuts. Clin. Microbiol. Rev. 2006, 19, 12–28. [Google Scholar] [CrossRef] [Green Version]
- Morotomi, M.; Nagai, F.; Sakon, H.; Tanaka, R. Dialister succinatiphilus sp. nov. and Barnesiella intestinihominis sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2008, 58, 2716–2720. [Google Scholar] [CrossRef] [Green Version]
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.M.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horan, T.C.; Gaynes, R.P.; Martone, W.J.; Jarvis, W.R.; Emori, T.G. CDC definitions of nosocomial surgical site infections, 1992: A modification of CDC definitions of surgical wound infections. Infect. Control Hosp. Epidemiol. 1992, 13, 606–608. [Google Scholar] [CrossRef]
- Fry, D.E.; Fry, R.V. Surgical Site Infection: The Host Factor. AORN J. 2007, 86, 801–814. [Google Scholar] [CrossRef] [Green Version]
- Negi, V.; Pal, S.; Juyal, D.; Sharma, M.K.; Sharma, N. Bacteriological Profile of Surgical Site Infections and Their Antibiogram: A Study from Resource Constrained Rural Setting of Uttarakhand State, India. J. Clin. Diagn. Res. 2015, 9, DC17–DC20. [Google Scholar] [CrossRef]
- Hassan, R.S.E.E.; Osman, S.O.S.; Aabdeen, M.A.S.; Mohamed, W.E.A.; Hassan, R.S.E.E.; Mohamed, S.O.O. Incidence and root causes of surgical site infections after gastrointestinal surgery at a public teaching hospital in Sudan. Patient Saf. Surg. 2020, 14, 45. [Google Scholar] [CrossRef]
- Ouedraogo, S.; Kambire, J.L.; Ouedraogo, S.; Ouangre, E.; Diallo, I.; Zida, M.; Bandre, E. Surgical Site Infection after Digestive Surgery: Diagnosis and Treatment in a Context of Limited Resources. Surg. Infect. 2020, 21, 547–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkaaki, A.; Al-Radi, O.O.; Khoja, A.; Alnawawi, A.; Alnawawi, A.; Maghrabi, A.; Altaf, A.; Aljiffry, M. Surgical site infection following abdominal surgery: A prospective cohort study. Can. J. Surg. 2019, 62, 111–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krezalek, M.A.; Alverdy, J.C. The role of the microbiota in surgical recovery. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Velasco, C.; Dunn, C.; Sturdy, C.; Izda, V.; Martin, J.; Rivas, A.; McNaughton, J.; Jeffries, M.A. Ear wound healing in MRL/MpJ mice is associated with gut microbiome composition and is transferable to non-healer mice via microbiome transplantation. PLoS ONE 2021, 16, e0248322. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Gómez, B.; McIntyre, M.; Dubick, M.; Christy, R.; Nicholson, S.; Burmeister, D. The Cutaneous Microbiome and Wounds: New Molecular Targets to Promote Wound Healing. Int. J. Mol. Sci. 2018, 19, 2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, A.D.G. Randomised clinical trial of synbiotic therapy in elective surgical patients. Gut 2004, 53, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Scarborough, J.E.; Mantyh, C.R.; Sun, Z.; Migaly, J. Combined Mechanical and Oral Antibiotic Bowel Preparation Reduces Incisional Surgical Site Infection and Anastomotic Leak Rates After Elective Colorectal Resection. Ann. Surg. 2015, 262, 331–337. [Google Scholar] [CrossRef]
- Hinchey, E.J.; Richards, G.K.; Prentis, J. Metronidazole as a prophylactic agent in wound infection after colon surgery. Surgery 1983, 93, 197–200. [Google Scholar]
| Surgery-Related | Patient-Related |
|---|---|
| Duration of surgery > 4 h | Male |
| Intraoperative blood transfusion | Advanced tumor stage, metastatic disease or local tumor size > 3 cm |
| Anastomosis of the large intestine | Pre-existing illnesses (vascular, hepatic, pulmonary, renal, diabetes) |
| Emergency surgery | (Ex)-smoker, alcohol abuse |
| Absorbable suture | History of radiotherapy or chemotherapy |
| Double-layer anastomosis | Current sepsis or infectious diseases |
| Poor viability of anastomosis | Current ileus |
| Extensive intravenous fluid intraoperatively | Cachexia or malnutrition |
| Late postoperative enteral nutrition | Obesity |
| Inexperienced surgeon | (Medicinal) immunosuppression |
| Composition of gut microbiota |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lederer, A.-K.; Chikhladze, S.; Kohnert, E.; Huber, R.; Müller, A. Current Insights: The Impact of Gut Microbiota on Postoperative Complications in Visceral Surgery—A Narrative Review. Diagnostics 2021, 11, 2099. https://doi.org/10.3390/diagnostics11112099
Lederer A-K, Chikhladze S, Kohnert E, Huber R, Müller A. Current Insights: The Impact of Gut Microbiota on Postoperative Complications in Visceral Surgery—A Narrative Review. Diagnostics. 2021; 11(11):2099. https://doi.org/10.3390/diagnostics11112099
Chicago/Turabian StyleLederer, Ann-Kathrin, Sophia Chikhladze, Eva Kohnert, Roman Huber, and Alexander Müller. 2021. "Current Insights: The Impact of Gut Microbiota on Postoperative Complications in Visceral Surgery—A Narrative Review" Diagnostics 11, no. 11: 2099. https://doi.org/10.3390/diagnostics11112099
APA StyleLederer, A.-K., Chikhladze, S., Kohnert, E., Huber, R., & Müller, A. (2021). Current Insights: The Impact of Gut Microbiota on Postoperative Complications in Visceral Surgery—A Narrative Review. Diagnostics, 11(11), 2099. https://doi.org/10.3390/diagnostics11112099






