Chronic Autoimmune Gastritis: Modern Diagnostic Principles
Abstract
:1. Introduction
1.1. Risk Factors
1.2. Clinical Manifestation
1.3. Gastrointestinal Symptoms
1.4. Vitamin B12 (Cobalamin) Deficiency
1.5. Iron Deficiency
1.6. Concomitant Diseases (Comorbidity)
2. Diagnostic Approaches
2.1. Laboratory Methods
2.2. Endoscopic Examination
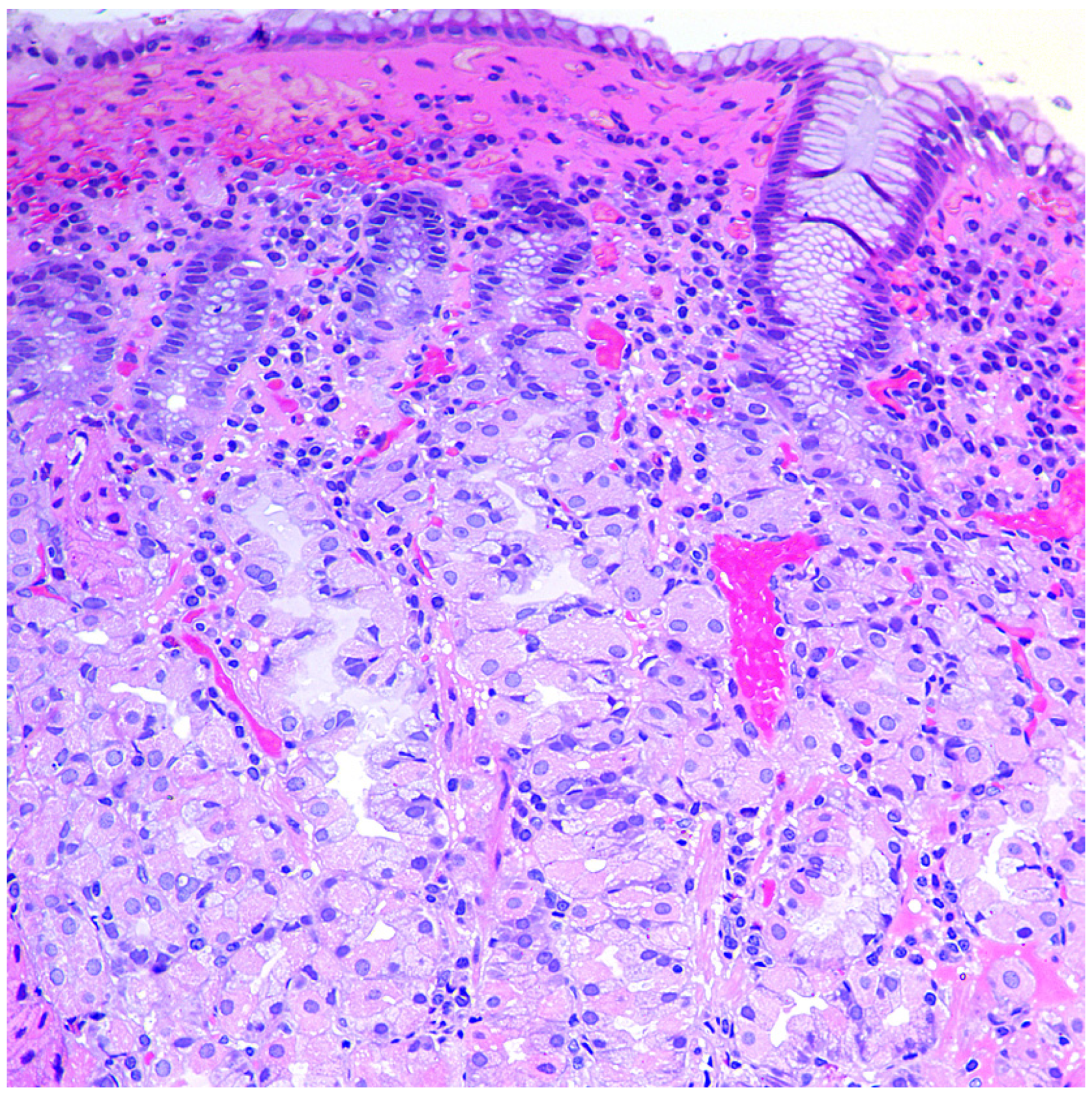
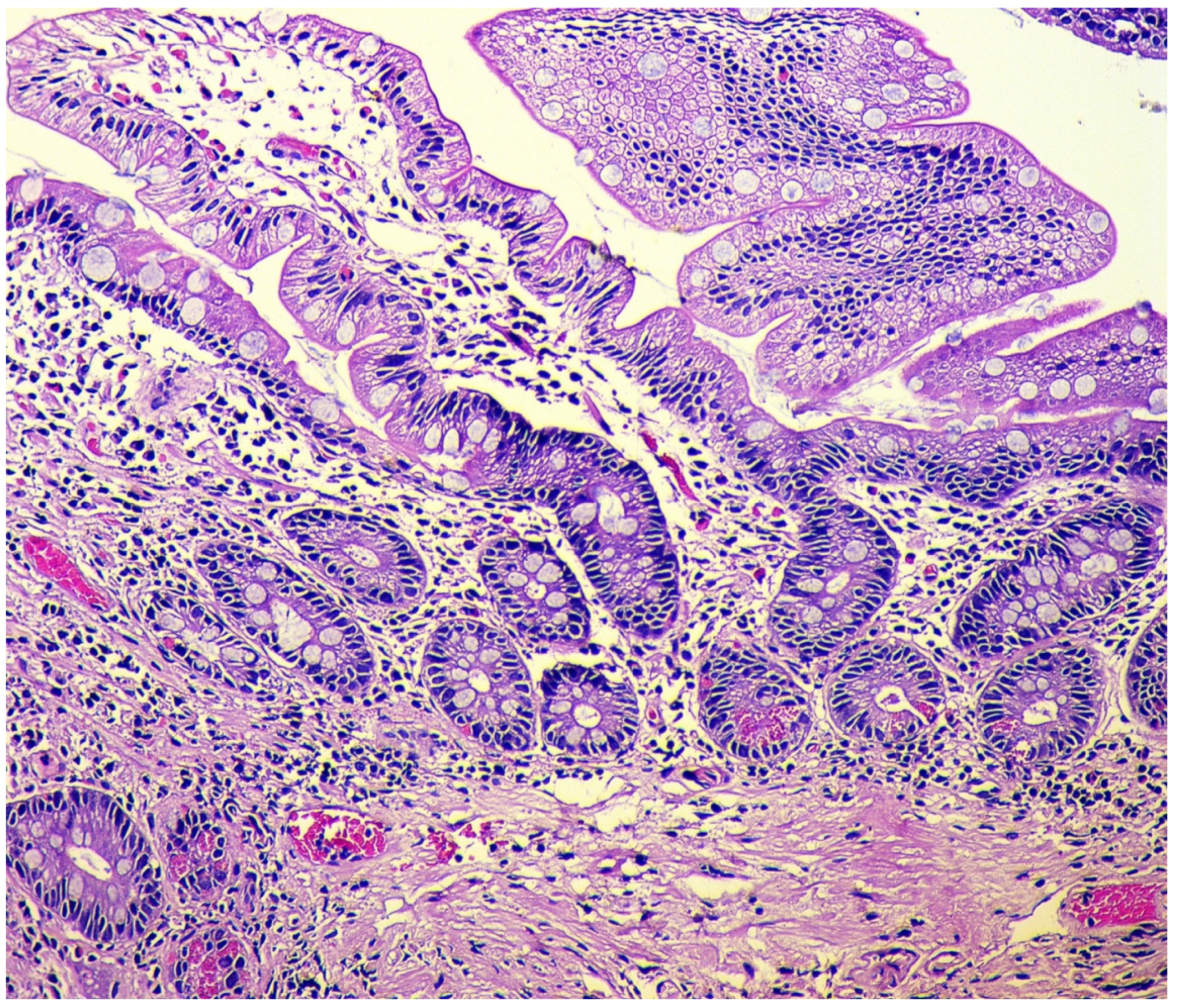
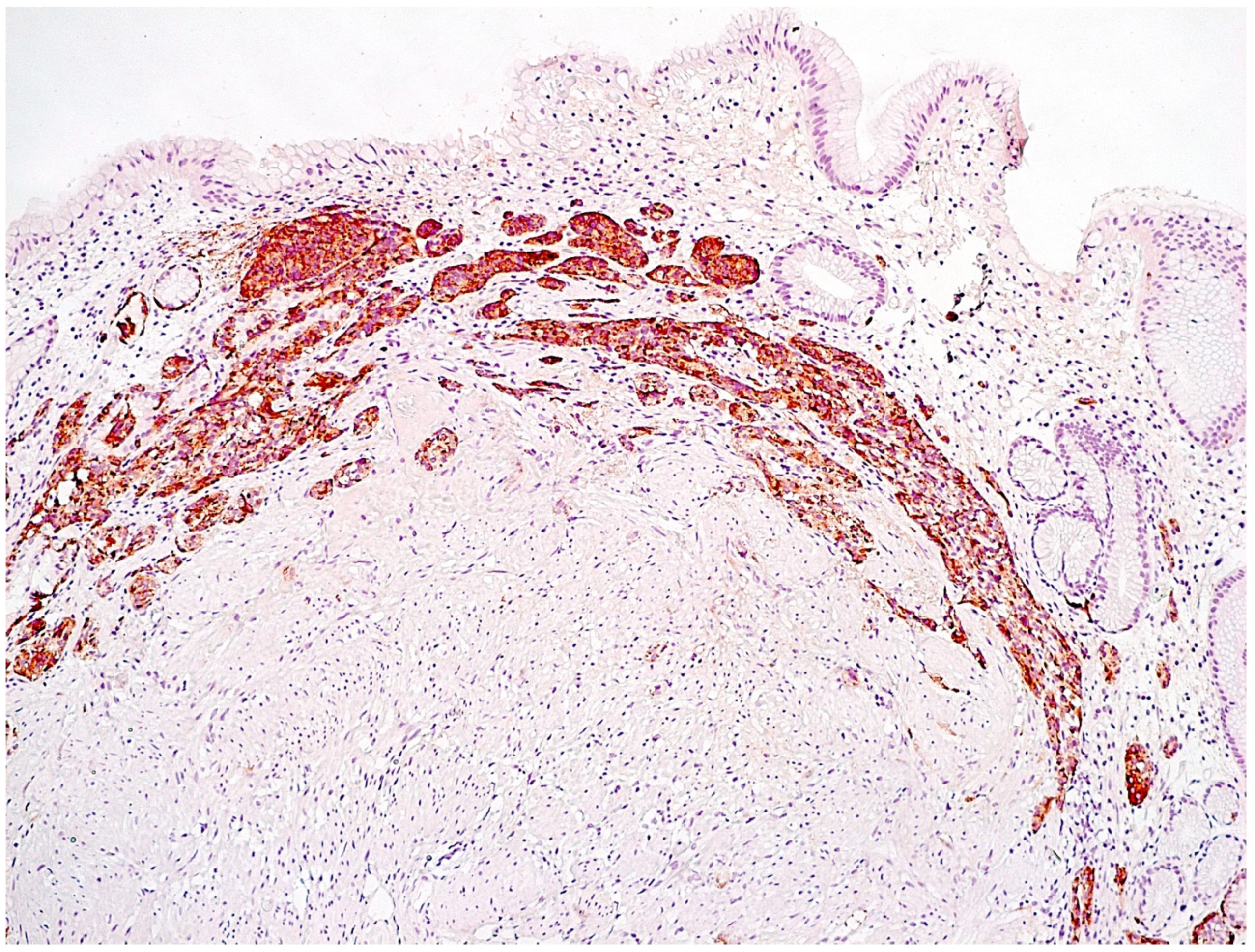
2.3. Morphological Examination
- Simple diffuse hyperplasia. This is characterized by a more than two-fold increase in the population of ECL cells. Diagnosis is difficult due to the lack of clear quantitative criteria. The diagnosis is poorly reproduced on biopsy material.
- Linear hyperplasia. The presence in one visual field of at least two groups of linearly located neuroendocrine cells, consisting of five or more cells. Usually, changes are diagnosed in the area of the neck glands (Figure 4).
- Micronodular hyperplasia. The presence of the cells’ cluster in contact with the basement membrane, but not exceeding the diameter of the gland, up to 150 μm in diameter, or a similar cluster located freely in the lamina propria of the mucous membrane.
- Adenomatous (adenomatoid) hyperplasia. The presence of an aggregate of five or more clusters (Figure 5).
- Neuroendocrine cells dysplasia. Merging clusters with diameters of more than 150 µm but less than 500 µm.
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malfertheiner, P.; Megraud, F.; O’morain, C.A.; Gisbert, J.; Kuipers, E.; Axon, A.T.; Bazzoli, P.; Gasbarrini, A.; Atherton, J.; Graham, D.Y. Management of Helicobacter pylori infection—The Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugano, K.; Tack, J.; Kuipers, E.J.; Graham, D.Y.; El-Omar, E.M.; Miura, S.; Haruma, K.; Asaka, M.; Uemura, N.; Malfertheiner, P.; et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coati, I.; Fassan, M.; Farinati, F.; Graham, D.Y.; Genta, R.M.; Rugge, M. Autoimmune gastritis: Pathologist’s viewpoint. World J. Gastroenterol. 2015, 21, 12179–12189. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Cavalcoli, F.; Rossi, R.E.; Conte, D.; Spampatti, M.P.; Ciafardini, C.; Verga, U.; Beck-Peccoz, P.; Peracchi, M. Chronic autoimmune atrophic gastritis associated with primary hyperparathyroidism: A transversal prospective study. Eur. J. Endocrinol. 2013, 168, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Lenti, M.V.; Rugge, M.; Lahner, E.; Miceli, E.; Toh, B.H.; Genta, R.M.; De Block, C.; Hershko, C.; Di Sabatino, A. Autoimmune gastritis. Nat. Rev. Dis. Primers 2020, 6, 56. [Google Scholar] [CrossRef]
- Neumann, W.L.; Coss, E.; Rugge, M.; Genta, R.M. Autoimmune atrophic gastritis—Pathogenesis, pathology and management. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 529–541. [Google Scholar] [CrossRef]
- Song, H.; Held, M.; Sandin, S.; Rautelin, H.; Eliasson, M.; Söderberg, S.; Hallmans, G.; Engstrand, L.; Nyrén, O.; Ye, W. Increase in the Prevalence of Atrophic Gastritis Among Adults Age 35 to 44 Years Old in Northern Sweden Between 1990 and 2009. Clin. Gastroenterol. Hepatol. 2015, 13, 1592–1600.e1. [Google Scholar] [CrossRef]
- Volynets, G.V. Klinicheskie i diagnosticheskie osobennosti i printsipy terapii autoimmunnogo gastrita u detey. Det. Gastroenterol. 2005, 3, 33–37. (In Russian) [Google Scholar]
- Youssefi, M.; Tafaghodi, M.; Farsiani, H.; Ghazvini, K.; Keikha, M. Helicobacter pylori infection and autoimmune diseases; Is there an association with systemic lupus erythematosus, rheumatoid arthritis, autoimmune atrophy gastritis and autoimmune pancreatitis? A systematic review and meta-analysis study. J. Microbiol. Immunol. Infect. 2021, 54, 359–369. [Google Scholar] [CrossRef]
- Faller, G.; Kirchner, T. Immunological and morphogenic basis of gastric mucosa atrophy and metaplasia. Virchows Arch. 2005, 446, 1–9. [Google Scholar] [CrossRef]
- D’Elios, M.M.; Appelmelk, B.J.; Amedei, A.; Bergman, M.P.; Del Prete, G. Gastric autoimmunity: The role of Helicobacter pylori and molecular mimicry. Trends Mol. Med. 2004, 10, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Claeys, D.; Faller, G.; Appelmelk, B.J.; Negrini, R.; Kirchner, T. The gastric H+,K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology 1998, 115, 340–347. [Google Scholar] [CrossRef]
- Venter, C.; Eyerich, S.; Sarin, T.; Klatt, K.C. Nutrition and the Immune System: A Complicated Tango. Nutrients 2020, 12, 818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzel, A.; Muller, D.N.; Hafler, D.A.; Erdman, S.E.; Linker, R.A.; Kleinewietfeld, M. Role of “Western diet” in inflammatory autoimmune diseases. Curr. Allergy Asthma Rep. 2014, 14, 404. [Google Scholar] [CrossRef] [Green Version]
- Abbott, R.D.; Sadowski, A.; Alt, A.G. Efficacy of the Autoimmune Protocol Diet as Part of a Multi-disciplinary, Supported Lifestyle Intervention for Hashimoto’s Thyroiditis. Cureus 2019, 11, e4556. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Castro, K.I.; Franceschi, M.; Noto, A.; Miraglia, C.; Nouvenne, A.; Leandro, G.; Meschi, T.; De’ Angelis, G.L.; Di Mario, F. Clinical manifestations of chronic atrophic gastritis. Acta Biomed. 2018, 89, 88–92. [Google Scholar]
- Kalkan, C.; Soykan, I. Differences between older and young patients with autoimmune gastritis. Geriatr. Gerontol. Int. 2017, 17, 1090–1095. [Google Scholar] [CrossRef]
- Carabotti, M.; Lahner, E.; Esposito, G.; Sacchi, M.C.; Severi, C.; Annibale, B. Upper gastrointestinal symptoms in autoimmune gastritis: A cross-sectional study. Medicine 2017, 96, e5784. [Google Scholar] [CrossRef]
- Kalkan, Ç.; Soykan, I.; Soydal, Ç.; Özkan, E.; Kalkan, E. Assessment of Gastric Emptying in Patients with Autoimmune Gastritis. Dig. Dis. Sci. 2016, 61, 1597–1602. [Google Scholar] [CrossRef]
- Miceli, E.; Lenti, M.V.; Padula, D.; Luinetti, O.; Vattiato, C.; Monti, C.M.; Di Stefano, M.; Corazza, G.R. Common features of patients with autoimmune atrophic gastritis. Clin. Gastroenterol. Hepatol. 2012, 10, 812–814. [Google Scholar] [CrossRef]
- Tenca, A.; Massironi, S.; Pugliese, D.; Consonni, D.; Mauro, A.; Cavalcoli, F.; Franchina, M.; Spampatti, M.; Conte, D.; Penagini, R. Gastro-esophageal reflux and antisecretory drugs use among patients with chronic autoimmune atrophic gastritis: A study with pH-impedance monitoring. Neurogastroenterol. Motil. 2016, 28, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.T.; Zhao, H.Y.; Kong, Y.; Sun, N.N.; Dong, A.Q. Correlation between serum vitamin B12 level and peripheral neuropathy in atrophic gastritis. World J. Gastroenterol. 2018, 24, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Devalia, V.; Hamilton, M.S.; Molloy, A.M.; British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br. J. Haematol. 2014, 166, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Hershko, C.; Ronson, A.; Souroujon, M.; Maschler, I.; Heyd, J.; Patz, J. Variable hematologic presentation of autoimmune gastritis: Age-related progression from iron deficiency to cobalamin depletion. Blood 2006, 107, 1673–1679. [Google Scholar] [CrossRef]
- Cavalcoli, F.; Zilli, A.; Conte, D.; Massironi, S. Micronutrient deficiencies in patients with chronic atrophic autoimmune gastritis: A review. World J. Gastroenterol. 2017, 23, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Toh, B.H. Diagnosis and classification of autoimmune gastritis. Autoimmun. Rev. 2014, 13, 459–462. [Google Scholar] [CrossRef]
- Rusak, E.; Chobot, A.; Krzywicka, A.; Wenzlau, J. Anti-parietal cell antibodies—Diagnostic significance. Adv. Med. Sci. 2016, 61, 175–179. [Google Scholar] [CrossRef]
- Lahner, E.; Norman, G.L.; Severi, C.; Encabo, S.; Shums, Z.; Vannella, L.; Delle Fave, G.; Annibale, B. Reassessment of intrinsic factor and parietal cell autoantibodies in atrophic gastritis with respect to cobalamin deficiency. Am. J. Gastroenterol. 2009, 104, 2071–2079. [Google Scholar] [CrossRef]
- Bizzaro, N.; Antico, A. Diagnosis and classification of pernicious anemia. Autoimmun. Rev. 2014, 13, 565–568. [Google Scholar] [CrossRef]
- Antico, A.; Tampoia, M.; Villalta, D.; Tonutti, E.; Tozzoli, R.; Bizzaro, N. Clinical usefulness of the serological gastric biopsy for the diagnosis of chronic autoimmune gastritis. Clin. Dev. 2012, 2012, 520970. [Google Scholar] [CrossRef]
- Lombardo, L.; Leto, R.; Molinaro, G.; Migliardi, M.; Ravarino, N.; Rocca, R.; Torchio, B. Prevalence of atrophic gastritis in dyspeptic patients in Piedmont. A survey using the GastroPanel test. Clin. Chem. Lab. Med. 2010, 48, 1327–1332. [Google Scholar] [CrossRef]
- Telaranta-Keerie, A.; Kara, R.; Paloheimo, L.; Härkönen, M.; Sipponen, P. Prevalence of undiagnosed advanced atrophic corpus gastritis in Finland: An observational study among 4,256 volunteers without specific complaints. Scand. J. Gastroenterol. 2010, 45, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- McNicholl, A.G.; Forné, M.; Barrio, J.; De la Coba, C.; González, B.; Rivera, R.; Esteve, M.; Fernandez-Bañares, F.; Madrigal, B.; Gras-Miralles, B.; et al. Accuracy of GastroPanel for the diagnosis of atrophic gastritis. Eur. J. Gastroenterol. Hepatol. 2014, 26, 941–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baudin, E.; Bidart, J.M.; Bachelot, A.; Ducreux, M.; Elias, D.; Ruffié, P.; Schlumberger, M. Impact of chromogranin A measurement in the work-up of neuroendocrine tumors. Ann. Oncol. 2001, 12, S79–S82. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, Y.; Long, J.; Yao, X.; Wang, J.; Zang, S.; Qu, W.; Wang, F. Serum chromogranin A for the diagnosis of gastroenteropancreatic neuroendocrine neoplasms and its association with tumour expression. Oncol. Lett. 2019, 17, 1497–1504. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yang, Q.C.; Lin, Y.; Xue, L.; Chen, M.H.; Chen, J. Chromogranin A as a marker for diagnosis, treatment, and survival in patients with gastroenteropancreatic neuroendocrine neoplasm. Medicine 2014, 93, e247. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Gao, J.; Li, N.; Li, Y.; Lu, M.; Li, Z.; Lu, Z.; Li, J.; Shen, L. Circulating Chromogranin, A as A Marker for Monitoring Clinical Response in Advanced Gastroenteropancreatic Neuroendocrine Tumors. PLoS ONE 2016, 11, e0154679. [Google Scholar] [CrossRef] [PubMed]
- Peracchi, M.; Gebbia, C.; Basilisco, G.; Quatrini, M.; Tarantino, C.; Vescarelli, C.; Massironi, S.; Conte, D. Plasma chromogranin A in patients with autoimmune chronic atrophic gastritis, enterochromaffin-like cell lesions and gastric carcinoids. Eur. J. Endocrinol. 2005, 152, 443–448. [Google Scholar] [CrossRef]
- Massironi, S.; Fraquelli, M.; Paggi, S.; Sangiovanni, A.; Conte, D.; Sciola, V.; Ciafardini, C.; Colombo, M.; Peracchi, M. Chromogranin A levels in chronic liver disease and hepatocellular carcinoma. Dig. Liver Dis. 2009, 41, 31–35. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar]
- Torbenson, M.; Abraham, S.C.; Boitnott, J.; Yardley, J.H.; Wu, T.T. Autoimmune gastritis: Distinct histological and immunohistochemical findings before complete loss of oxyntic glands. Mod. Pathol. 2002, 15, 102–109. [Google Scholar] [CrossRef]
- Stolte, M.; Baumann, K.; Bethke, B.; Ritter, M.; Lauer, E.; Eidt, H. Active autoimmune gastritis without total atrophy of the glands. Z. Gastroenterol. 1992, 30, 729–735. [Google Scholar] [PubMed]
- Itsuno, M.; Watanabe, H.; Iwafuchi, M.; Ito, S.; Yanaihara, N.; Sato, K.; Kikuchi, M.; Akiyama, N. Multiple carcinoids and endocrine cell micronests in type A gastritis. Their morphology, histogenesis, and natural history. Cancer 1989, 63, 881–890. [Google Scholar] [CrossRef]
- Graham, D.Y.; Rugge, M.; Genta, R.M. Diagnosis: Gastric intestinal metaplasia—What to do next? Curr. Opin. Gastroenterol. 2019, 35, 535–543. [Google Scholar] [CrossRef]
- Wada, Y.; Nakajima, S.; Kushima, R.; Takemura, S.; Mori, N.; Hasegawa, H.; Nakayama, T.; Mukaisho, K.I.; Yoshida, A.; Umano, S.; et al. Pyloric, pseudopyloric, and spasmolytic polypeptide-expressing metaplasias in autoimmune gastritis: A case series of 22 Japanese patients. Virchows Arch. 2021, 479, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Rugge, M. Biologic profiles meet clinical priorities: Incorporating pseudopyloric, and spasmolytic-expressing metaplasia in the assessment of gastric atrophy. Virchows Arch. 2020, 477, 487–488. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Filipe, M.I.; Pinho, A. Variants of intestinal metaplasia in the evolution of chronic atrophic gastritis and gastric ulcer. A follow up study. Gut 1990, 31, 1097–1104. [Google Scholar] [CrossRef] [Green Version]
- Jencks, D.S.; Adam, J.D.; Borum, M.L.; Koh, J.M.; Stephen, S.; Doman, D.B. Overview of Current Concepts in Gastric Intestinal Metaplasia and Gastric Cancer. Gastroenterol. Hepatol. 2018, 14, 92–101. [Google Scholar]
- Rugge, M.; Fassan, M.; Pizzi, M.; Zorzetto, V.; Maddalo, G.; Realdon, S.; De Bernard, M.; Betterle, C.; Cappellesso, R.; Pennelli, G.; et al. Autoimmune gastritis: Histology phenotype and OLGA staging. Aliment. Pharmacol. Ther. 2012, 35, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Zullo, A.; Hassan, C.; Romiti, A.; Giusto, M.; Guerriero, C.; Lorenzetti, R.; Campo, S.M.; Tomao, S. Follow-up of intestinal metaplasia in the stomach: When, how and why. World J. Gastrointest. Oncol. 2012, 4, 30–36. [Google Scholar] [CrossRef]
- Vannella, L.; Lahner, E.; Osborn, J.; Bordi, C.; Miglione, M.; Delle Fave, G.; Annibale, B. Risk factors for progression to gastric neoplastic lesions in patients with atrophic gastritis. Aliment. Pharmacol. Ther. 2010, 31, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Capelle, L.G.; de Vries, A.C.; Haringsma, J.; Ter Borg, F.; de Vries, R.A.; Bruno, M.J.; van Dekken, H.; Meijer, J.; van Grieken, N.C.; Kuipers, E.J. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest. Endosc. 2010, 71, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Piazuelo, M.B.; Kuipers, E.J.; Li, D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology 2021, 161, 1325–1332.e7. [Google Scholar] [CrossRef] [PubMed]
- Rugge, M.; Fassan, M.; Pizzi, M.; Farinati, F.; Sturniolo, G.C.; Plebani, M.; Graham, D.Y. Operative link for gastritis assessment vs operative link on intestinal metaplasia assessment. World J. Gastroenterol. 2011, 17, 4596–4601. [Google Scholar] [CrossRef]
- Kononov, A.V.; Mozgovoĭ, S.I.; Shimanskaia, M.V.; Markelova, M.V.; Osintseva, I.L.; Vas’kina, T.V.; Paniushkin, L.V. The Russian revision of chronic gastritis classification: Reproducibility of the pathomorphologic picture. Arkhiv Patol. 2011, 73, 52–56. (In Russian) [Google Scholar]
- Wei, N.; Zhong, Z.; Shi, R. A novel method of grading gastric intestinal metaplasia based on the combination of subtype and distribution. Cancer Cell Int. 2021, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, S.; Vanoli, A. Gastric neuroendocrine neoplasms and related precursor lesions. J. Clin. Pathol. 2014, 67, 938–948. [Google Scholar] [CrossRef]
- Cockburn, A.N.; Morgan, C.J.; Genta, R.M. Neuroendocrine proliferations of the stomach: A pragmatic approach for the perplexed pathologist. Adv. Anat. Pathol. 2013, 20, 148–157. [Google Scholar] [CrossRef]
- Hsing, A.W.; Hansson, L.E.; McLaughlin, J.K.; Nyren, O.; Blot, W.J.; Ekbom, A.; Fraumeni, J.F. Pernicious anemia and subsequent cancer. A population-based cohort study. Cancer 1993, 71, 745–750. [Google Scholar] [CrossRef]
- Borch, K.; Ahren, B.; Ahlman, H.; Falkmer, S.; Granerus, G.; Grimelius, L. Gastric carcinoids: Biologic behavior and prognosis after differentiated treatment in relation to type. Ann. Surg. 2005, 242, 64–73. [Google Scholar] [CrossRef]


| Hematological Manifestations |
|
| Gastroenterological Manifestations |
|
| Psychoneurological Manifestations |
|
| Micronutrient | Development Frequency | Development Mechanisms | Manifestations |
|---|---|---|---|
| Vitamin B12 | 37–69% | Decreased production of intrinsic Castle’s factor by parietal cells of the gastric body and decreased absorption of vitamin B12 in the ileum | Hematological, gastroenterological, neuropsychiatric disorders |
| Iron | 52% | The presence of erosive lesions of the mucous membrane and possible latent blood loss, concomitant infection with H. pylori and bacteria competition for dietary iron, hypochlorhydria, and increased hepcidin synthesis against a background of a concomitant inflammatory process | Microcytic anemia |
| Vitamin C | Unknown | Breakdown of ascorbic acid in the stomach due to increased pH (hypo-, achlorhydria) and concomitant bacterial overgrowth in the small intestine | Decreased antioxidant defense, immunity, and protein synthesis |
| Calcium | Unknown | Dissolution, ionization, and absorption of calcium salts decrease under conditions of hypo-, achlorhydria | Osteopenia/Osteoporosis |
| Vitamin D | 12.1% | Not determined | Secondary hyperparathyroidism, osteopenia/osteoporosis, decreased immunity, increased risk of autoimmune disease development |
| Disease | Association Degree |
|---|---|
| Autoimmune thyroiditis (Hashimoto’s thyroiditis) and Graves’ disease | ++++ (multiple cohort studies, two crossover studies, and two case–control studies) |
| Type 1 diabetes mellitus | +++ (one case–control study and several cohort studies) |
| Vitiligo | ++ (one cohort study and several clinical observations) |
| Alopecia | ++ (several cohort studies) |
| Celiac disease | ++ (several cohort studies) |
| Myasthenia gravis | + (single clinical observations) |
| Connective tissue disease | ++ (one cohort study and several clinical observations) |
| Primary biliary cholangitis | ++ (one cohort study and several clinical observations) |
| Primary sclerosing cholangitis | + (single clinical observations) |
| Autoimmune hepatitis | + (single clinical observations) |
| Addison’s disease | + (single clinical observations) |
| Primary ovarian insufficiency | + (single clinical observations) |
| Primary hypoparathyroidism | + (single clinical observations) |
| Lambert-Eaton syndrome | + (single clinical observations) |
| Oral lichen planus | + (single clinical observations) |
| Indicant | Value | Comments |
|---|---|---|
| Antibodies to parietal cells | ↑ | They are found in 80–90% of patients with CAG, but they can also be present in 7.8–19.5% of healthy adults, as well as in people infected with HP or with other autoimmune diseases. They may not be determined in the later stages of the disease, as atrophy of the gastric mucosa progresses. |
| Antibodies to the intrinsic Castle’s factor | ↑ | Specificity—98.6%, sensitivity—30–60%. They correlate with atrophy of the gastric mucosa. |
| Pepsinogen I | ↓ | It is produced by the main cells of the stomach’s body. A decrease in the indicator shows the presence of atrophy in the mucous membrane of the stomach’s body. Serum level decreases in proportion to the severity of atrophy. |
| Pepsinogen II | Not changed | It is produced mainly in the stomach’s antrum and duodenum; therefore, it does not change with CAG. |
| Pepsinogen I/Pepsinogen II | ↓ | They decrease linearly with increasing severity of atrophy of the stomach’s body glands. |
| Gastrin-17 | ↑ | It is produced by gastrin-producing cells of the stomach’s antrum. With a decrease in acid production against the background of the stomach’s body glands atrophy, it increases by the mechanism of a negative feedback loop. |
| Chromogranin A | ↑ | It correlates with the degree of ECL cell hyperplasia under conditions of hypo- and achlorhydria. Due to the low sensitivity and specificity, its widespread use in routine practice is limited. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Livzan, M.A.; Gaus, O.V.; Mozgovoi, S.I.; Bordin, D.S. Chronic Autoimmune Gastritis: Modern Diagnostic Principles. Diagnostics 2021, 11, 2113. https://doi.org/10.3390/diagnostics11112113
Livzan MA, Gaus OV, Mozgovoi SI, Bordin DS. Chronic Autoimmune Gastritis: Modern Diagnostic Principles. Diagnostics. 2021; 11(11):2113. https://doi.org/10.3390/diagnostics11112113
Chicago/Turabian StyleLivzan, Maria A., Olga V. Gaus, Sergei I. Mozgovoi, and Dmitry S. Bordin. 2021. "Chronic Autoimmune Gastritis: Modern Diagnostic Principles" Diagnostics 11, no. 11: 2113. https://doi.org/10.3390/diagnostics11112113
APA StyleLivzan, M. A., Gaus, O. V., Mozgovoi, S. I., & Bordin, D. S. (2021). Chronic Autoimmune Gastritis: Modern Diagnostic Principles. Diagnostics, 11(11), 2113. https://doi.org/10.3390/diagnostics11112113








